Abstract
Objective
To determine the serum levels of Dickkopf-1 (DKK-1) and sclerostin, as well as their correlations with the structural damage assessed by modified stoke ankylosing spondylitis spine score (mSASSS) and the disease activity evaluated by ankylosing spondylitis disease activity score (ASDAS) in patients with ankylosing spondylitis (AS).
Methods
Eighty-eight AS patients, 26 rheumatoid arthritis (RA) patients, and 26 age- and gender-matched healthy controls (HC) were collected from rheumatic clinic of the Second Affiliated Hospital of Zhejiang University, School of Medicine, between March 2015 and July 2015. Demographic data, parameters of ASDAS, and image evaluations of spine (i.e., mSASSS) were collected. The serum levels of DKK-1 and sclerostin were measured using commercially available ELISA kits.
Results
Both DKK-1 and sclerostin were significantly higher in the AS patients than in the controls (1855 ± 84.58 vs. 1406 ± 99.76 pg/ml and 106 ± 6.75 vs. 62.78 ± 6.39 pmol/l, respectively, P < 0.05). The correlation analysis suggested a negative correlation between serum sclerostin and mSASSS (P = 0.019, r2 = 0.062). DKK-1 had a trend of positive correlation with mSASSS, but was not statistically significant (P > 0.05). There was no association between the serum levels of DKK-1 or sclerostin and disease activity assessed by ASDAS (P > 0.05). DKK-1 and sclerostin had a negative correlation (P = 0.013, r2 = 0.07).
Conclusion
In the present study, the expressions of serum DKK-1 and sclerostin were independent of disease activity. Sclerostin was negatively correlated with the mSASSS, which suggests that sclerostin may be a potential marker indicating the spine ossification process in AS. The specific mechanism remains to be investigated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ankylosing spondylitis (AS) is the prototype of immune-mediated inflammatory rheumatic diseases named as spondyloarthritis (SpA) which is characterized by new bone formation and progressively leads to ankylosis and functional disability [1,2,3]. The mechanisms of ossification in AS have not been fully understood. In recent years, the impact of Wnt/β-catenin signaling pathway in AS has been investigated intensively for its crucial modulating effect on bone formation [4]. This pathway is involved in bone morphogenesis and homeostasis and plays a key role in the development of AS [5, 6]. The canonical signaling was activated by the binding of Wnt ligands to the Frizzled receptor and the co-receptors low-density lipoprotein receptor–related proteins 5 and 6 (LRP-5 and LRP-6), thus maintaining the structural stability of β-catenin, which acts as a prominent component in the signaling pathway. Subsequently, β-catenin increases in cytoplasm and translocates into nucleus to modulate target genes transcription [7]. Both DKK-1 and sclerostin have been proved to be upstream potent antagonist of Wnt/β-catenin signaling which work as suppressors of LRP-5 and LRP-6 co-receptors and subsequently block their interaction with Wnt, resulting in β-catenin degradation [8]. Evidences from animal models of arthritis have proved that DKK-1 is a main regulator of joint remodeling shifting the balance toward new bone formation when its expression is decreased and toward erosion/joint destruction when its expression is increased [9,10,11]. However, it seems not the same story in human beings. The results were inconsistent about those studies evaluating Wnt pathway modulators in AS [12, 13]. Therefore, the aim of our study is to comprehensively estimate the role of serum DKK-1 and sclerostin in the development of AS.
Materials and methods
Patients and samples
A total of 88 AS patients, 26 RA patients, and 26 age- and gender-matched healthy controls were collected from rheumatic clinic of the Second Affiliated Hospital of Zhejiang University, School of Medicine, between March 2015 and July 2015. The demographic data and disease-specific data were recorded. All the AS patients recruited fulfilled the modified New York criteria of AS and the RA diagnosis was made according to the 2010 American College of Rheumatology (ACR) criteria. Patients with underlying bone metabolism diseases such as low phosphorus rickets and osteoporosis with pathological fracture as well as malignant diseases were excluded. The study was performed according to the Declaration of Helsinki, and approved by the ethics committee of the Second Affiliated Hospital of Zhejiang University, School of Medicine.
Serum collection and biomarker testing
Serum samples were obtained from all participants and were stored in aliquots of 200 μl at − 20 °C. The erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) level were measured in all patients and controls. Commercially available enzyme-linked immunosorbent assay (ELISA) kits (Biomedica, Austria) were used to measure DKK-1, sclerostin, and BMP-2 (bone morphogenetic protein 2) in each group according to the manufacturer’s instructions. All measurements were performed in duplicate for each sample in the same time, and a mean value was calculated.
AS disease activity measure
AS disease activity was calculated using ASDAS by software automatically (RheumaHelper v2.7.1). ASDAS < 1.3 indicates inactive disease or remission stage and ASDAS ≥ 3.5 suggests a very high disease activity, while ASDAS ranging from 1.3 to 2.1 (not included) and from 2.1 to 3.5 (not included) are defined as moderate and high disease activities, respectively.
Modified stoke AS spine score
The radiographic assessment was performed using the modified stoke ankylosing spondylitis spine score (mSASSS) defined in the literature [14] by two independent radiologists.
Statistical analysis
Statistical analysis was performed using SPSS v.13.0 and GraphPad Prism 6.01. Measurement data was illustrated as mean value ± standard deviation (X ± SD) or median (quartile 1, quartile 3) as appropriate and enumeration data was showed as number (percentage). The Mann-Whitney U test was used for group comparisons. Correlations between circulating levels of DKK-1 and sclerostin were calculated by unitary linear regression analysis. For all statistical analysis, P values less than 0.05 were considered significant.
Results
This study included 66 (75.0%) male and 22 (25.0%) female AS patients, with the average age of 36.5 ± 13.5 years old. The median disease duration was 8.5 years. In all, 93.2% of patients were HLA-B27 positive. The patient distribution according to disease activity assessed by ASDAS-CRP and ASDAS-ESR is illustrated in Table 1.
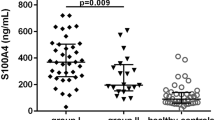
Serum DKK-1 was significantly higher in the AS patients than in the RA patients (1855 ± 84.58 vs. 1416 ± 115.20 pg/ml, P < 0.05) and in healthy controls (1855 ± 84.58 vs. 1406 ± 99.76 pg/ml, P < 0.05). Compared to the healthy controls, the serum sclerostin increased significantly in the AS patients (106 ± 6.75 vs. 62.78 ± 6.39 pmol/l, P < 0.05) but not in the RA group (95.23 ± 13.00 vs. 62.78 ± 6.39 pmol/l, P > 0.05), as showed in Fig. 1. Although there was no association between the serum levels of DKK-1 or sclerostin and disease activity scores assessed by ASDAS-CRP or ASDAS-ESR (Fig. 2), the correlation analysis revealed a negative association between serum sclerostin and mSASSS (P = 0.019, r2 = 0.062), as illustrated in Fig. 3. DKK-1 had a trend of positive correlation with mSASSS, but was not statistically significant (P > 0.05). mSASSS was not correlated with other bone turnover markers such as BMP-2 or vitamin D, but had a positive association with disease duration (P < 0.0001, r2 = 0.20). DKK-1 was negatively related to sclerostin (P = 0.013, r2 = 0.07). Both DKK-1 and sclerostin were not related to disease duration or BMP-2 (Fig. 4).
Serum DKK-1, sclerostin, BMP-2, and vitamin D levels in AS, RA, and HC subgroups. The serum concentrations of DKK-1 and sclerostin in AS subgroup were significantly higher compared with controls (P < 0.05). The BMP and vitamin D levels were similar among all three subgroups. AS, ankylosing spondylitis; RA, rheumatoid arthritis; HC, healthy controls; DKK-1, Dickkopf-1; SOST, sclerostin; BMP-2, bone morphogenetic protein 2; VitD, vitamin D
Serum DKK-1 and SOST in AS patients with different disease activities scored by ASDAS-CRP and ASDAS-ESR. The expressions of DKK-1 or sclerostin were not associated with ASDAS-CRP or ASDAS-ESR. DKK-1, Dickkopf-1; SOST, sclerostin; AS, ankylosing spondylitis; ASDAS, ankylosing spondylitis disease activity score; inactive, ASDAS < 1.3; moderate, 1.3 ≤ ASDAS < 2.1; high, 2.1 ≤ ASDAS < 3.5; very high, ASDAS ≥ 3.5
The correlation between serum levels of DKK-1 and SOST as well as other parameters and mSASSS in AS patients. It revealed a negative correlation between serum SOST and mSASSS (P = 0.019, r2 = 0.062); DKK-1 had a trend of positive correlation with mSASSS, but was not statistically significant (P > 0.05); mSASSS was not correlated with BMP-2 or vitamin D, but had a positive association with disease duration (P < 0.0001, r2 = 0.20). mSASSS, modified stoke ankylosing spondylitis spine score; DKK-1, Dickkopf-1; SOST, sclerostin; VitD, vitamin D; BMP-2, bone morphogenetic protein 2
The correlation between DKK-1 and SOST, BMP2, and disease duration as well as vitamin D in AS patients. In AS patient group, there was a negative correlation between DKK-1 and SOST (P = 0.013, r2 = 0.07). DKK-1 and vitamin D were weakly and positively related (P = 0.027, r2 = 0.061). DKK-1 had relationship neither with BMP-2 nor with disease duration. DKK-1, Dickkopf-1; SOST, sclerostin; BMP-2, bone morphogenetic protein 2; VitD, vitamin D
Discussion
As the prototype of SpA, the pathological process of AS includes three stages: inflammation, erosion, and syndesmophytes [15]. In the inflammation stage, TNF-α is the principal cytokine involved and anti-TNF-α agents have achieved great success in recent years [16, 17]. However, erosion and abnormal bony outgrowth can lead to dysfunction and disability of AS patients and need more investigations [3, 18]. The effect of Wnt signaling on the AS pathogenesis has generated tremendous interest recently and the most commonly studied secreted Wnt inhibitors are DKK-1 and sclerostin [19, 20]. In the present study, both DKK-1 and sclerostin levels were significantly increased in patients with AS (Fig. 1). Similar results have been reported by Daoussis and Korksz, respectively [21, 22]. However, studies on the correlation between Wnt pathway inhibitors and AS did not come to an agreement, even the meta-analysis reached controversial conclusions [10, 12, 23,24,25]. The discrepancy among those studies mentioned above may be explained in several aspects. Firstly, while sclerostin expression was confined exclusively to osteocytes, the expression of DKK-1 is not restricted to bone [26]. In fact, other origins such as platelets are also important sources of circulating DKK-1. It is not appropriate to attribute the fluctuation of serum DKK-1 to AS only. Secondly, the role of DKK-1 in modulating Wnt pathway might be oversimplified. New evidences have showed that DKK-1 was linked to the abnormal activation of β-catenin-independent Wnt signaling by binding less avidly to LRP6 and was dysfunctional in AS [21]. Thus, the differences in the technology used in individual study which determine the total or functional DKK-1 was tested would result in opposite conclusions [27]. Similarly, the commercial ELISA kits for sclerostin measurements have not demonstrated consistent internal agreement in addition to which there are differences between serum and plasma levels [28]. Obviously, standardization of testing technology is very important to reveal the effect of Wnt suppressors in AS. Thirdly, it has been reported that patients with AS studied serially prior to and following anti-TNF alpha administration exhibited a significant increase in serum DKK-1 levels [21]. So the background treatment should be clarified.
The relationship between Wnt modulators and ASDAS as well as image evaluation in publication was not in accordance, too. ASDAS is well accepted to access the disease activity of AS using CRP (ASDAS-CRP) or ESR (ASDAS-ESR) as the major parameter [29, 30]. Besides inflammation, radiographic damage is another core outcome in AS. mSASSS is the most appropriate method by which to score the radiographic progression in AS patients in clinical trials [14]. Although Rossini M’s work revealed a significant positive correlation between DKK-1 serum levels and CRP [31], it showed no association between serum levels of DKK-1 or sclerostin and ASDAS in the present study (Fig. 2). Similar results had been published by Muntean L [32]. But sclerostin was related to mSASSS (Fig. 3), as reported by Appel H and his colleagues [33]. In our opinion, inflammation and ossification are two independent processes in the pathogenesis of AS and Wnt pathway is involved mostly in the new bone formation rather than in inflammation. As mSASSS reflected the osteophyte-forming process, sclerostin may be a potential marker indicating the spine ossification process in AS.
Interestingly, we found a negative relationship between DKK-1 and sclerostin (Fig. 4). As DKK-1 and sclerostin were both inhibitors of Wnt pathway, they were kindly competitive. But the R value was low. Maybe it’s the functional DKK-1 but not the total DKK-1 is the true competitor with sclerostin. The specific mechanism remains to be investigated.
Several limitations should be considered in this study. The number of patients is relatively small. The total DKK-1 but not the functional DKK-1 was tested. The different performances of commercial ELISA kits for sclerostin measurements should be considered and the background treatments should be clarified.
In conclusion, the correlation between serum Wnt suppressors and AS is still a matter of ongoing discussion. So, further investigations are needed to comprehensively understand the mechanisms of DKK-1 and sclerostin in the development of AS.
References
Braun J, Sieper J (2007) Ankylosing spondylitis. Lancet 369:1379–1390
Sieper J, Poddubnyy D (2017) Axial spondyloarthritis. Lancet 390:73–84
Smith JA (2015) Update on ankylosing spondylitis: current concepts in pathogenesis. Curr Allergy Asthma Rep 15:489
Zhou Y, Wang T, Hamilton JT, Chen D (2017) Wnt/β-catenin signaling in osteoarthritis and in other forms of arthritis. Curr Rheumatol Rep 19:53
Tsui FW, Tsui HW, Las Heras F, Pritzker KP, Inman RD (2014) Serum levels of novel noggin and sclerostin-immune complexes are elevated in ankylosing spondylitis. Ann Rheum Dis 73:1873–1879
Corr M (2014) Wnt signaling in ankylosing spondylitis. Clin Rheumatol 33:759–762
MacDonald BT, Tamai K, He X (2009) Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell 17:9–26
Xie W, Zhou L, Li S, Hui T, Chen D (2016) Wnt/β-catenin signaling plays a key role in the development of spondyloarthritis. Ann N Y Acad Sci 1364:25–31
Klavdianou K, Liossis SN, Sakkas L, Daoussis D (2017) The role of Dickkopf-1 in joint remodeling and fibrosis: a link connecting spondyloarthropathies and scleroderma? Semin Arthritis Rheum 46:430–438
Wu M, Chen M, Ma Y, Yang J, Han R, Yuan Y, Hu X, Wang M, Zhang X, Xu S, Liu R, Jiang G, Xu J, Shuai Z, Zou Y, Pan G, Pan F (2018) Dickkopf-1 in ankylosing spondylitis: review and meta-analysis. Clin Chim Acta 481:177–183
Daoussis D, Andonopoulos AP, Liossis SN (2010) Wnt pathway and IL-17: novel regulators of joint remodeling in rheumatic diseases. Looking beyond the RANK-RANKL-OPG axis. Semin Arthritis Rheum 39:369–383
Zhang L, Ouyang H, Xie Z, Liang ZH, Wu XW (2016) Serum DKK-1 level in the development of ankylosing spondylitis and rheumatic arthritis: a meta-analysis. Exp Mol Med 48:e228
Niu CC, Lin SS, Yuan LJ, Chen LH, Yang CY, Chung AN, Lu ML, Tsai TT, Lai PL, Chen WJ (2017) Correlation of blood bone turnover biomarkers and Wnt signaling antagonists with AS, DISH, OPLL, and OYL. BMC Musculoskelet Disord 18:61
Wanders AJ, Landewé RB, Spoorenberg A, Dougados M, van der Linden S, Mielants H et al (2004) What is the most appropriate radiologic scoring method for ankylosing ? Arthritis Rheum 50:2622–2632
Chou CT (2013) How to translate basic knowledge into clinical application of biologic therapy in spondyloarthritis. Clin Dev Immunol 2013:369202
Liang H, Li WR, Zhang H, Tian X, Wei W, Wang CM (2015) Concurrent intervention with exercises and stabilized tumor necrosis factor inhibitor therapy reduced the disease activity in patients with ankylosing spondylitis. Medicine (Baltimore) 94:e2254
Osman MS, Maksymowych WP (2017) An update on the use of tumor necrosis factor alpha inhibitors in the treatment of ankylosing spondylitis. Expert Rev Clin Immunol 13:125–131
Uderhardt S, Diarra D, Katzenbeisser J, David JP, Zwerina J, Richards W, Kronke G, Schett G (2010) Blockade of Dickkopf (DKK)-1 induces fusion of sacroiliac joints. Ann Rheum Dis 69:592–597
Heiland GR, Appel H, Poddubnyy D, Zwerina J, Hueber A, Haibel H, Baraliakos X, Listing J, Rudwaleit M, Schett G, Sieper J (2012) High level of functional dickkopf-1 predicts protection from syndesmophyte formation in patients with ankylosing spondylitis. Ann Rheum Dis 71:572–574
Sakellariou GT, Iliopoulos A, Konsta M, Kenanidis E, Potoupnis M, Tsiridis E, Gavana E, Sayegh FE (2017) Serum levels of DKK-1, sclerostin and VEGF in patients with ankylosing spondylitis and their association with smoking, and clinical, inflammatory and radiographic parameters. Jt Bone Spine 84:309–315
Daoussis D, Liossis SN, Solomou EE, Tsanaktsi A, Bounia K, Karampetsou MEE et al (2010) Evidence that DKK-1 is dysfunctional in ankylosing spondylitis. Arthritis Rheum 62:150–158
Korkosz M, Gąsowski J, Leszczyński P, Pawlak-Buś K, Jeka S, Kucharska E, Grodzicki T (2013) High disease activity in ankylosing spondylitis is associated with increased serum sclerostin level and decreased wingless protein-3a signaling but is not linked with greater structural damage. BMC Musculoskelet Disord 14:99–104
Ustun N, Tok F, Kalyoncu U, Motor S, Yuksel R, Yagiz AE, Guler H, Turhanoglu AD (2014) Sclerostin and Dkk-1 in patients with ankylosing spondylitis. Acta Reumatol Port 39:146–151
Kwon SR, Lim MJ, Suh CH, Park SG, Hong YS, Yoon BY, Kim HA, Choi HJ, Park W (2012) Dickkopf-1 level is lower in patients with ankylosing spondylitis than in healthy people and is not influenced by anti-tumor necrosis factor therapy. Rheumatol Int 32:2523–2527
Klavdianou K, Liossis SN, Papachristou DJ, Theocharis G, Sirinian C, Kottorou A, Filippopoulou A, Andonopoulos AP, Daoussis D (2016) Decreased serotonin levels and serotonin-mediated osteoblastic inhibitory signaling in patients with ankylosing spondylitis. J Bone Miner Res 31:630–639
Ke HZ, Richards WG, Li X, Ominsky MS (2012) Sclerostin and Dickkopf-1 as therapeutic targets in bone diseases. Endocr Rev 33:747–783
Yucong Z, Lu L, Shengfa L, Yongliang Y, Ruguo S, Yikai L (2014) Serum functional dickkopf-1 levels are inversely correlated with radiographic severity of ankylosing spondylitis. Clin Lab 60:1527–1531
Costa AG, Cremers S, Bilezikian JP (2017) Sclerostin measurement in human disease: validity and current limitations. Bone 96:24–28
Sellas I, Fernandez A, Juanola Roura X, Alonso Ruiz A, Rosas J, Medina Luezas J, Collantes Estevez E et al (2017) Clinical utility of the ASDAS index in comparison with BASDAI in patients with ankylosing spondylitis (Axis Study). Rheumatol Int 37:1817–1823
Machado PM, Landewé R, Heijde DV, Assessment of SpondyloArthritis international Society (ASAS) (2018) Ankylosing Spondylitis Disease Activity Score (ASDAS): 2018 update of the nomenclature for disease activity states. Ann Rheum Dis 77:1539–1540
Rossini M, Viapiana O, Idolazzi L, Ghellere F, Fracassi E, Troplini S, Povino MR, Kunnathully V, Adami S, Gatti D (2016) Higher level of Dickkopf-1 is associated with low bone mineral density and higher prevalence of vertebral fractures in patients with ankylosing spondylitis. Calcif Tissue Int 98:438–445
Muntean L, Lungu A, Gheorghe SR, Valeanu M, Craciun AM, Felea I et al (2016) Elevated serum levels of Sclerostin are associated with high disease activity and functional impairment in patients with axial spondyloarthritis. Clin Lab 62:589–597
Appel H, Ruiz-Heiland G, Listing J, Zwerina J, Herrmann M, Mueller R, Haibel H, Baraliakos X, Hempfing A, Rudwaleit M, Sieper J, Schett G (2009) Altered skeletal expression of sclerostin and its link to radiographic progression in ankylosing spondylitis. Arthritis Rheum 60:3257–3262
Funding
The study was sponsored by fund from the Jinhua city Science and Technology Bureau (2015-3-040).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was performed according to the Declaration of Helsinki, and approved by the ethics committee of the Second Affiliated Hospital of Zhejiang University, School of Medicine.
Disclosures
None.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sun, W., Tian, L., Jiang, L. et al. Sclerostin rather than Dickkopf-1 is associated with mSASSS but not with disease activity score in patients with ankylosing spondylitis. Clin Rheumatol 38, 989–995 (2019). https://doi.org/10.1007/s10067-018-4356-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-018-4356-z