Abstract
The clinical significance of C–C motif chemokine11 (CCL11) in bone metabolism in ankylosing spondylitis (AS) is not clearly elucidated. Thus, this cross-sectional study aimed to compare serum levels of CCL11 between patients with AS and healthy controls and to investigate the relationship between serum levels of CCL11 and radiographic spinal damage in patients with AS. We consecutively recruited 55 male patients with AS and 26 age- and sex-matched healthy controls. Serum levels of CCL11, tumor necrosis factor-α (TNF-α), interleukin-17, and Dickkopf-1 (DKK-1) were measured with commercially available enzyme-linked immunosorbent assay kits. Radiographs were scored according to the modified Stoke ankylosing spondylitis spine score (mSASSS), and syndesmophytes were defined as mSASSS ≥ 2. The serum levels of CCL11 in AS patients with syndesmophytes were significantly higher than those in AS patients without syndesmophytes (p = 0.007) and healthy controls (p = 0.006). In AS patients, the serum levels of CCL11 were significantly and positively correlated with mSASSS (p = 0.006), number of syndesmophytes (p = 0.029). After adjusting for confounding factors, elevated serum levels of CCL11 were associated with increased mSASSS (β = 0.007, p = 0.03) and higher risk for the presence of syndesmophytes (OR 2.34 per 50 pg/ml increase, p = 0.012) in AS patients. We found that the serum level of CCL11 was associated with structural damage in patients with AS, suggesting that CCL11 may serve as a promising biomarker for new bone formation in AS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ankylosing spondylitis (AS) is a chronic inflammatory rheumatic disease that predominantly affects the axial skeleton such as the sacroiliac joint and spine. New bone formation in the form of syndesmophytes and bony bridges occurring at the cortical bone compartment of the axial skeleton is a distinguishing characteristic of AS, potentially leading to decreased spinal mobility and loss of function [1, 2]. Otherwise, inflammation-associated trabecular bone loss in the spine is another important clinical and pathophysiological features of AS [3], which is strongly associated with increased risk of fragility fracture [4]. Accordingly, impaired bone metabolism is evident during the disease course and can contribute to a significant clinical burden for patients with AS. New bone formation leading to structural damage is assumed to occur as a result of repair process secondary to inflammation-associated bone loss [1, 5], but the mechanism underlying bone metabolism in AS remains to be clarified. In addition, many studies have attempted to identify promising molecules for bone-related outcomes in AS including Dickkopf-1 (DKK-1), sclerostin, and adipokines [6,7,8,9,10]. However, these markers have not been validated and are not yet available in clinical practice [11,12,13]. Thus, further research is necessary to explore novel biomarkers that reflect structural bone damage in patients with AS.
C–C motif chemokine11 (CCL11), also known as eotaxin-1, exerts its action by binding CCR3 and is a potent chemoattractant for eosinophils. Thus, it plays an important role in the pathogenesis of allergic conditions such as bronchial asthma [14] and allergic rhinitis [15]. CCL11 can also recruit mast cells, Th2 cells, basophils, and macrophages and is produced by various cell types including eosinophils, endothelial cells, epithelial cells, fibroblasts, and chondrocytes [16]. Beyond its association with allergic diseases, elevated levels of CCL11 have also been identified in patients with other inflammatory diseases such as inflammatory bowel disease [17] and rheumatoid arthritis (RA) [18]. Although less is known about the effect of CCL11 on bone metabolism, a recent study has reported that osteoblasts express CCL11 during inflammatory conditions and osteoclastic bone resorption is activated by CCL11, suggesting a potential role of CCL11 in inflammatory bone diseases [16]. The association between elevated serum levels of CCL11 and less radiographic progression in early RA was previously reported [19], but the clinical significance of CCL11 in bone metabolism in AS remains poorly defined. Hence, in the present study, we aimed to compare the serum levels of CCL11 between patients with AS and healthy controls and to investigate the relationship between serum levels of CCL11 and radiographic spinal damage in patients with AS.
Methods
Study design and subjects
This observational and cross-sectional study involved 55 consecutively recruited male patients with AS and 26 age- and sex-matched healthy controls. The participants were recruited from a university-affiliated rheumatology center in Korea from March 2016 to December 2017. All patients with AS fulfilled the modified New York criteria for AS [20] and were aged ≥ 20 years. Exclusion criteria were as follows: (1) patients with other autoimmune rheumatic diseases except for AS; (2) AS patients having known allergic diseases including asthma, allergic rhinitis, and atopic dermatitis; (3) AS patients with chronic diseases of kidney, liver, thyroid, or parathyroid; (4) AS patients with active infection; (5) AS patients taking bisphosphonates or other anti-osteoporosis medications which could affect bone metabolism; and (6) AS patients who refuse to participate in the present study. Healthy controls had no history of autoimmune rheumatic diseases or any chronic diseases and were not receiving any medications which could affect bone metabolism. The present study was approved by the Research and Ethical Review Board of Pusan National University Hospital (IRB No. 1603-005-039). All study participants provided written informed consent in accordance with the principles of the Declaration of Helsinki.
Clinical, laboratory and radiographic evaluations
Demographic data, such as age, body mass index (BMI), and current smoking status, and laboratory markers, including erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), CCL11, tumor necrosis factor-α (TNF-α), IL-17, and DKK-1, in patients with AS and healthy controls were obtained at baseline visit. BMI was determined as weight divided by squared height (kg/m2). All laboratory assays were performed with blood samples obtained after overnight fasting. CRP levels were measured using particle-enhanced immunoturbidimetric assay (Tina-Quant C-reactive protein assay; Roche Diagnostics, Zurich, Switzerland) with a P800 Module (Roche Diagnostics). The blood samples were centrifuged at 3000 rpm for 10 min at 4 °C, and the serum was separated and stored at − 80 °C until quantitative assessment of chemokine and cytokines. The serum levels of CCL11, TNF-α, IL-17, and DKK-1 were measured with commercially available enzyme-linked immunosorbent assay kits (R&D Systems, Minneapolis, MN, USA).
The following additional clinical parameters were assessed in patients with AS: disease duration, HLA-B27 status, Bath ankylosing spondylitis disease activity index (BASDAI), Bath ankylosing spondylitis functional index, Bath ankylosing spondylitis metrology index (BASMI), ankylosing spondylitis disease activity score (ASDAS)-CRP [21], ASDAS-ESR [21] and current medications.
Radiographs of lateral cervical and lumbar spine of all patients with AS were obtained at baseline and scored according to the modified Stoke ankylosing spondylitis spine score (mSASSS) [22]. Briefly, the anterior corners of C2 lower to T1 upper and T12 lower to S1 upper were scored for squaring, erosion, and/or sclerosis (1 point), nonbridging syndesmophyte (2 points), and bridging syndesmophyte (3 points) [22]. Syndesmophytes were defined as non-bridging or bridging syndesmophytes (mSASSS of 2 or 3, respectively) in at least 1 vertebral corner, and the number of syndesmophytes per patient with AS was recorded. All radiographs were scored and analyzed by a highly experienced rheumatologist (Lee) who was blinded to clinical data.
Assessment of bone mineral density
Bone mineral density at the lumbar spine (L1–L4) and left hip (femoral neck and total hip) was measured in a subgroup of 27 patients with AS and 11 healthy subjects using dual energy X-ray absorptiometry (DEXA) equipment (GE-Lunar Prodigy or Lunar Prodigy advance, Madison, MA, USA). BMD was presented as g/cm2 and the standard deviation (SD) from the age-matched healthy population (Z-score). Unlike women, there is no consensus regarding appropriate reference data in the assessment of BMD in male patients. Use of women reference population when calculating Z-scores in men is supported by the WHO [23], Scientific Advisory Council of Osteoporosis Canada [24], National Osteoporosis Guideline Group [25] and International Society for Clinical Densitometry [26]. Thus, calculation of the Z-score in this study was performed based on South Korean female reference data provided by the manufacturers. BMD examinations were carried out according to the standardized procedures provided by the manufacturer. The coefficient of variance was 0.33% at the spine and 0.4% at the hip in our center.
Statistical analyses
Quantitative variables are presented as mean ± SD or median (interquartile range; IQR) as appropriate and qualitative variables as numbers (percentages). To evaluate the normality of data distribution, we performed Kolmogorov–Smirnov test. For group comparisons, the Student’s t test, Mann–Whitney U test, or Kruskal–Wallis test for quantitative variables and the Chi-squared test or Fisher’s exact test for qualitative variables were performed as appropriate. Spearman’s correlation analyses were conducted to assess the correlations among clinical and laboratory variables. To investigate independent relationship between the serum levels of CCL11 and mSASSS in patients with AS, we used stepwise multivariable linear regression models including variables with p < 0.1 in univariable analysis and a priori confounding factors such as TNF-α blockers. Because mSASSS was not normally distributed, log-transformed mSASSS determined as \(\ln ({\text{mSASSS}}+1)\) was included as an outcome variable (dependent variable) in our multivariable linear regression models. The association between the serum levels of CCL11 and the presence of syndesmophytes was assessed using backward multivariable logistic regression models. All statistical analyses were carried out with PASW version 18.0 (SPSS Inc., Chicago, IL, USA) and STATA version 11.0 (StataCorp LP, College Station, TX, USA). A p value less than 0.05 was considered statistically significant.
Results
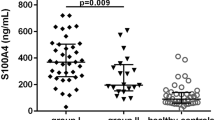
Comparisons of clinical and laboratory characteristics between patients with AS and healthy controls are summarized in Table 1. For patients with AS, the mean ± SD disease duration and BADAI were 77.8 ± 51.7 months and 4.2 ± 2.2, respectively. All but one of the patients with AS showed positive results for HLA-B27, and the median (IQR) mSASSS was 5 (2–16). Twenty-six (47.3%) patients had one or more syndesmophytes. At baseline visit, 28 (50.9%) patients with AS were receiving TNF-α blockers and 16, 8, 2, 1, 1 and 1 patients were treated with adalimumab, etanercept, infliximab biosimilar, infliximab and golimumab, respectively. Serum levels of ESR, CRP, and DKK-1 in patients with AS were significantly higher than those in healthy subjects, whereas serum levels of TNF-α and IL-17 did not differ between these two groups. No statistically significant difference was observed in the serum levels of CCL11 between patients with AS and controls. However, the median serum levels of CCL11 in AS patients with syndesmophytes were significantly higher than those in AS patients without syndesmophytes [158.5 (131.3–185.8) vs. 106.1 (92.8–139.6) pg/mL, p = 0.007] and healthy controls [158.5 (131.3–185.8) vs. 105.6 (94.1–148.6) pg/mL, p = 0.006], as depicted in Fig. 1. In addition, as compared with healthy controls, patients with AS showed significantly lower total hip BMD and Z-score but exhibited comparable lumbar spine BMD and Z-score.
Table 2 shows differences in clinical and laboratory characteristics in patients with AS according to the presence of syndesmophytes. AS patients with syndesmophytes had significantly longer disease duration and a higher BASMI than those without syndesmophytes. In contrast to CCL11, no significant differences were noted in the serum levels of TNF-α, IL-17, and DKK-1 in patients with AS according to the presence of syndesmophytes. The Z-scores of the lumbar spine in AS patients with syndesmophytes were significantly higher than those in AS patients without syndesmophytes. However, no statistically significant differences were found in BMD and Z-scores at the femoral neck and total hip. We also compared laboratory markers between AS patients with BADAI ≥ 4 and those with < 4, but the differences in the serum levels of CCL11, TNF-α, IL-17, and DKK-1 were not statistically significant according to disease activity (data not shown).
Correlations of serum levels of CCL11 with other clinical and laboratory variables in patients with AS and healthy subjects are described in Table 3. In patients with AS, the serum levels of CCL11 were significantly and positively correlated with mSASSS (ρ = 0.366, p = 0.006), number of syndesmophytes (ρ = 0.295, p = 0.029), and BASMI (ρ = 0.348, p = 0.01). However, the serum levels did not show correlations with disease activity indices, such as BASDAI, ASDAS-ESR, and ASDAS-CRP, or acute-phase reactants, such as ESR and CRP. In addition, the serum levels of CCL11 showed significant positive correlations with TNF-α (ρ = 0.331, p = 0.013) and DKK-1 (ρ = 0.32, p = 0.017) and a trend of positive correlation with IL-17 (ρ = 0.257, p = 0.059) in patients with AS. The serum levels of CCL11 were significantly positively correlated with lumbar spine BMD (ρ = 0.408, p = 0.035) and Z-scores (ρ = 0.488, p = 0.01) in patients with AS, but showed a trend of negative correlation with lumbar spine Z-scores (ρ = − 0.41, p = 0.091) in healthy controls.
Results of linear regression analyses for the log-transformed mSASSS in patients with AS are described in Table 4. In univariable linear regression models, increased serum levels of CCL11 were associated with higher log-transformed mSASSS in patients with AS. This association remained significant in multivariable linear regression analyses after adjusting for confounding factors including disease duration and use of medications, such as nonsteroidal anti-inflammatory drugs, and TNF-α blockers (β = 0.007, SE = 0.003, p = 0.03). Table 5 shows logistic regression models for the presence of syndesmophytes in patients with AS. After adjusting for confounding factors, the odds ratio (95% confidence interval) for the presence of syndesmophytes was 2.34 (1.2–4.53) per 50 pg/mL increase in the serum levels of CCL11 in patients with AS.
Discussion
In the present cross-sectional study, AS patients with syndesmophytes had significantly higher serum levels of CCL11 than those without syndesmophytes and healthy subjects. In patients with AS, elevated serum levels of CCL11 were independently associated with higher risk for increased mSASSS as well as the presence of syndesmophytes after adjusting for confounding factors. This result indicates a potential role of this chemokine in the pathogenesis of new bone formation in AS. In addition, the serum levels of CCL11 in patients with AS were positively correlated with the serum levels of TNF-α, DKK-1, and IL-17, all of which are known to be associated with bone metabolism in AS. Otherwise, no correlations of serum levels of CCL11 with disease activity markers, such as BASDAI and ASDAS, or acute-phase reactants, such as ESR and CRP, were observed. This finding suggests that the effect of CCL11 on inflammatory process in AS may be minimal. We also performed a subgroup analysis to investigate the association between serum levels of CCL11 and BMD. The result showed that the serum levels of CCL11 were positively correlated with lumbar spine Z-scores in patients with AS but showed a trend of negative correlation with lumbar spine Z-scores in healthy controls, suggesting a contradictory effect of CCL11 in patients with AS and healthy subjects on BMD.
To our knowledge, this study is the first to evaluate the serum levels of CCL11 in patients with AS. The major finding of our study was that increased serum levels of CCL11 were associated with structural damage, suggesting that CCL11 may contribute to osteoproliferation in AS. Although a recent experimental study has shown that CCL11 increases pre-osteoclast migration and concomitant bone resorption [16], a significant association was also reported between higher baseline serum levels of CCL11 and less radiographic progression in patients with early RA [19], which supports our hypothesis that CCL11 contributes to osteogenesis in AS. The reasons for this discrepancy remain unclear, but it may be due to a complex process of bone metabolism in AS. The pathologic process of new bone formation in AS is not well established. However, it has been recently proposed that inflammatory and biomechanical stress initially leads to bone loss and reduces bone strength via activation of osteoclast, which subsequently triggers a stabilizing anabolic effort resulting in new bone formation during the disease course [1]. Based on this concept, our results may be interpreted that CCL11 primarily activates osteoclast at early stage and sequentially induces osteoproliferation as a reactive process at later stage in AS. Taken together, the effect of CCL11 on bone metabolism in inflammatory arthritis, such as AS and RA, seems to be extremely complex and warrants clarification in further studies.
In this study, the serum levels of CCL11 were significantly and positively correlated with those of TNF-α and showed a trend of positive correlation with the serum levels of IL-17 in patients with AS. Although CCL11 may have a direct and specific role in osteogenesis, this finding suggests that the biologic action of CCL11 in bone metabolism may be connected with or mediated by TNF-α and/or IL-17, which are key inflammatory cytokines in AS. TNF-α and IL-17 are considered to have a mostly negative impact on bone formation in AS [1] because they have potent osteoclastogenic activity by upregulating the receptor activator of NF-κB ligand, which promotes differentiation and proliferation of osteoclast precursors [27] and inhibiting osteogenesis by suppressing the WNT signaling pathway, which is responsible for osteoblastogenesis [28,29,30]. In contrast to their classical action in osteoclastic bone loss, recent data have suggested that TNF-α and IL-17 could possibly enhance osteogenesis [31,32,33]. Lower levels of TNF-α moderately increase the expression of osteogenic transcription factors although higher levels of TNF-α treatment inhibit osteogenic differentiation [31]. IL-17 enhances bone matrix formation on human bone marrow-derived mesenchymal stem cells [32] and this effect is synergistic with bone morphogenetic protein 2 [33]. Thus, the effect of TNF-α and IL-17 on bone metabolism in AS may be heterogeneous. Further studies are needed to elucidate the exact role of CCL11 along with TNF-α and IL-17 in the pathogenesis of bone formation in AS.
In addition to new bone formation at the cortical bone compartment, AS is characterized by trabecular bone loss [34]. In subgroup analyses of our study, the serum levels of CCL11 were positively correlated with lumbar spine BMD and Z-scores in patients with AS, but this correlation was not observed in the femoral neck and total hip. Because lumbar BMD measured by DEXA can be overestimated by syndesmophytes and/or bony bridges, which were associated with serum levels of CCL11 in our data, whether CCL11 directly acts as a protective factor for systemic bone loss at the trabecular bone or simply reflects the osteoproliferation at the cortical bone in AS is unclear. In contrast to patients with AS, a trend of negative correlation between serum levels of CCL11 and lumbar Z-scores was found in healthy controls. Thus, CCL11 seems to have different effect on BMD between patients with AS and healthy population. However, due to the small sample size that underwent BMD examination in our data, this interpretation should be taken with care, and larger studies should be performed to clarify the relationship between CCL11 and BMD in AS.
CCL11 has long been recognized as a proinflammatory chemokine involved in allergic and other inflammatory diseases including bronchial asthma [14], allergic rhinitis [15], atomic dermatitis [35] and inflammatory bowel disease [17]. With regard to chronic inflammatory arthritis, recent experimental studies have shown the increased level of CCL11 in synovial fluid and elevated expression of CCR3 in fibroblast-like synoviocytes in patients with RA [36] and osteoarthritis (OA) [37]. This finding implicates that CCL11–CCR3 may play an important role in the pathogenesis of synovitis in these conditions. In contrast to its association with bone metabolism, our data showed that the serum level of CCL11 was not significantly related to markers for disease activity, such as BASDAI, ASDAS, ESR, and CRP, in patients with AS. Thus, in contrast to RA and OA, the contribution of CCL11 to inflammation in AS may be minimal.
The present study has several limitations. First, due to its cross-sectional nature, our data could not provide evidence whether CCL11 can lead to radiographic progression in patients with AS and thus further longitudinal studies are needed to confirm our findings. Second, this study only evaluated male patients with AS; the role of CCL11 in bone metabolism in female counterparts also needs to be determined in further studies. Third, besides allergic diseases, production of CCL11 and its circulating levels can be affected by psychiatric disorders, such as schizophrenia, major depression, and bipolar disorder [38,39,40]. However, this study did not fully adjust the effect of psychiatric diseases on the serum levels of CCL11 in study subjects. Finally, due to the small size of the examined sample in the present study, further largers studies are needed to validate our results.
In conclusion, we found that the serum levels of CCL11 in AS patients with syndesmophytes were significantly higher than those in AS patients without syndesmophytes or healthy subjects. In addition, the elevated serum levels of CCL11 were independently associated with increased mSASSS and higher risk for the presence of syndesmophytes, which reflect spinal damage in patients with AS. This notion suggests that CCL11 may be exploited as a promising biomarker for new bone formation of AS. Although in vitro and animal studies as well as longitudinal studies are required to validate our results more conclusively, we believe that this study provides a novel insight into a potential role of chemokines in the pathogenesis involved in bone metabolism of AS.
References
Neerinckx B, Lories RJ (2017) Structural disease progression in axial spondyloarthritis: still a cause for concern? Curr Rheumatol Rep 19(3):14. https://doi.org/10.1007/s11926-017-0639-7
Park EK, Pak K, Park JH, Kim K, Kim SJ, Kim IJ, Kim GT, Lee SG (2017) Baseline increased 18F-fluoride uptake lesions at vertebral corners on positron emission tomography predict new syndesmophyte development in ankylosing spondylitis: a 2-year longitudinal study. Rheumatol Int 37(5):765–773. https://doi.org/10.1007/s00296-017-3660-2
Neerinckx B, Lories R (2017) Mechanisms, impact and prevention of pathological bone regeneration in spondyloarthritis. Curr Opin Rheumatol 29(4):287–292. https://doi.org/10.1097/BOR.0000000000000404
Zhang M, Li XM, Wang GS, Tao JH, Chen Z, Ma Y, Li XP (2017) The association between ankylosing spondylitis and the risk of any, hip, or vertebral fracture: a meta-analysis. Medicine (Baltimore) 96(50):e8458. https://doi.org/10.1097/MD.0000000000008458
Van Mechelen M, Gulino GR, de Vlam K, Lories R (2017) Bone disease in axial spondyloarthritis. Calcif Tissue Int. https://doi.org/10.1007/s00223-017-0356-2
Sakellariou GT, Iliopoulos A, Konsta M, Kenanidis E, Potoupnis M, Tsiridis E, Gavana E, Sayegh FE (2017) Serum levels of Dkk-1, sclerostin and VEGF in patients with ankylosing spondylitis and their association with smoking, and clinical, inflammatory and radiographic parameters. Joint Bone Spine 84(3):309–315. https://doi.org/10.1016/j.jbspin.2016.05.008
Korkosz M, Gasowski J, Leszczynski P, Pawlak-Bus K, Jeka S, Kucharska E, Grodzicki T (2013) High disease activity in ankylosing spondylitis is associated with increased serum sclerostin level and decreased wingless protein-3a signaling but is not linked with greater structural damage. BMC Musculoskelet Disord 14:99. https://doi.org/10.1186/1471-2474-14-99
Kim KJ, Kim JY, Park SJ, Yoon H, Yoon CH, Kim WU, Cho CS (2012) Serum leptin levels are associated with the presence of syndesmophytes in male patients with ankylosing spondylitis. Clin Rheumatol 31(8):1231–1238. https://doi.org/10.1007/s10067-012-1999-z
Syrbe U, Callhoff J, Conrad K, Poddubnyy D, Haibel H, Junker S, Frommer KW, Muller-Ladner U, Neumann E, Sieper J (2015) Serum adipokine levels in patients with ankylosing spondylitis and their relationship to clinical parameters and radiographic spinal progression. Arthritis Rheumatol 67(3):678–685. https://doi.org/10.1002/art.38968
Park JH, Lee SG, Jeon YK, Park EK, Suh YS, Kim HO (2017) Relationship between serum adipokine levels and radiographic progression in patients with ankylosing spondylitis: a preliminary 2-year longitudinal study. Medicine (Baltimore) 96(33):e7854. https://doi.org/10.1097/MD.0000000000007854
Danve A, O’Dell J (2015) The ongoing quest for biomarkers in Ankylosing Spondylitis. Int J Rheum Dis 18(8):826–834. https://doi.org/10.1111/1756-185X.12779
Maksymowych WP (2015) Biomarkers in axial spondyloarthritis. Curr Opin Rheumatol 27(4):343–348. https://doi.org/10.1097/BOR.0000000000000180
Prajzlerova K, Grobelna K, Pavelka K, Senolt L, Filkova M (2016) An update on biomarkers in axial spondyloarthritis. Autoimmun Rev 15(6):501–509. https://doi.org/10.1016/j.autrev.2016.02.002
Wu D, Zhou J, Bi H, Li L, Gao W, Huang M, Adcock IM, Barnes PJ, Yao X (2014) CCL11 as a potential diagnostic marker for asthma? J Asthma 51(8):847–854. https://doi.org/10.3109/02770903.2014.917659
Paplinska M, Hermanowicz-Salamon J, Nejman-Gryz P, Bialek-Gosk K, Rubinsztajn R, Arcimowicz M, Placha G, Gora J, Chazan R, Grubek-Jaworska H (2012) Expression of eotaxins in the material from nasal brushing in asthma, allergic rhinitis and COPD patients. Cytokine 60(2):393–399. https://doi.org/10.1016/j.cyto.2012.07.001
Kindstedt E, Holm CK, Sulniute R, Martinez-Carrasco I, Lundmark R, Lundberg P (2017) CCL11, a novel mediator of inflammatory bone resorption. Sci Rep 7(1):5334. https://doi.org/10.1038/s41598-017-05654-w
Rehman MQ, Beal D, Liang Y, Noronha A, Winter H, Farraye FA, Ganley-Leal L (2013) B cells secrete eotaxin-1 in human inflammatory bowel disease. Inflamm Bowel Dis 19(5):922–933. https://doi.org/10.1097/MIB.0b013e3182802950
Hueber W, Tomooka BH, Zhao X, Kidd BA, Drijfhout JW, Fries JF, van Venrooij WJ, Metzger AL, Genovese MC, Robinson WH (2007) Proteomic analysis of secreted proteins in early rheumatoid arthritis: anti-citrulline autoreactivity is associated with up regulation of proinflammatory cytokines. Ann Rheum Dis 66(6):712–719. https://doi.org/10.1136/ard.2006.054924
Syversen SW, Goll GL, Haavardsholm EA, Boyesen P, Lea T, Kvien TK (2008) A high serum level of eotaxin (CCL 11) is associated with less radiographic progression in early rheumatoid arthritis patients. Arthritis Res Ther 10(2):R28. https://doi.org/10.1186/ar2381
van der Linden S, Valkenburg HA, Cats A (1984) Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 27(4):361–368
van der Heijde D, Lie E, Kvien TK, Sieper J, Van den Bosch F, Listing J, Braun J, Landewe R (2009) ASDAS, a highly discriminatory ASAS-endorsed disease activity score in patients with ankylosing spondylitis. Ann Rheum Dis 68(12):1811–1818. https://doi.org/10.1136/ard.2008.100826
Creemers MC, Franssen MJ, van’t Hof MA, Gribnau FW, van de Putte LB, van Riel PL (2005) Assessment of outcome in ankylosing spondylitis: an extended radiographic scoring system. Ann Rheum Dis 64(1):127–129. https://doi.org/10.1136/ard.2004.020503
Kanis JA, McCloskey EV, Johansson H, Oden A, Melton LJ, Khaltaev N (2008) A reference standard for the description of osteoporosis. Bone 42(3):467–475. https://doi.org/10.1016/j.bone.2007.11.001
Papaioannou A, Morin S, Cheung AM, Atkinson S, Brown JP, Feldman S, Hanley DA, Hodsman A, Jamal SA, Kaiser SM, Kvern B, Siminoski K, Leslie WD, Osteoporosis SAC (2010) 2010 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: summary. Can Med Assoc J 182(17):1864–1873. https://doi.org/10.1503/cmaj.100771
Compston J, Bowring C, Cooper A, Cooper C, Davies C, Francis R, Kanis JA, Marsh D, McCloskey EV, Reid DM, Selby P (2013) Diagnosis and management of osteoporosis in postmenopausal women and older men in the UK: National Osteoporosis Guideline Group (NOGG) update 2013 (vol 75, pg 392, 2013). Maturitas 76(4):387–387. https://doi.org/10.1016/j.maturitas.2013.08.006
Watts NB, Leslie WD, Foldes AJ, Miller PD (2013) 2013 international society for clinical densitometry position development conference: task force on normative databases. J Clin Densitom 16(4):472–481. https://doi.org/10.1016/j.jocd.2013.08.001
Rossini M, Viapiana O, Adami S, Idolazzi L, Fracassi E, Gatti D (2016) Focal bone involvement in inflammatory arthritis: the role of IL17. Rheumatol Int 36(4):469–482. https://doi.org/10.1007/s00296-015-3387-x
Diarra D, Stolina M, Polzer K, Zwerina J, Ominsky MS, Dwyer D, Korb A, Smolen J, Hoffmann M, Scheinecker C, van der Heide D, Landewe R, Lacey D, Richards WG, Schett G (2007) Dickkopf-1 is a master regulator of joint remodeling. Nat Med 13(2):156–163. https://doi.org/10.1038/nm1538
Li J, Sarosi I, Cattley RC, Pretorius J, Asuncion F, Grisanti M, Morony S, Adamu S, Geng Z, Qiu W, Kostenuik P, Lacey DL, Simonet WS, Bolon B, Qian X, Shalhoub V, Ominsky MS, Zhu Ke H, Li X, Richards WG (2006) Dkk1-mediated inhibition of Wnt signaling in bone results in osteopenia. Bone 39(4):754–766. https://doi.org/10.1016/j.bone.2006.03.017
Uluckan O, Wagner EF (2016) Role of IL-17A signalling in psoriasis and associated bone loss. Clin Exp Rheumatol 34(4 Suppl 98):17–20
Huang H, Zhao N, Xu X, Xu Y, Li S, Zhang J, Yang P (2011) Dose-specific effects of tumor necrosis factor alpha on osteogenic differentiation of mesenchymal stem cells. Cell Prolif 44(5):420–427. https://doi.org/10.1111/j.1365-2184.2011.00769.x
Osta B, Lavocat F, Eljaafari A, Miossec P (2014) Effects of interleukin-17A on osteogenic differentiation of isolated human mesenchymal stem cells. Front Immunol 5:425. https://doi.org/10.3389/fimmu.2014.00425
Croes M, Oner FC, van Neerven D, Sabir E, Kruyt MC, Blokhuis TJ, Dhert WJA, Alblas J (2016) Proinflammatory T cells and IL-17 stimulate osteoblast differentiation. Bone 84:262–270. https://doi.org/10.1016/j.bone.2016.01.010
Arends S, Spoorenberg A, Efde M, Bos R, Leijsma MK, Bootsma H, Veeger NJ, Brouwer E, van der Veer E (2014) Higher bone turnover is related to spinal radiographic damage and low bone mineral density in ankylosing spondylitis patients with active disease: a cross-sectional analysis. PLoS ONE 9(6):e99685. https://doi.org/10.1371/journal.pone.0099685
Rankin SM, Conroy DM, Williams TJ (2000) Eotaxin and eosinophil recruitment: implications for human disease. Mol Med Today 6(1):20–27
Liu X, Zhang H, Chang X, Shen J, Zheng W, Xu Y, Wang J, Gao W, He S (2017) Upregulated expression of CCR3 in rheumatoid arthritis and CCR3-dependent activation of fibroblast-like synoviocytes. Cell Biol Toxicol 33(1):15–26. https://doi.org/10.1007/s10565-016-9356-7
Chang X, Shen J, Yang H, Xu Y, Gao W, Wang J, Zhang H, He S (2016) Upregulated expression of CCR3 in osteoarthritis and CCR3 mediated activation of fibroblast-like synoviocytes. Cytokine 77:211–219. https://doi.org/10.1016/j.cyto.2015.09.012
Kang WS, Kim YJ, Park HJ, Kim SK, Paik JW, Kim JW (2018) Association of CCL11 promoter polymorphisms with schizophrenia in a Korean population. Gene 656:80–85. https://doi.org/10.1016/j.gene.2018.02.053
Hong S, Lee EE, Martin AS, Soontornniyomkij B, Soontornniyomkij V, Achim CL, Reuter C, Irwin MR, Eyler LT, Jeste DV (2017) Abnormalities in chemokine levels in schizophrenia and their clinical correlates. Schizophr Res 181:63–69. https://doi.org/10.1016/j.schres.2016.09.019
Magalhaes PV, Jansen K, Stertz L, Ferrari P, Pinheiro RT, da Silva RA, Kapczinski F (2014) Peripheral eotaxin-1 (CCL11) levels and mood disorder diagnosis in a population-based sample of young adults. J Psychiatr Res 48(1):13–15. https://doi.org/10.1016/j.jpsychires.2013.10.007
Acknowledgements
We specially thank the late Professor Sung-Il Kim who devoted himself to education, research, and patient care in Division of Rheumatology, Department of Internal Medicine, Pusan National University School of Medicine (1963 to 2011).
Funding
This work was supported by the research fund of Rheumatology Research Foundation (RRF-2017-01). Also, this research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2016R1D1A1B03934716).
Author information
Authors and Affiliations
Contributions
DHS: research concept and study design, performance of the tests, statistical analysis, data analysis and interpretation, and drafting the article; HJ: performance of the tests and data analysis; JSR: performance of the tests and data analysis; HNL: radiographic data analysis; EK: clinical examination, sample collection and data acquisition; JHK: data interpretation; SGL: research concept and study design, study subject recruitment, statistical analysis, data analysis and interpretation, and drafting the article, manuscript editing, substantial supervision and coordination of the study. All authors approved the final version of the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflict of interest.
Ethical approval
The present study was approved by the Research and Ethical Review Board of Pusan National University Hospital (IRB No. 1603-005-039). All study participants provided written informed consent in accordance with the principles of the Declaration of Helsinki.
Rights and permissions
About this article
Cite this article
Sohn, D.H., Jeong, H., Roh, J.S. et al. Serum CCL11 level is associated with radiographic spinal damage in patients with ankylosing spondylitis. Rheumatol Int 38, 1455–1464 (2018). https://doi.org/10.1007/s00296-018-4073-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-018-4073-6