Abstract
Idiopathic antineutrophil cytoplasmic antibody (ANCA)-associated vasculitides (AAV) are a group of diseases that are often difficult to diagnose due to the wide range of clinical manifestations. Notably, renal involvement is a serious organ complication, which usually requires intensive immunosuppressive therapy and is prone to recurrence. In recent years, there has been some progress regarding the understanding of the pathogenesis of the diseases. It has been shown that both cocaine and levamisole, which is a common adulterant of cocaine, can trigger the formation of ANCAs and lead to the corresponding symptoms. We report two cases of AAV with different renal manifestations associated with cocaine consumption. Furthermore, we performed a review of the literature to identify, characterize and describe histologically documented cases of renal involvement in AAV, related to cocaine abuse. Cocaine/levamisole-induced vasculitis may, therefore, mimic idiopathic AAV. Although the detection of ANCA and anti-PR3 (proteinase 3, PR3) as well as anti-MPO antibodies (myeloperoxidase, MPO) are the serological hallmark of idiopathic AAV, certain clinical- and antibody constellations should lead to consideration of illicit drugs as inductors of the disease. Especially in young patients, certain serologic constellations (e.g., PR3 and MPO double positivity, positive antinuclear antibodies, low complement level, and positive testing for antiphospholipid antibodies), skin involvement, musculoskeletal symptoms and hematologic (anemia, leukopenia) affections should prompt testing for cocaine and levamisole consumption via urine drug testing. Treatment includes both immunosuppressive approaches and drug cessation but is difficult since many patients continue cocaine consumption.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cocaine is an illicit drug with potent stimulating effects. According to the European Monitoring Centre For Drugs And Drug Addiction (EMCDDA), in 2018 the estimated lifetime prevalence of cocaine use in adults (15–65 years) in the European Union was 5.2%, ranking second after cannabis consumption (26.3%) [1]. Apart from its sympathomimetic effect and well-known cardiovascular complications, recent literature describes a wide range of clinical symptoms including rheumatic manifestations [2,3,4,5]. As early as 1996, Armstrong and colleagues described anti-neutrophil cytoplasmic antibody (ANCA)-associated nasal septal ulcerations in association with cocaine consumption, mimicking granulomatosis with polyangiitis (GPA) [6]. This manifestation is termed cocaine-induced midline destructive lesion (CIMDL) and thought to be mainly caused by the vasoconstrictive effects of the substance, as well as its traumatic effect on the mucosa and the recurrent local infections. In cases with ANCA positivity and elevated inflammatory parameters, clinical differentiation between CIMDL and local manifestations of GPA can be extremely challenging [3].
Certain toxic effects can be attributed directly to cocaine, but the cutting of illicit drugs with a variety of adulterants is common [7]. In 2009, 69% of seized cocaine in the US was contaminated with levamisole, an immune modulating and anthelmintic substance, with similar texture and taste as the drug, and potentiating effects on it [8, 9]. Data from 2015 reveal that up to 83% of the cocaine in Germany is adulterated by the substance, which was formerly used in human and nowadays veterinary medicine, to treat inflammatory conditions, cancer and infections [8, 10]. Since the late 1970s, levamisole-induced adverse effects have been described, especially leukopenia, agranulocytosis and cutaneous necrotizing vasculitis, resolving with discontinuation of the substance [11].
In recent literature, both cocaine and levamisole have been suspected as triggers of multi-organ ANCA-associated vasculitis (AAV) via induction of the formation of neutrophil extracellular traps (NETs), resulting in the presentation of proinflammatory mitochondrial DNA (mtDNA) [8, 12]. The clinical picture of cocaine- or levamisole-induced AAV closely resembles idiopathic AAV, with constitutional symptoms, cutaneous leukocytoclastic vasculitis (LCV), ear, nose and throat (ENT) involvement and, although rare, pulmonary and renal disease, including pauci-immune necrotizing glomerulonephritis (GN) and pulmonary–renal syndrome [5]. Certain autoantibody patterns, such as anti-PR3- and anti-MPO-ANCA double positivity with mainly perinuclear staining and high anti-MPO-titers, in combination with antinuclear antibody (ANA) positivity can suggest drug-induced manifestation [13].
We report two cases of AAV with renal manifestations associated with cocaine consumption and present a review of the literature describing histologically documented cases of renal involvement.
Case presentations
Case 1
A 19-year-old Caucasian male was referred to our center from an outpatient clinic to commence biologic therapy for a recently diagnosed AAV with ENT and renal involvement. He initially presented with LCV of the skin (extremities and trunk, no biopsy was performed), sinusitis with crusty, bloody nasal discharge and, glomerular hematuria with mild proteinuria accompanied by pain and swelling of the right elbow. Serologic parameters in Table 1 show that c-ANCA (cytoplasmic ANCA) was positive with no specificity for PR3 or MPO. Infectious diseases (HIV, hepatitis B and C, tuberculosis) were ruled out, and renal function and metabolic panel were normal. At the time of presentation, therapy consisted of daily prednisolone 80 mg and methotrexate (MTX) 20 mg subcutaneously weekly. Pulmonary manifestation was excluded by computer tomography (CT) and, cranial magnetic resonance imaging (MRI) showed maxillary sinusitis and ethmoid mucosal swelling. Repeat ANCA testing (IFT) was negative. A renal biopsy was performed, and histologic examination showed crescentic pauci-immune GN without signs of acute glomerular necrosis, but with intratubular erythrocyte casts, consistent with ANCA-associated GN (Fig. 2). The patient admitted transnasal cocaine abuse 2 weeks prior to disease manifestation. A drug screen at first presentation was not performed. We postulated cocaine-induced AAV and administered induction therapy with rituximab (RTX) 1000 mg twice with an interval of 2 weeks, and corticosteroids could be tapered within a period of 3 months. ENT- and skin manifestations resolved within weeks, mild proteinuria and hematuria persisted for 6 months, when complete remission was achieved. Follow-up drug screens were negative for cocaine.
Case 2
In 2012 a 39-year-old male presented with nasal septum perforation. The patient admitted occasional cocaine consumption in the past but denied recent use.
Autoimmune serology was positive for perinuclear-ANCA (p-ANCA) (1:80) and PR3 (700 U/mL). Further laboratory analysis is shown in Table 1. Pulmonary involvement was excluded by CT. MRI of the head showed maxillary sinusitis and biopsy of the nasal septum revealed necrotizing inflammation with lymphocytic inflammatory infiltrate without granulomas (Fig. 1). Localized ANCA-associated vasculitis was diagnosed and treatment with co-trimoxazole initiated. Due to progression of ENT-involvement prednisolone in combination with MTX 15 mg subcutaneously weekly was added in 2013.
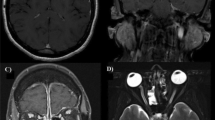
Case 2: Magnet-resonance imaging (MRI), clinical picture and biopsy specimen. a MRI orbita, T2 weighted; coronary plane, inflammatory pannus breaking through right sided orbital wall, affecting medial rectus muscle (→). b MRI orbita, T2 weighted axial plane, inflammatory mass entering right sided orbital cavity (→). c Massive necrosis of the hard palate. d Biopsy specimen (hematoxylin–eosin stain) of nasal ulceration showing active ulcerating and necrotic rhinitis without granuloma formation
In 2017, decreasing renal function and erythrocyturia were noted. Kidney biopsy revealed mesangial proliferative IgA-nephritis (Fig. 2). Therefore, treatment was switched to azathioprine, but as renal insufficiency progressed intravenous cyclophosphamide was initiated. After 12 doses of cyclophosphamide (cumulative dose: 14.4 g), maxillary granulation tissue perforated the right orbital cavity and caused blindness of the right eye (Fig. 1). Therapy was again intensified with an increase in corticosteroid dosage and oral cyclophosphamide in combination with the induction of RTX 1000 mg twice with an interval of 2 weeks. Surgical decompression was considered too risky. Markers of renal function remained stable throughout this period of the disease. Due to local infections (including multidrug-resistant Pseudomonas aeruginosa), the patient had to be hospitalized several times. As the hard palate also perforated, he lost over 40 kg of body weight within a few months due to feeding problems. As part of an outpatient follow-up, continued cocaine use was repeatedly confirmed in urine samples, though levamisole testing was repeatedly negative. The patient was unwilling to participate in a drug withdrawal program and was finally lost to further follow-up.
Representative images of the histological pattern of case 1 and case 2. a Case 1: Periodic acid–Schiff (PAS) stain showing crescentic pauci-immune glomerulonephritis. Note a fibrocellular crescent inside the bowmann space (→). b Case 1: Masson–Goldner stain, showing numerous erythrocyte cylinders inside tubuli due to rupture of the glomerular basement membrane (→). c Case 2: PAS stain, please note mesangial proliferation due to the deposition of IgA inside the mesangium (→). d Case 2: immunohistochemical stain for immunoglobulin A (IgA). Please note IgA deposits (stained red) inside the glomerular mesangium (→)
Literature search
A literature search with no restriction to publication date was conducted on 24 July 2019. The databases PubMed and Scopus were searched for case reports and case series that examined the role of cocaine/levamisole-induced ANCA-associated renal disease (detailed search terms are listed in the supplementary material).
Study selection
Primary studies providing empirical data from individual patients regarding the role of cocaine/levamisole-induced ANCA-associated renal disease were included. Two of the authors independently reviewed the identified studies in abstract format. If the abstracts did not provide sufficient data for a decision (e.g., ANCA status not reported within the abstract), full texts were reviewed instead.
The following inclusion criteria were applied: (1) article published in English language; (2) original paper was published in a peer-reviewed journal; (3) study type: case report or case series; (4) biopsy-proven renal involvement; and (5) positive ANCA testing. Studies that did not match the above-defined criteria were excluded from further analysis. Overall, there was no disagreement between the two reviewers throughout the selection process.
Data extraction
Individual patient data described within the case reports and case series were analyzed and extracted: clinical data; laboratory data; renal biopsy results; treatment; outcome; and follow-up. The extracted data were summarized (see supplementary material).
Results of the literature search
The reported search strategy (see supplementary material) initially identified 1089 publications. 513 publications were identified via PubMed, 558 publications via Scopus and two publications through other sources. Taken together, the search identified 635 publications (after removing duplicates) that were assessed for eligibility. 610 publications did not match the inclusion criteria. In total, we report the data of 41 cases from 25 publications (39 cases) and our own cases (2 cases) [5, 14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37].
Discussion
Renal involvement in cocaine-induced AAV is rare, and a renal biopsy was only performed in a minority of cases [5]. We present two cases of AAV with renal involvement associated with cocaine consumption and searched PubMed and Scopus for case reports and case series. More than half of the 41 cases (25/41, 60.9%) showed histological evidence of pauci-immune GN, which represents the typically encountered renal manifestation in AAV [38]. In 14.6% (6/41) of all cases, pauci-immune GN was associated with a concomitant renal pathology, such as membranous nephropathy in 5 cases and interstitial nephritis in 1 subject [30]. Interstitial nephritis has been attributed to cocaine consumption without association with positive ANCA titers in several case reports. Other acute kidney pathologies commonly seen in relation to cocaine consumption are renal infarction, thrombotic microangiopathy and acute kidney injury attributable to rhabdomyolysis [28, 39, 40].
Surprisingly, in association with cocaine consumption, more than one-third (39.0%, 16/41) of the renal biopsies revealed a variety of different pathologies, not commonly associated with AAV (Table 2). One-fifth (21.9%, 9/41) of the patients were diagnosed with membranous nephropathy (MN) and five of them with concomitant pauci-immune GN. Collister et al. presented two of these cases [14]. According to the authors, a combination of these two glomerulopathies has already been described in patients suffering from idiopathic AAV and in ANCA- as well as anti-GBM-antibody (glomerular basement membrane, GBM)-negative subjects without clinical signs of systemic lupus erythematosus (SLE).
One of our patients showed histological signs of mesangial proliferative nephritis with IgA deposits, which seems to be a rare entity in cocaine-induced AAV. In our review, there is only one similar case reported by Subesinghe et al. [23] (patient 9)—which showed mesangial proliferative GN with crescents and IgA deposits with concomitant cutaneous LCV, resembling the clinical picture of IgA vasculitis. This patient showed transient c-ANCA positivity with no specificity in PR3 or MPO testing. Another case, reported by Rowshani et al. [25], suffered from longstanding CIMDL and developed crescentic glomerulonephritis and mesangial IgA deposition with serological signs of GPA (c-ANCA, PR3-positive) and LCV of the skin. Our patient suffered from previous longstanding cocaine-induced nasal septal perforation with recurring bacterial superinfection. IgA nephropathy has been associated with upper respiratory tract infection [41]. Furthermore, IgA nephropathy is the most common primary GN worldwide. IgA nephropathy with ANCA positivity is a rare but known entity: in a retrospective study by Yang et al. [42], 1.2% of 1729 patients with IgA nephropathy showed concomitant ANCA positivity, with p-ANCA in 80%. This finding was associated with a more severe clinical picture, but followed by a better response to immunosuppressive therapy and renal outcome in short term. In our case, one has to consider that the occurrence of IgA deposition in pre-existing cocaine-induced AAV could also be a coincidence.
Three patients were diagnosed with immune-complex-mediated GN, and other patterns included focal segmental glomerulosclerosis, complement component 3 (C3)-associated GN, lupus nephritis and interstitial fibrosis, which were each demonstrated in one patient, respectively. In two case reports, description of the histological findings is somewhat unclear (‘proliferative glomerulonephritis’ and ‘focal segmental necrotizing glomerulitis’) and immunohistochemistry results are not provided; hence, they are listed as ‘other’ [27, 29].
Patients with cocaine-induced AAV showed p-ANCA staining in more than half of the reported cases (54.5%, 18/33). In 18.2% (6/33) of the subjects, c-ANCA staining was positive and in 27.3% (9/33) staining differentiation was not observed (Table 3). The testing for the corresponding antigen showed that MPO clearly predominated with a positivity in 74.2% (23/31) of the reported cases. Nearly half of the reported subjects (45.2%, 14/31) were double positive for MPO- and PR3-antibodies. Isolated PR3 positivity was only detected in four patients (12.9%, 4/31). Our second case showed p-ANCA staining with discordant anti-PR3 positivity, in accordance with previous other cases of CIMDL [3]. This paradoxical ANCA-constellation is typical for this manifestation. Furthermore, anti-human neutrophil elastase (HNE) antibodies are found in up to 80% of CIMDL individuals, with relatively low sensitivity but high specificity [11, 43]. The patients we report on were HNE negative in single testing.
Three case reports showed isolated positivity of PR3 in cocaine-induced AAV, with pauci-immune GN on kidney biopsy [25, 28, 31]. These cases are extremely difficult to distinguish from idiopathic AAV. Muñoz-Vahos et al. list eight patients with biopsy-proven renal manifestation, but specific antibody information for each patient was not provided [26]. As in our first case, 35.7% (10/28) of the subjects in this review were ANA positive. Only 17 subjects (17/41, 41.4%) were tested for antiphospholipid antibodies (APS-Abs), but in 47.1% (8/17) of them testing was positive. Low complement component (C) 3 was observed in 45.8% (11/24) and low C4 in 39.1% (9/23); in many subjects, complement components were not reported (C3 not reported in 17/41, C4 not reported in 18/41). In this review, no cryoglobulins were detected, and testing was performed in 12 patients.
Interestingly, 34 (82.9%) patients in our review were reported to have levamisole-induced AAV, but only 9 were effectively tested with positive results in 6 (6/41, 14.6%) subjects. This resembles a well-known problem: Larocque and Hoffmann [11] published a review of 206 cases with complications of levamisole-adulterated cocaine, where only 28% (57/203) had confirmed the presence of levamisole. Most of the authors rely on clinical and serological parameters to assume levamisole as a culprit. High titer MPO positivity and p-ANCA, as well as anti-PR3- and anti-MPO-antibody double positivity seem to be indicative, especially in concomitant APS-Abs positivity [3, 5, 44]. Furthermore, APS-Abs and leukopenia are rarely observed in GPA and MPA [44,45,46].
Idiopathic as well as cocaine-induced AAV presented with a wide range of clinical manifestations [3,4,5, 45]. 84.6% (33/39) of the patients in our review presented with cutaneous symptoms. As cited by Muñoz-Vahos et al., LCV of the skin is the most frequent observation in levamisole-induced AAV. The study even presents a typical clinical pattern with the appearance of retiform purpura of the extensor surfaces of the limbs, buttocks, face and abdomen in 83% of the patients. 73% had ear necrosis, which resembles the classic manifestation in association with levamisole intake [26].
Case 1 in our presentation showed arthritis of the elbow and LCV of the skin; 63.3% (19/30) of the subjects reviewed suffered from arthritis or arthralgia. In 11 patients (11/41, 26.8%), musculoskeletal symptoms were not reported. This result is consistent with the findings of the largest reviews performed on cocaine/levamisole-induced AAV [5, 26, 44].
64.8% (24/37) of the subjects in our review presented with anemia. In 21.6% (8/37), leukopenia was documented. One patient developed thrombocytopenia. These numbers are considerably lower than in the case series presented by Muñoz-Vahos et al., as well as in a recently published review of levamisole-induced systemic vasculopathy [44]. Pulmonary affection was observed in five patients (17.9%, 5/28), and pulmonary–renal syndrome (PRS) diagnosed in four cases (9.7%) [14, 15, 29, 30], which is less frequent than in GPA [45].
All except two patients in our review were initially treated with corticosteroids, accompanied by additional immunosuppressants [MMF 3.1% (1/32), MTX 6.2% (2/32), CYC 59.3% (19/32), RTX 25.0% (8/32)]. In 9 subjects (9/41, 21.9%), treatment was not reported. Eleven patients (11/41, 26.8%) received hemodialysis (HD) and 5 of them with additional plasmapheresis due to the severity of renal affection.
So far, there are no data or guidelines concerning treatment strategies in cocaine/levamisole-induced AAV with renal manifestation. Drug abstinence may be the mainstay of therapy. In general, re-exposure to the drug is present as a major challenge, as 45.2% (14/31) of the patients in our review were reported to have continued cocaine intake in follow-up consultations. Analysis of outcomes in different subgroups of patients was difficult and was, therefore, performed and discussed by two of the authors. Specific data were missing in a substantial number of cases (22/41, 53.6%). In 19 patients (19/41, 46.3%), follow-up concerning drug continuation and related outcome is comprehensible. In all the seven patients who stopped cocaine consumption, the clinical outcome was either stable or improved under treatment with immunosuppressants. In the remaining 12 cases, persistent substance abuse was observed. In seven of these cases, the clinical course was poor (relapse, ongoing dialysis dependence) and in four cases the situation stabilized (stable creatinine, protein and hematuria). Only one patient with ongoing cocaine consumption improved clinically, which underscores the therapeutic importance of drug cessation (Table 3).
Cocaine can be detected in blood samples up to 48 h after ingestion [47]. Cocaine metabolites can also be found up to 96 h after a single dose (urine testing). Preston et al. were able to demonstrate that in cases of increased doses or chronic abuse, detection in urine samples may even be possible up to 14 days after the last consumption [48].
Unfortunately, levamisole testing is more time consuming and expensive, since it is not offered by default by most laboratories. Since the half-life in blood and urine is only 5.6 h, only very recent consumption can be detected [49].
Another way to detect less recent cocaine use is a hair test. On average, hair grows 1 cm per month, so hair analysis can detect both cocaine abuse and in some cases levamisole [50, 51].
Our work has some limitations, such as the lack of laboratory proof of cocaine intake in case 1 and the absence of proof of levamisole and cocaine intake in many patients in the reviewed articles. We cannot exclude misdiagnoses between idiopathic AAV and cocaine-related disease in the review.
Based on this review, Table 4 displays major characteristics of cocaine and levamisole-induced AAV and provides implications for diagnosis and treatment.
Conclusion
Through rising cocaine consumption, cocaine and levamisole-induced AAV has become a frequently encountered form of vasculitis. Still, renal manifestation is rare and may present with atypical manifestations in a high proportion of patients, as observed in one of our reported cases. Although positive ANCA testing and anti-PR3 as well as anti-MPO are the serological hallmark of idiopathic AAV, the interpretation of the serologic markers and clinical manifestation should always lead to consideration of drugs as inductors of the disease. Especially in younger patients with certain serologic constellations (anti-PR3 and anti-MPO double positivity, concomitant ANA positivity, low complement level as well as APS-Abs) and skin as well as musculoskeletal symptoms and hematologic (anemia, leukopenia) affections should prompt testing for cocaine consumption via urine drug testing, and if positive with subsequent testing for levamisole. In the absence of established treatment guidelines, treatment consists of drug cessation. Despite this, in reality many patients continue to consume cocaine.
References
EMCDDA (2018) European drug report—trends and developments 2018. European drug report. Publications Office of the European Union, Luxembourg. https://doi.org/10.2810/800331
Lange RA, Hillis LD (2001) Cardiovascular complications of cocaine use. N Engl J Med 345(5):351–358. https://doi.org/10.1056/NEJM200108023450507
Graf J (2013) Rheumatic manifestations of cocaine use. Curr Opin Rheumatol 25(1):50–55. https://doi.org/10.1097/BOR.0b013e32835b4449
Espinoza LR, Perez Alamino R (2012) Cocaine-induced vasculitis: clinical and immunological spectrum. Curr Rheumatol Rep 14(6):532–538. https://doi.org/10.1007/s11926-012-0283-1
McGrath MM, Isakova T, Rennke HG, Mottola AM, Laliberte KA, Niles JL (2011) Contaminated cocaine and antineutrophil cytoplasmic antibody-associated disease. Clin J Am Soc Nephrol 6(12):2799–2805. https://doi.org/10.2215/CJN.03440411
Armstrong M Jr, Shikani AH (1996) Nasal septal necrosis mimicking Wegener’s granulomatosis in a cocaine abuser. Ear Nose Throat J 75(9):623–626
Cole C, Jones L, McVeigh J, Kicman A, Syed Q, Bellis M (2011) Adulterants in illicit drugs: a review of empirical evidence. Drug Test Anal 3(2):89–96. https://doi.org/10.1002/dta.220
Pendergraft WF 3rd, Niles JL (2014) Trojan horses: drug culprits associated with antineutrophil cytoplasmic autoantibody (ANCA) vasculitis. Curr Opin Rheumatol 26(1):42–49. https://doi.org/10.1097/BOR.0000000000000014
Centers for Disease C, Prevention (2009) Agranulocytosis associated with cocaine use—four States, March 2008–November 2009. MMWR Morb Mortal Wkly Rep 58(49):1381–1385
Dziadosz M, Klintschar M, Teske J (2015) Letter to the editor—consumption of levamisole in cocaine preparations. J Forensic Sci 60(2):538. https://doi.org/10.1111/1556-4029.12674
Larocque A, Hoffman RS (2012) Levamisole in cocaine: unexpected news from an old acquaintance. Clin Toxicol (Phila) 50(4):231–241. https://doi.org/10.3109/15563650.2012.665455
Lood C, Hughes GC (2017) Neutrophil extracellular traps as a potential source of autoantigen in cocaine-associated autoimmunity. Rheumatology (Oxford) 56(4):638–643. https://doi.org/10.1093/rheumatology/kew256
Wiik A (2008) Drug-induced vasculitis. Curr Opin Rheumatol 20(1):35–39. https://doi.org/10.1097/BOR.0b013e3282f1331f
Collister D, Sathianathan C, Ryz K, Karpinski M, Bernstein K, Gibson IW (2017) ANCA associated vasculitis secondary to levamisole-adultered cocaine with associated membranous nephropathy: a case series. Am J Nephrol 45(3):209–216. https://doi.org/10.1159/000456553
Carlson AQ, Tuot DS, Jen KY, Butcher B, Graf J, Sam R, Imboden JB (2014) Pauci-immune glomerulonephritis in individuals with disease associated with levamisole-adulterated cocaine: a series of 4 cases. Medicine (Baltimore) 93(17):290–297. https://doi.org/10.1097/MD.0000000000000090
Chawdhary K, Parke A (2015) Levamisole-induced vasculitis with renal involvement. Conn Med 79(6):343–346
Moinuddin I, Madhrira M, Bracamonte E, Thajudeen B, Sussman A (2016) Membranous nephropathy with crescents associated with levamisole-induced MPO-ANCA vasculitis. Pathol Res Pract 212(7):650–653. https://doi.org/10.1016/j.prp.2016.03.008
Sirvent AE, Enriquez R, Andrada E, Sanchez M, Millan I, Gonzalez C (2016) Necrotising glomerulonephritis in levamisole-contaminated cocaine use. Nefrologia 36(1):76–78. https://doi.org/10.1016/j.nefro.2015.10.008
Liu YW, Mutnuri S, Siddiqui SB, Weikle GR, Oladipo O, Ganti N, Beach RE, Afrouzian M (2016) Levamisole-adulterated cocaine nephrotoxicity: ultrastructural features. Am J Clin Pathol 145(5):720–726. https://doi.org/10.1093/ajcp/aqw029
Carrara C, Emili S, Lin M, Alpers CE (2016) Necrotizing and crescentic glomerulonephritis with membranous nephropathy in a patient exposed to levamisole-adulterated cocaine. Clin Kidney J 9(2):234–238. https://doi.org/10.1093/ckj/sfv141
Veronese FV, Dode RS, Friderichs M, Thome GG, da Silva DR, Schaefer PG, Sebben VC, Nicolella AR, Barros EJ (2016) Cocaine/levamisole-induced systemic vasculitis with retiform purpura and pauci-immune glomerulonephritis. Braz J Med Biol Res 49(5):e5244. https://doi.org/10.1590/1414-431X20165244
Olives TD, Kornas RL, Fujisawa R, Cole JB (2017) Unexpected complication of cocaine-associated anti-neutrophil cytoplasmic antibody vasculitis related to persistent in-hospital cocaine use. J Addict Med 11(2):157–160. https://doi.org/10.1097/ADM.0000000000000290
Subesinghe S, van Leuven S, Yalakki L, Sangle S, D’Cruz D (2018) Cocaine and ANCA associated vasculitis-like syndromes—a case series. Autoimmun Rev 17(1):73–77. https://doi.org/10.1016/j.autrev.2017.11.011
Kumar D, Batal I, Jim B, Mendez B, Anis K (2018) Unusual case of levamisole-induced dual-positive ANCA vasculitis and crescentic glomerulonephritis. BMJ Case Rep. https://doi.org/10.1136/bcr-2018-225913
Rowshani AT, Schot LJ, ten Berge IJ (2004) c-ANCA as a serological pitfall. Lancet 363(9411):782. https://doi.org/10.1016/S0140-6736(04)15694-8
Muñoz-Vahos CH, Herrera-Uribe S, Arbelaez-Cortes A, Jaramillo-Arroyave D, Gonzalez-Naranjo LA, Vasquez-Duque G, Restrepo-Escobar M, Correa-Londono LA, Arias-Restrepo LF, Vanegas-Garcia AL (2019) Clinical profile of levamisole-adulterated cocaine-induced vasculitis/vasculopathy: a 30-case series. J Clin Rheumatol 25(3):e16–e26. https://doi.org/10.1097/RHU.0000000000000813
Alqalyoobi S, Vaidya O, Abu Ghanimah AM, Elkhanany A, Gohar A (2015) Cocaine induced pleural and pericardial effusion syndrome. Case Rep Pulmonol 2015:321539. https://doi.org/10.1155/2015/321539
Filho J, Ogawa MY, de Souza Andrade TH, de Andrade Cordeiro Gadelha S, Fernandes P, Queiroz AL, Daher EF (2019) Spectrum of acute kidney injury associated with cocaine use: report of three cases. BMC Nephrol 20(1):99. https://doi.org/10.1186/s12882-019-1279-0
Berlioz AR, Garner O, Wiesner E, Iardino A, Bhairavarasu K (2017) Severe ANCA associated vasculitis in the setting of cocaine abuse: a case report. Clin Med Rev Case Rep 4(7):1–6. https://doi.org/10.23937/2378-3656/1410177
Baptiste GG, Alexopoulos AS, Masud T, Bonsall JM (2015) Systemic levamisole-induced vasculitis in a cocaine user without cutaneous findings: a consideration in diagnosis. Case Rep Med 2015:547023. https://doi.org/10.1155/2015/547023
Neynaber S, Mistry-Burchardi N, Rust C, Samtleben W, Burgdorf WH, Seitz MA, Messer G, Wollenberg A (2008) PR3-ANCA-positive necrotizing multi-organ vasculitis following cocaine abuse. Acta Derm Venereol 88(6):594–596. https://doi.org/10.2340/00015555-0514
van der Veer T, Pennings E, Tervaert JW, Korswagen LA (2015) Levamisole-contaminated cocaine: a hairy affair. BMJ Case Rep. https://doi.org/10.1136/bcr-2015-210970
Shiue Z, McNicholas B, Cormack F, Akilesh S (2015) Antineutrophil cytoplasmic antibody mediated glomerulonephritis associated with levamisole-adulterated cocaine. Clin Nephrol Case Stud 3:37–41. https://doi.org/10.5414/CNCS108385
Roca-Argente L, Moll-Guillen JL, Espi-Reig J, Blanes-Julia M, Garcia-Martinez AM, Pujol-Marco C, Hernandez-Jaras J (2015) Membranous glomerulonephritis and cellular crescents induced by levamisole-adulterated cocaine abuse: a case report. Ann Transl Med 3(18):271. https://doi.org/10.3978/j.issn.2305-5839.2015.10.29
Garg L, Gupta S, Swami A, Zhang P (2015) Levamisole/Cocaine induced systemic vasculitis and immune complex glomerulonephritis. Case Rep Nephrol 2015:372413. https://doi.org/10.1155/2015/372413
Gulati S, Donato AA (2012) Lupus anticoagulant and ANCA associated thrombotic vasculopathy due to cocaine contaminated with levamisole: a case report and review of the literature. J Thromb Thrombolysis 34(1):7–10. https://doi.org/10.1007/s11239-012-0711-0
Posada AF, Neri ID, Bustos MF, Castellanos M, Calderón M (2015) Systemic lupus erythematosus associated with chronic cocaine use [Lupus eritematoso sistémico asociado al consumo crónico de cocaína]. Rev Colomb Rheumatol 22(3):174–179. https://doi.org/10.1016/j.rcreu.2015.06.002
Jennette JC, Wilkman AS, Falk RJ (1989) Anti-neutrophil cytoplasmic autoantibody-associated glomerulonephritis and vasculitis. Am J Pathol 135(5):921–930
Bahaa Aldeen M, Talibmamury N, Alalusi S, Nadham O, Omer AR, Smalligan RD (2014) When coke is not hydrating: cocaine-induced acute interstitial nephritis. J Investig Med High Impact Case Rep 2(3):2324709614551557. https://doi.org/10.1177/2324709614551557
Goel N, Pullman JM, Coco M (2014) Cocaine and kidney injury: a kaleidoscope of pathology. Clin Kidney J 7(6):513–517. https://doi.org/10.1093/ckj/sfu092
Wyatt RJ, Julian BA (2013) IgA nephropathy. N Engl J Med 368(25):2402–2414. https://doi.org/10.1056/NEJMra1206793
Yang YZ, Shi SF, Chen YQ, Chen M, Yang YH, Xie XF, Zou R, Lv JC, Liu LJ, Zhang H (2015) Clinical features of IgA nephropathy with serum ANCA positivity: a retrospective case-control study. Clin Kidney J 8(5):482–488. https://doi.org/10.1093/ckj/sfv078
Néel A, Agard C, Hamidou M (2018) Vasculitides induced by cocaine and/or levamisole. Jt Bone Spine 85(1):9–14. https://doi.org/10.1016/j.jbspin.2017.05.022
Dartevel A, Chaigne B, Moachon L, Grenier F, Dupin N, Guillevin L, Bouillet L, Mouthon L (2019) Levamisole-induced vasculopathy: a systematic review. Semin Arthritis Rheum 48(5):921–926. https://doi.org/10.1016/j.semarthrit.2018.07.010
Almouhawis HA, Leao JC, Fedele S, Porter SR (2013) Wegener’s granulomatosis: a review of clinical features and an update in diagnosis and treatment. J Oral Pathol Med 42(7):507–516. https://doi.org/10.1111/jop.12030
Villiger PM, Guillevin L (2010) Microscopic polyangiitis: clinical presentation. Autoimmun Rev 9(12):812–819. https://doi.org/10.1016/j.autrev.2010.07.009
Blaho K, Logan B, Winbery S, Park L, Schwilke E (2000) Blood cocaine and metabolite concentrations, clinical findings, and outcome of patients presenting to an ED. Am J Emerg Med 18(5):593–598. https://doi.org/10.1053/ajem.2000.9282
Preston KL, Epstein DH, Cone EJ, Wtsadik AT, Huestis MA, Moolchan ET (2002) Urinary elimination of cocaine metabolites in chronic cocaine users during cessation. J Anal Toxicol 26(7):393–400
Lynch KL, Dominy SS, Graf J, Kral AH (2011) Detection of levamisole exposure in cocaine users by liquid chromatography–tandem mass spectrometry. J Anal Toxicol 35(3):176–178
Lazareth H, Peytavin G, Polivka L, Dupin N (2012) The hairy-print for levamisole-induced vasculitis. BMJ Case Rep. https://doi.org/10.1136/bcr-2012-006602
Polivka L, Peytavin G, Franck N, Mouthon L, Dupin N (2015) Testing for levamisole and cocaine in hair samples for the diagnosis of levamisole-related panniculitis. J Eur Acad Dermatol Venereol 29(12):2487–2489. https://doi.org/10.1111/jdv.12582
Author information
Authors and Affiliations
Contributions
FL and MK share the first authorship and equally contributed to the draft of the manuscript. FL and NR performed the review of the literature. TK and IK reviewed the manuscript. FP provided and commented on the histopathologic examination. All the authors reviewed the draft and provided comments for change. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lötscher, F., Krusche, M., Ruffer, N. et al. Cocaine-induced ANCA-associated renal disease: a case-based review. Rheumatol Int 39, 2005–2014 (2019). https://doi.org/10.1007/s00296-019-04410-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-019-04410-9