Abstract
Purpose of review
(Levamisole adulterated) cocaine can cause a number of symptoms. One of the most severe is cocaine-induced vasculitis, which is hard to both diagnose and treat. We conducted a review to summarize the most recent findings on symptomatology, treatment options, anti-neutrophil cytoplasmic antibodies (ANCA) positivity and pathophysiology.
Recent findings
In the past years multiple large cohort studies have been published extensively describing the symptomology and rates of ANCA positivity in patients with (levamisole-adulterated) cocaine-induced vasculitis. These studies also give more insight into the effects of different treatment strategies.
Summary
The mainstay of treatment is abstinence of cocaine supported by antibiotics in case of concomitant infections and/or immunosuppressive medication depending on symptoms. ANCA positivity is a hallmark of more extensive disease and is a characteristic of immune system activation. In cocaine-induced vasculitis, dual positivity for both proteinase 3(PR3)- and myeloperoxidase (MPO)-ANCA is described and some patients are found to have human elastase type (HNE-)ANCA. HNE-ANCA positivity varies in patients with cocaine-induced midline destruction (CIMDL) from 28-84%, but has not been researched thoroughly in patients with cocaine-induced vasculitis. We present our hypothesis of a “sliding-scale” by which CIMDL turns into cocaine-induced systemic vasculitis based upon recent literature and we describe the mechanisms by which cocaine-induced vasculitis develops.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cocaine is an extract from the leaves of Coca plants native to South-America. In leaf form Coca has been chewed on by the indigenous people of South America for more than a thousand years. Traditionally it was used to cope with tiredness, hunger and altitude sickness. In 1855 a German scientist managed to isolate the alkaloid that was the main cause of these effects and cocaine was born [1]. Western medicine quickly adapted the new drug and began to use it as an anesthetic in various fields. One of the most vocal advocates for cocaine was Sigmund Freud, who described it as a “magical substance”.
For the public cocaine really caught on when in 1886 American pharmacist John Sith Pemberton created a sugary drink with added coca leaves, also known as Coca-Cola. Since then both legal and illegal use of cocaine skyrocketed with the drug being added to all sorts of agents. This however also made the dangers of cocaine apparent in short time. The addictiveness and physical side effects of prolonged use became more and more clear, which caused the US government to require products containing cocaine to be labeled as such in 1906. Under public pressure cocaine was already removed from Coca-Cola in 1903 and in 1914 possession and distribution for (non-registered) personal use became illegal [1]. This caused cocaine use to lessen until the 1970’s/1980’s when it saw a resurgence in illegal use, partially due to the discovery of “crack” cocaine, a form in which cocaine can be smoked by dissolving it in bicarbonate and water.
Nowadays cocaine is the second most popular illegal recreational drug in Europe and the US with a reported yearly use of 2.3% of the European population aged 15–34 years old. 68% of cocaine used in Europe is snorted, while 28% is smoked [2]. Increased amounts of cocaine in wastewater analyses, increased medical care related to cocaine use and a historically high amount of cocaine busts show signs for an increasing usage of cocaine in the European population. In these seizures the average purity was between 48–85% [2]. Cocaine is often adulterated with sugar, cornstarch or fentanyl, but most often with levamisole, with studies suggesting that around 83% of all cocaine in Germany and 88% of all cocaine in the US contains this anthelmintic drug mostly used in veterinary medicine [3].
Cocaine is a stimulant drug that has diverse effects on multiple neurotransmitters. The most known effect is the blockade of the dopamine transporter protein. This causes dopamine to gather in the synaptic cleft and activates reward mechanisms which leads to euphoria and dependence. Due to diverse mechanisms in the body, which include increase in norepinephrine levels and changes in calcium channel currents cocaine also has a number of different physical effects, some of these are increased energy and motor activity, tachycardia, decreased appetite and vasoconstriction.
Exposure to cocaine has significant negative effects, which increase with prolonged use. Chronic intranasal usage can lead to destruction of mucosal and osseocartilaginous structures inside the nose, a condition known as cocaine-induced midline destructive lesions (CIMDL). The use of (in particular levamisole-adulterated) cocaine can lead to cocaine-induced vasculitis, a potentially life-threatening disease of which we currently have limited knowledge. Because of the limited knowledge there is an active discussion about the pathophysiology, diagnostics and treatment of cocaine-induced vasculitis. We review what is currently known about cocaine-induced vasculitis and present hypotheses regarding the pathophysiological mechanism by which this disease develops.
General symptomology
A one-time use of cocaine can lead to cardiac arrythmias, angina, heart failure, paranoid delusions, seizures or epistaxis. Chronic cocaine use increases the chance of aforementioned complications, leads to withdrawal symptoms when stopping use and has specific long-term complications depending on method of delivery.
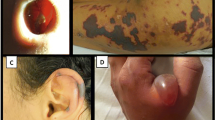
The most common symptoms are arthralgia (40–83%), rhinological symptoms such as sinusitis or CIMDL (44–98%, especially when cocaine is used intranasally), skin manifestations (61–83%) such as retiform purpura, ear necrosis and cutaneous ulcers, and constitutional symptoms (27–72%) such as fever, night sweats, weight loss, malaise, or myalgia [4,5,6]. Chronic smoking of cocaine can lead to bronchospasms, hemoptysis, asthma and systemic eosinophilia. Less common, but potentially life-threatening symptoms are nephritis/renal failure, alveolar haemorrhage and pulmonary-renal syndrome [6, 7].
These symptoms often go together with abnormal haematologic lab and urinalysis results. In previous studies leukopenia (both lymphopenia and neutropenia), anaemia, thrombocytosis, or thrombocytopenia, proteinuria and haematuria have been described [4, 6].
Pathophysiology of cocaine-induced vasculitis: what is known
The pathogenesis of cocaine-induced vasculitis remains elusive. The main difference between heavy cocaine users with symptoms of cocaine-induced vasculitis and heavy users without these symptoms appears to be positive ANCA serology. Although there are reported cases in which symptoms of cocaine-induced vasculitis present without ANCA-positive serology, most cases present with at least one ANCA-positive blood result.
There are a few theories as to why levamisole adulterated cocaine causes an auto-immune response with the formation of ANCAs. The main theory concerns the release of neutrophil extracellular traps (NETs). Activated neutrophils release NETs (a process also called NETosis) which contain dsDNA, histones and granules with MPO, PR3, elastase and lactoferrin among others, which induces ANCAs [8]. Levamisole-adulterated cocaine has been proven to cause the release of NET-associated neutrophil elastase in the neutrophils of healthy donors and NET targeted antibodies have been found in patients with use of levamisole adulterated cocaine with and without systemic symptoms [8, 9]. NETs cause inflammation through a number of mechanisms, they:
-
Cause direct endothelial damage.
-
Activate the alternative complement pathway.
-
Make up a big part of thrombi and give structural stability to the formation of clots.
-
Activate the coagulation cascade through both the extrinsic and intrinsic pathway.
Due to this damage the process of NETosis with the release of ANCAs gets activated again, which causes NETs to once again be released, which causes a vicious cycle. What potentiates the vicious cycle of NETosis is the concomitant bacterial infection which is found in almost all CIMDL/cocaine-induced vasculitis patients. Some bacteria such as S. aureus and certain streptococcal species are known to release toxins known as superantigens which activate the immune system in an unrestricted manner [10, 11]. According to our hypothesis this unrestricted immune response again induces NETosis. Studies performed in the past have shown that the destruction of neutrophils caused by levamisole seems immune mediated rather than through the direct cytotoxic effect of levamisole [12]. The concomitant infection found in cocaine-induced vasculitis is comparable to the concomitant infection found in (other) ANCA-associated vasculitides such as granulomatosis with polyangiitis (GPA). In GPA S. aureus infection seems to play a role in triggering auto-immune inflammatory responses in a multitude of ways [13]. This shows the importance of also treating concomitant infections with bacteria known to release superantigens, aside from stopping cocaine use, in patients with CIMDL/cocaine -induced vasculitis.
The above-described theories illustrate how cocaine-induced vasculitis inflicts damage, but doesn’t give an explanation as to why some people develop it and some don’t. It has been suggested that the above-described sequence of NETosis with the release of ANCAs mainly happens in genetically susceptible individuals. Multiple studies point to the genes that code for humane leukocyte antigen (HLA) B27 which would give susceptible patients a genetic predisposition to the negative effects of mainly levamisole which would make these people more likely to develop cocaine-induced vasculitis [12]. Notably, HLA-B(27) does not emerge as a gene locus linked to GPA or microscopic polyangiitis (MPA), but does seem to have an association with eosinophilic GPA (eGPA) and chronic sinusitis with nasal polyps (CRSwNP) as is shown by comprehensive whole genome-wide association studies (GWAS) across patient cohorts [14, 15].
To summarize, levamisole-adulterated cocaine seems to induce systemic vasculitis in genetically susceptible patients due to a combination of factors. These mainly consist of immune mediated damage caused by levamisole, which induces NETosis, which releases ANCAs, which induces NETosis and causes a vicious cycle. This entire process is exacerbated by a concomitant infection with bacteria that secrete superantigens. (Fig. 1a) We think that there is a sliding scale of response to levamisole adulterated cocaine in genetically susceptible patients, which you can roughly break down into four categories. (Fig. 1b).
The first of these categories is patients with almost no genetical susceptibility to the negative effects of levamisole. These patients just experience the usual side effects of cocaine use, but don’t have complaints severe enough to go to a doctor despite potential heavy usage of cocaine. These patients are unlikely to form ANCAs but no evidence is available to confirm this suspicion.
The second category is patients with some susceptibility and therefore symptoms severe enough to go to a doctor. These patients usually have isolated CIMDL, pulmonary symptoms or skin lesions, but symptoms are limited to one (external) organ system such as the skin, the nose or the lungs (when crack cocaine is used). In this category ANCA positivity is quite common. Despite the positive ANCA serology these patients have limited systemic symptoms.
The third category would be patients with systemic vasculitis induced by levamisole-adulterated cocaine, presenting itself as a transitional phase from a local to a systemic disease. Patients develop symptoms over multiple organ systems such as CIMDL in combination with skin manifestations and/or arthralgia and/or constitutional symptoms. In this stage internal organs generally are still spared but the first signs of renal failure such as increased creatinine may already be showing. ANCAs are (almost) always found and dual positivity with MPO and/or PR3 (and/or HNE, however this has not been tested) is found more often, yet still not common.
The fourth category is patients with a full-blown systemic vasculitis with damage to internal organs. These patients transition to this phase because of genetic susceptibility combined with prolonged use of cocaine (in high doses). In this phase internal organs start failing and patients get symptoms such as alveolar haemorrhages, kidney failure or signs of pulmonary-renal syndrome. Dual positive ANCA serology is found often, yet still not in all patients.
Diagnosis of cocaine-induced vasculitis
The average patient presenting themselves at an outpatient clinic with symptoms due to their cocaine use is male (cocaine-induced vasculitis affects males and females equally, but in Europe cocaine use among males is 1.5 to 2 times higher than use among females) and between 30–50 years old (but ages range between 18–57 years), see Table 1 [2, 5, 6, 16]. Patients usually present themselves at an ENT or dermatology outpatient clinic, depending on whether they have rhinological symptoms or skin manifestations [5, 8, 17]. When patients present with more systemic complaints they will often be referred to rheumatologists and/or immunologists for diagnosis of an underlying systemic disease. Diagnostic biopsies often return non-specific findings of necrosis making the diagnostic process difficult with worsening symptoms until the patient’s cocaine use comes to light.
Establish cocaine use
When a patient presents with a vasculitis-like disorder and cocaine use is suspected, it is important to try and confirm / disprove this suspicion. However, this can be quite challenging since patients often don’t want to admit their drug use. There are a multitude of reasons like being ashamed, being afraid of legal consequences or being afraid of not being helped (in the way that they want, i.e. wanting to get rid of the symptoms without wanting to stop their drug use). The first step in this process is to establish a basis of trust, which can be a long and arduous process. Even when a patient admits their previous use, it is good to realize that cocaine addiction is a chronic disorder with many relapses of use. To be able to provide the right advice and counselling toxicology tests can be of aid. In previous studies reliability of patient self-reported use was highly inaccurate with 21–40% falsely reporting abstinence > 1 year or non-lifetime use [5, 18]. It is of note that patients who are asked about illicit drug use in the past year are more likely to accurately report drug use than patients who are straight up asked if they have used any form of cocaine, so this should be taken into account when taking a patient’s medical history.
The most commonly used toxicology tests are blood tests and urinalysis. Benzoylecgonine, one of the main metabolites of cocaine, can be detected in urine for 48–72 h after use, depending on dosage taken, way of administration and individual patient characteristics. With chronic, high dosage use (10 g a day, for comparison the usual intranasal dose is 20-100 mg) the drug has been detected for up to 22 days after the last consumption. In blood cocaine is detectable for 4–6 h if the user has taken 20 mg and up to 12 h if 100 mg was taken [19]. These detection times are useful for identifying recent use, but to get a more complete insight into a patient’s drug use hair tests have been getting more and more popular. This is because hair tests should be able to detect cocaine use for up to 3 months after last use [20]. Hair tests should however be interpreted with care since up to half of all users can still be missed with a hair test (sensitivity ranges from 50%-97%) [21,22,23]. Even though the number of false positives in clinical setting is generally very low (specificity > 90%), external contamination of hair with cocaine can lead to false positive results [24]. Since hair testing is the most available technique that can analyse cocaine use with decent reliability for a period longer than 48 h, we still recommend hair testing at least once if urinalysis is negative and doubts about cocaine use still persist. Periodic testing can also be considered in consultation with patients who admit to use of cocaine in the past but are likely to have a relapse as a way to ensure that the patient continues their abstinence (since they know tests are being done periodically) [25].
Vasculitis diagnostic procedures
Biopsies are the gold standard for diagnosing vasculitis. However, biopsies of the nasal cavity in cocaine-induced vasculitis often return with non-specific findings like necrosis and generalized inflammation. Skin biopsies can show leukocytoclastic vasculitis, thrombotic vasculopathy or pseudovasculitis, but these findings are seen in less than half of all patients [6]. Nasal/sinus biopsy does not seem to be more specific, often showing generalized necrosis, acute and chronic inflammatory changes. Less commonly found are signs of leukoclastic/small vessel vasculitis and fibrinoid necrosis [5, 26]. The only consistent finding in studies that have performed nasal biopsies is that no granulomatous inflammation was visualised, which might make nasal biopsy a valid diagnostic option for differentiation of cocaine-induced vasculitis from granulomatosis with polyangiitis (GPA) but still can’t differentiate from other types of vasculitis [5, 16, 27]. It is however important to keep in mind that the absence of granulomatosis can also be a sampling error, so one biopsy without granulomatosis does not definitively rule out GPA.
Anti-neutrophil cytoplasmic antibody (ANCA) tests have varying degrees of positivity in patients with CIMDL or cocaine-induced vasculitis, with ANCA observed in as few as 28% of CIMDL patients [10]. (Table 2) ANCA positivity is not prevalent in patients that use cocaine, but do not have CIMDL or cocaine-induced vasculitis, with a maximum of 4% in one large study examining a group of 2740 patients [28]. With immunofluorescence p-ANCA is more often found (38–100%) than c-ANCA (6–50%). ELISA testing shows varying degrees of MPO- and PR3-ANCA without a predominance of one of the two types. Interestingly, in studies that have found positive MPO-ANCA, the number of patients is often > 80%, but there are also studies in which no patients have MPO-ANCA positivity [4, 6]. This might be because the studies that have more positive cases of MPO-ANCA involve patients with cocaine-induced vasculitis instead of CIMDL. It could therefore be theorised that MPO-ANCA can be a sign of systemic involvement / vasculitis, but this isn’t certain because the differences can also be explained by differences in testing methodology or another unknown factor. In contrast PR3-ANCA is found in half of all patients in almost all performed studies. One multi-patient study reported the amount elastase ANCA found in patients with CIMDL, which was 28% [10]. In 2004, a study done by Wiesner et al. showed a high number of patients with a positive human neutrophil elastase (HNE) ANCA, with 84% having a positive test result. The frequency of HNE ANCA in autoimmune diseases is thought to be very low, this is why this article has often been referenced as proof that the presence of HNE ANCA is the most distinguishing feature of CIMDL. Even though it certainly is a very distinguishing feature of CIMDL, it should not be used as a golden standard for proving cocaine-induced vasculitis. A later study performed by Peikert et al. showed that HNE ANCA was only positive in 21 out of 37 (57%) of their patients [29]. Larger studies evaluating the presence of HNE ANCA have not been performed. Another problem with HNE ANCA testing is that it is not widely available in every country. Concluding, ANCA serology can be of help in differentiating CIMDL or cocaine-induced vasculitis from auto-immune diseases, however results need to be used in conjunction with other available data to make a definitive diagnosis. We recommend first performing regular (MPO/PR3) ANCA tests and when the suspicion arises that a patient’s symptoms are due to cocaine abuse a HNE ANCA test can be performed if available.
Treatment of cocaine-induced vasculitis
The main principle of treatment is cessation of cocaine use. Symptoms such as skin lesions and CIMDL have shown either recovery or diminishment (sometimes within weeks to months) after stopping cocaine use [5, 26]. The moment cocaine is recognized as the culprit for the symptoms a patient is having, counseling should be focused towards stopping. Patients should also be offered to start a contingency management program or some other form of psychosocial intervention to assist in ceasing cocaine use since these increase the chance of successful discontinuation of use [32].
Aside from stopping use, therapy is mostly supportive. For CIMDL an ENT surgeon specialized in (cocaine) rhinology should be consulted. Supportive therapy for CIMDL should consist of nasal rinsing with saline solution, long term antibiotics to treat chronic sinusitis concomitant with CIMDL and eventually nasal reconstruction if the patient has long-term reduction of symptoms and proven discontinuation of cocaine use [5, 33]. For the initial antibiotic treatment trimethoprim/sulfamethoxazole is recommended due to positive effects seen in GPA and the experience the authors have using this antibiotic in patients with CIMDL/cocaine-induced vasculitis [34, 35]. Antibiotic therapy should be reconsidered if cultures show reduced effectiveness or when infection worsens during initial therapy. Dependent on the severity of skin lesions, an experienced Wound Care Nurse or dermatologist should be consulted for appropriate treatment and supportive care [36]. Besides CIMDL and skin lesions, cocaine-induced vasculitis can present with a multitude of symptoms. Depending on symptoms an internist and/or rheumatologist should be consulted.
Literature does not provide a clear consensus on whether immunosuppression with steroids or other immunosuppressive medication should be started in all patients with cocaine-induced vasculitis. In a review concerning cocaine/levamisole vasculopathy published by Pearson et al. 31 out of 35 patients showed improvement or resolution of symptoms after cessation of cocaine use [37]. However only 60% of these patients were treated with systemic corticosteroids, while 40% of patients were being treated conservatively. In a case series done by Gill et al. evaluating patients with CIMDL, all patients who continued cocaine use stayed symptomatic regardless of immunosuppressive therapy [5]. A few patients who continued cocaine use did show (temporary) reduction in symptoms with immunosuppressive therapy. The conclusion that can be drawn from all available literature is that symptoms will only disappear completely by ceasing cocaine use. Immunosuppressive therapy doesn’t seem to be applicable in all cases, but should be prescribed on an individual basis, mostly for bouts of inflammation. Immunosuppressive therapy should match the severity of symptoms; for example, prednisone and methotrexate might be used for mild cases, with rituximab reserved for more severe cases. Current studies do not show preferred immunosuppressive therapy so we recommend prescribing immunosuppressive therapy according to the experience of the physician. The role for immunosuppressive therapy after stopping cocaine treatment is unclear as both curation with conservative therapy and immunosuppressive therapy have been described, but no studies compare the two after stopping cocaine use.
Immunosuppressive therapy does seem to be applicable in all patients who have involvement of internal organs [7, 38]. In a review done by Jin et al. 15 out of 21 patients with pauci-immune glomerulonephritis had improvement or stabilisation of symptoms under immunosuppressive therapy, even though the majority of patients continued to use cocaine [8].
Conclusions
Healthcare professionals who come into contact with cocaine users (ENT, dermatology, pulmonology and rheumatology/immunology) should recognize the symptoms of cocaine-induced vasculitis. Once there is a suspicion of cocaine-induced vasculitis it is preferred to (dis)prove recent cocaine use with a hair test. Biopsies and (elastase) ANCA serology can help in ruling out auto-immune diseases but should be used in combination with other available data. The main principle of treatment is cessation of cocaine use. It is crucial to offer patients a contingency management program or some other form of psychosocial intervention as this increases the chances of successful cessation. Apart from cessation of cocaine use, treatment is mostly supportive, with antibiotic treatment of bacterial superinfections being the most important part of supportive therapy. It is advised to consult specialists for a multidisciplinary treatment of symptoms if necessary. Immunosuppressive treatment / steroids should mostly be reserved for an exacerbation of symptoms or involvement of internal organs since the currently available evidence doesn’t seem to show a beneficial effect of immunosuppressive therapy on all patients.
Levamisole-adulterated cocaine seems to induce systemic vasculitis in genetically susceptible patients due to immune mediated damage caused by levamisole, which induces NETosis, which releases ANCAs, which induces NETosis and causes a vicious cycle. This entire process is exacerbated by a concomitant infection with bacteria that secrete superantigens so it is of the outmost importance to treat any concomitant infections in patients with cocaine-induced vasculitis. We present a sliding scale hypothesis of response to levamisole adulterated cocaine in genetically susceptible patients, but future research needs to be done to further get a grasp on this disease, it’s pathophysiology and the best treatment options.
Data availability
No datasets were generated or analyzed during the current study.
References
Johanson CE, Fischman MW. The pharmacology of cocaine related to its abuse. Pharmacol Rev. 1989;41(1):3–52.
European Monitoring Centre for Drugs and Drug Addiction. European Drug Report 2023: Trends and Developments. 2023. https://www.emcdda.europa.eu/publications/european-drug-report/2023_en. https://doi.org/10.2810/161905.
Dziadosz M, Klintschar M, Teske J. Letter to the editor-Consumption of levamisole in cocaine preparations. J Forensic Sci. 2015;60(2):538. https://doi.org/10.1111/1556-4029.12674.
McGrath MM, Isakova T, Rennke HG, Mottola AM, Laliberte KA, Niles JL. Contaminated cocaine and antineutrophil cytoplasmic antibody-associated disease. Clin J Am Soc Nephrol. 2011;6(12):2799–805. https://doi.org/10.2215/cjn.03440411.
Gill C, Sturman J, Ozbek L, Henderson SR, Burns A, Hamour S, et al. Cocaine-induced granulomatosis with polyangiitis-an under-recognized condition. Rheumatol Adv Pract. 2023;7(1):rkad027027. https://doi.org/10.1093/rap/rkad027.
Muñoz-Vahos CH, Herrera-Uribe S, Arbeláez-Cortés Á, Jaramillo-Arroyave D, González-Naranjo LA, Vásquez-Duque G, et al. Clinical Profile of Levamisole-Adulterated Cocaine-Induced Vasculitis/Vasculopathy: A 30-Case Series. J Clin Rheumatol. 2019;25(3):e16–26. https://doi.org/10.1097/rhu.0000000000000813.
Bucur P, Weber M, Agrawal R, Madera-Acosta AI, Elam RE. Pulmonary-Renal Syndrome from Levamisole-Adulterated Cocaine-Induced Antineutrophil Cytoplasmic Antibody (ANCA)-Associated Vasculitis: A Systematic Review. Pharmaceuticals (Basel). 2023;16(6). https://doi.org/10.3390/ph16060846.
Jin Q, Kant S, Alhariri J, Geetha D. Levamisole adulterated cocaine associated ANCA vasculitis: review of literature and update on pathogenesis. J Community Hosp Intern Med Perspect. 2018;8(6):339–44. https://doi.org/10.1080/20009666.2018.1536242.
Lood C, Hughes GC. Neutrophil extracellular traps as a potential source of autoantigen in cocaine-associated autoimmunity. Rheumatology (Oxford). 2017;56(4):638–43. https://doi.org/10.1093/rheumatology/kew256.
Trimarchi M, Gregorini G, Facchetti F, Morassi ML, Manfredini C, Maroldi R, et al. Cocaine-induced midline destructive lesions: clinical, radiographic, histopathologic, and serologic features and their differentiation from Wegener granulomatosis. Medicine (Baltimore). 2001;80(6):391–404. https://doi.org/10.1097/00005792-200111000-00005.
Trimarchi M, Bussi M, Sinico RA, Meroni P, Specks U. Cocaine-induced midline destructive lesions - an autoimmune disease? Autoimmun Rev. 2013;12(4):496–500. https://doi.org/10.1016/j.autrev.2012.08.009.
Cascio MJ, Jen KY. Cocaine/levamisole-associated autoimmune syndrome: a disease of neutrophil-mediated autoimmunity. Curr Opin Hematol. 2018;25(1):29–36. https://doi.org/10.1097/moh.0000000000000393.
Dekkema GJ, Rutgers A, Sanders JS, Stegeman CA, Heeringa P. The Nasal Microbiome in ANCA-Associated Vasculitis: Picking the Nose for Clues on Disease Pathogenesis. Curr Rheumatol Rep. 2021;23(7):54. https://doi.org/10.1007/s11926-021-01015-9.
Lyons PA, Rayner TF, Trivedi S, Holle JU, Watts RA, Jayne DR, et al. Genetically distinct subsets within ANCA-associated vasculitis. N Engl J Med. 2012;367(3):214–23. https://doi.org/10.1056/NEJMoa1108735.
Asano K, Ueki S, Tamari M, Imoto Y, Fujieda S, Taniguchi M. Adult-onset eosinophilic airway diseases. Allergy. 2020;75(12):3087–99. https://doi.org/10.1111/all.14620.
Subesinghe S, van Leuven S, Yalakki L, Sangle S, D’Cruz D. Cocaine and ANCA associated vasculitis-like syndromes - A case series. Autoimmun Rev. 2018;17(1):73–7. https://doi.org/10.1016/j.autrev.2017.11.011.
Viedma-Martinez M, Gallo-Pineda G, Recio-Monescillo M, Jimenez-Gallo D, Lopez-Sanz P, Drake-Monfort M, et al. Retrospective Case Series of Cocaine-Associated Plasma Cell Orificial Mucositis. JAMA Dermatol. 2024. https://doi.org/10.1001/jamadermatol.2023.5692.
McNagny SE, Parker RM. High prevalence of recent cocaine use and the unreliability of patient self-report in an inner-city walk-in clinic. JAMA. 1992;267(8):1106–8.
Verstraete AG. Detection times of drugs of abuse in blood, urine, and oral fluid. Ther Drug Monit. 2004;26(2):200–5. https://doi.org/10.1097/00007691-200404000-00020.
Garcia-Bournissen F, Moller M, Nesterenko M, Karaskov T, Koren G. Pharmacokinetics of disappearance of cocaine from hair after discontinuation of drug use. Forensic Sci Int. 2009;189(1–3):24–7. https://doi.org/10.1016/j.forsciint.2009.04.004.
Haller DL, Acosta MC, Lewis D, Miles DR, Schiano T, Shapiro PA, et al. Hair analysis versus conventional methods of drug testing in substance abusers seeking organ transplantation. Am J Transplant. 2010;10(5):1305–11. https://doi.org/10.1111/j.1600-6143.2010.03090.x.
Ursitti F, Klein J, Sellers E, Koren G. Use of hair analysis for confirmation of self-reported cocaine use in users with negative urine tests. J Toxicol Clin Toxicol. 2001;39(4):361–6. https://doi.org/10.1081/clt-100105156.
Gryczynski J, Schwartz RP, Mitchell SG, O’Grady KE, Ondersma SJ. Hair drug testing results and self-reported drug use among primary care patients with moderate-risk illicit drug use. Drug Alcohol Depend. 2014;141:44–50. https://doi.org/10.1016/j.drugalcdep.2014.05.001.
Hart ED, Vikingsson S, Winecker RE, Evans AL, Cone EJ, Mitchell JM, et al. Performance of Hair Testing for Cocaine Use-Comparison of Five Laboratories Using Blind Reference Specimens. J Anal Toxicol. 2023;47(2):154–61. https://doi.org/10.1093/jat/bkac066.
Sánchez-Hervás E, ZacarésRomaguera F, Santonja Gómez FJ, Secades-Villa R, García-Rodríguez O, Martín Yanez E. Urine testing during treatment predicts cocaine abstinence. J Psychoactive Drugs. 2010;42(3):347–52. https://doi.org/10.1080/02791072.2010.10400697.
Berman M, Paran D, Elkayam O. Cocaine-Induced Vasculitis. Rambam Maimonides Med J. 2016;7(4). https://doi.org/10.5041/rmmj.10263.
Nolan AL, Jen KY. Pathologic manifestations of levamisole-adulterated cocaine exposure. Diagn Pathol. 2015;10:48. https://doi.org/10.1186/s13000-015-0279-z.
Morcos MB, Lood C, Hughes GC. Demographic, Clinical, and Immunologic Correlates among a Cohort of 50 Cocaine Users Demonstrating Antineutrophil Cytoplasmic Antibodies. J Rheumatol. 2019;46(9):1151–6. https://doi.org/10.3899/jrheum.180771.
Peikert T, Finkielman JD, Hummel AM, McKenney ME, Gregorini G, Trimarchi M, et al. Functional characterization of antineutrophil cytoplasmic antibodies in patients with cocaine-induced midline destructive lesions. Arthritis Rheum. 2008;58(5):1546–51. https://doi.org/10.1002/art.23469.
Wiesner O, Russell KA, Lee AS, Jenne DE, Trimarchi M, Gregorini G, et al. Antineutrophil cytoplasmic antibodies reacting with human neutrophil elastase as a diagnostic marker for cocaine-induced midline destructive lesions but not autoimmune vasculitis. Arthritis Rheum. 2004;50(9):2954–65. https://doi.org/10.1002/art.20479.
Pendolino AL, Benshetrit G, Navaratnam AV, To C, Bandino F, Scarpa B, et al. The role of ANCA in the management of cocaine-induced midline destructive lesions or ENT pseudo-granulomatosis with polyangiitis: a London multicentre case series. Laryngoscope. 2024;134(6):2609–16. https://doi.org/10.1002/lary.31219.
Bentzley BS, Han SS, Neuner S, Humphreys K, Kampman KM, Halpern CH. Comparison of Treatments for Cocaine Use Disorder Among Adults: A Systematic Review and Meta-analysis. JAMA Netw Open. 2021;4(5):e218049. https://doi.org/10.1001/jamanetworkopen.2021.8049.
Trimarchi M, Nicolai P, Lombardi D, Facchetti F, Morassi ML, Maroldi R, et al. Sinonasal osteocartilaginous necrosis in cocaine abusers: experience in 25 patients. Am J Rhinol. 2003;17(1):33–43.
Monti S, Delvino P, Riboli M, Rebuffi C, Xoxi B, De Silvestri A, et al. The role of trimethoprim/sulfametoxazole in reducing relapses and risk of infections in ANCA-associated vasculitis: a meta-analysis. Rheumatology (Oxford). 2021;60(8):3553–64. https://doi.org/10.1093/rheumatology/keab267.
Cohen Tervaert JW. Trimethoprim-sulfamethoxazole and antineutrophil cytoplasmic antibodies-associated vasculitis. Curr Opin Rheumatol. 2018;30(4):388–94. https://doi.org/10.1097/bor.0000000000000508.
Chung C, Tumeh PC, Birnbaum R, Tan BH, Sharp L, McCoy E, et al. Characteristic purpura of the ears, vasculitis, and neutropenia–a potential public health epidemic associated with levamisole-adulterated cocaine. J Am Acad Dermatol. 2011;65(4):722-5.e2. https://doi.org/10.1016/j.jaad.2010.08.024.
Pearson T, Bremmer M, Cohen J, Driscoll M. Vasculopathy related to cocaine adulterated with levamisole: A review of the literature. Dermatol Online J. 2012;18(7):1.
Ruffer N, Krusche M, Holl-Ulrich K, Kötter I, Lötscher F. Cocaine-induced vasculitis and mimics of vasculitis. Z Rheumatol. 2023;82(7):606–14. https://doi.org/10.1007/s00393-022-01217-1.
Author information
Authors and Affiliations
Contributions
JD, RF and BR were responsible for the conceptualization of the article. JD wrote the original draft for the manuscript with review and editing performed by all authors (JD, RF and BR). Figure 1a was prepared by JD and BR, Fig. 1b was prepared by JD. All authors have read and agreed to the submitted version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Donkers, J.W.J., Feijen, R.A. & Rutgers, A.(. (Levamisole Adulterated) Cocaine-Induced Vasculitis: What Is Known/Current Evidence. Curr Treat Options in Rheum 10, 35–42 (2024). https://doi.org/10.1007/s40674-024-00215-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40674-024-00215-5