Abstract
An insidious progression of cardiovascular (CV) involvement is generally associated with rheumatologic diseases and finally regarded as a major source of morbidity and mortality in Juvenile idiopathic arthritis (JIA) patients. JIA could involve all of the cardiac structures, including pericardium, myocardium, endocardium; coronary vessels; valves and conduction system. Development of pericarditis, myocarditis, endocarditis and ventricular dysfunction are not unexpected issues in the progress of JIA. It is essential to ensure a comprehensive follow-up with advanced and up-to-date diagnostic and therapeutic modalities for prevention of CV complications in JIA patients. Since these are all associated with an unfavorable prognosis, it is necessary to detect subclinical cardiac involvement in CV asymptomatic patients, in order to start adequate management and treatment. Furthermore, controlling chronic inflammatory state of JIA by new treatment modalities will also significantly reduce the overall morbidity and mortality related to CV diseases. In this review, we aimed to investigate CV involvement patterns in patients with JIA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Juvenile idiopathic arthritis (JIA) is one of the most commonly seen chronic diseases of childhood. The incidence of new cases ranges from 2 to 20 per 100,000 population per year. Despite new therapeutic options including biologic agents, burden of JIA remains considerable. Earlier diagnosis and aggressive treatment as well as understanding the environmental triggers and genetic predisposition are necessary to achieve an improvement in JIA outcome [1].
JIA is a heterogeneous group of disorders present with chronic arthritis and many extraarticular manifestations including CV disorders. According to literature data, CV system-related diseases constitute the second common cause of mortality in JIA patients [2]. Unfortunately, there are only a few report regarding the longitudinal follow-up and mortality of JIA patients. The most comprehensive study reports the standardized mortality ratio of males as 3.4 (95 % CI 2.0–5.5) and of females 5.1 (95 % CI 3.2–7.8) from a Scottish record. Musculoskeletal/connective tissue and circulatory manifestations were the leading causes of death [3].
Pericardial, myocardial or endocardial involvements are well-known entities that are seen in patients with JIA. The most common and also benign involvement pattern of the heart is pericarditis that is seen in 30–36 % of the patients although rare, endocardial and myocardial involvement contribute to the morbidity and mortality of the disease [4]. Most patients with endocarditis have aortic regurgitation, and others have mitral regurgitation, which can sometimes require valve surgery [5]. Myocarditis can be life threatening with congestive heart failure and dysrhythmias [4].
Additionally, in recent years, ischemic coronary artery diseases in rheumatologic disorders are more frequently pronounced at younger ages as a consequence of premature atherosclerosis. Together with the classical already known CV risk factors, systemic inflammatory molecules further contribute to the progression of atherosclerosis. In the last 15 years, survival of the JIA patients has continued to increase by the emerging of new therapeutic drugs such as biologics. However, this result induced an increase in prevalence of coronary artery disease.
Both insidious and increased prevalence of CV manifestations during the course of autoimmune diseases necessitate advanced screening methods and early treatment modalities.
Timely recognition and instituting treatment of CV disorders in JIA with DMARDs and biologic agents are crucial to prevent long-term damage and to achieve good physical and functional outcome.
In this review, we have focused on valvular, myocardial and pericardial involvement patterns together with vascular and rhythm disorders in JIA. We also aimed to increase the awareness about the interface between heart and rheumatology.
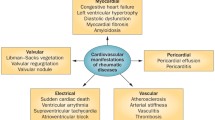
Knowledge of the involvement patterns of all of the cardiac elements is necessary for understanding various heart-associated manifestations such as endocarditis, myocarditis, pericarditis, myocardial and pericardial fibrosis, rhythm disturbances, coronary artery diseases, valvular lesions, pulmonary hypertension and finally heart failure.
Since all of these consequences have a poor prognosis, ongoing efforts for timely diagnosis of children with cardiac involvement even in the absence of any complaint are critical to better provide an effective treatment and improve health-related quality of life [6]. To prevent the poor cardiac outcome of JIA patients, subclinical cardiac deformations should be recognized and managed timely before establishment of apparent cardiac manifestations.
Search strategy and selection criteria
We searched PubMed for studies with the terms “juvenile idiopathic arthritis AND (cardiovascular OR heart OR cardiac OR pericarditis OR myocarditis OR endocarditis OR ventricle OR ventricular OR valvular OR electrical OR conduction).” Subsequently, we have called “juvenile idiopathic arthritis” and “juvenile arthritis” together with the same words. We preferentially handled the studies published from January 2000 to June 2016, without excluding main reports published before.
The research strategy in preparation of this review was generated from the scientific search guidelines [7].
Aims of this review are to outline the cardiac involvement patterns in JIA and methods for detection of subclinical cardiac involvement as well as to summarize the current treatment approaches from a comprehensive literature analysis.
Traditional and specific risk factors for cardiac events in JIA
Before defining the cardiac involvement patterns, documenting the risk factors for cardiac disease in JIA will provide a better understanding of this review.
Increased frequency of atherosclerosis as a consequence of continuous inflammatory environment is a well-known and specific risk factor for CV disease in JIA as in RA. While prevalence of already known traditional CV risk factors is reported to be increased in RA, on the other hand, the effects of these risk factors to cardiac structures remain unclear in JIA. These traditional risk factors are hypertension, diabetes mellitus, obesity, smoking, reduced physical activity (disease-related pain and functional disability further limit the patient). Since apparent CV disease due to JIA is generally not an issue of childhood, further prospective studies investigating the effects of traditional risk factors in adults with JIA are needed [8, 9].
Pericardial disease
Besides inflammation of the three layers of heart, subsequent development of effusion in the pericardium and valvular problems are not rare in JIA and categorized in extraarticular manifestations [10]. Among these, pericarditis is the most frequently involved part with the frequency in JIA is reported as 30 % [4].
Pericarditis is an extraarticular manifestation of JIA producing often mild complaints, and then, spontaneous regression takes place in most children. The quiescent nature of pericarditis makes the diagnosis less possible. This phenomenon elucidates the significant difference in the incidences of real-life studies (3–9 %) and postmortem reports (30–50 %) [11]. Some authors have consistently reported the incidence of pericarditis as 30–36 %, even in the absence of an apparent clinical sign [12, 13]. On the other hand, neither clinical nor echocardiographic evidence of effusion was detected in the studies of some authors [4, 10, 14].
While autopsy studies demonstrate pericarditis in as many as 45 % of JIA patients, clinically pericarditis occurs in only 7–36 % of the cases. Fortunately, this frequent involvement pattern generally follows a benign course [15].
Valvular involvement
Endocardial inflammation generally results with aortic or mitral regurgitation, which rarely needs valvular operation [5]. Valvular heart disease seldom occurs, however, when present leads to life-threatening consequences. Alkady et al. [16] reported thickened mitral valve with regurgitation in 24.3 % of patients, while aortic valve thickened solely in 5.4 % of children without regurgitation. Consistent with this result, in another study, regurgitating and thickened mitral valve was found to be 10 and 8 %, respectively [17].
Huppertz et al. [10] have detected aortic valve regurgitation in 4 children among 40 JIA patients. Various studies have also confirmed the mitral and aortic valve involvement in JIA [18, 19]. On the contrary, valvular involvement could not be identified in the studies of Oguz et al. [4] and Bharti et al. [14]. Aortic and mitral valve regurgitation (Video 1–2) may occur as a consequence of endocarditis, and clinicians should take into consideration the valve surgery procedure.
Heart failure
Myocarditis is possible life-threatening condition with congestive heart failure and arrhythmias [20]. Myocarditis is rare in JRA with frequency being reported as 1.2–10 % in different series [18, 19, 21, 22].
Although within normal range, increased resting heart rate and diastolic LV dimensions together with insignificantly decreased EF and FS in JIA patients compared to healthy controls may be an indicator of subclinical myocarditis [16]. Similar conclusions have been consistently found by different authors. Oguz et al. [4], Bharti et al. [14] and Huppertz et al. [10] showed a significant increase in the end-diastolic flow velocity in children with HLA B27-associated JIA at the termination of exercises. This is in contrast to Alkady et al. that reported impaired diastolic function even at rest [16]. However, another study did not find sufficient evidence of impaired diastolic functions in JIA patients and proposed that impairment of diastolic functions is a late finding that develops as the disease progresses [23].
Gupta et al. [24] demonstrated the correlation between impaired diastolic function with duration of disease. However, the exact mechanism of this inadequate ventricular filling in diastolic phase is still not clarified. It may be due to either a decrease in preload or an increase in afterload. Furthermore, impaired LV relaxation may lead to diastolic dysfunction. Although afterload increases as a result of increased blood pressure and heart rate, the relaxation of myocardium could not occur properly as a consequence of inadequate time.
In a chronic active JIA patient, fibrosis of myocardium further impair diastolic functions. In summary, impaired relaxation of myocardium may be due to various etiologies, including thickened and stiff pericardium, hypertrophy of LV, fibrosis, ischemic heart diseases and amyloid accumulation [24].
Oğuz et al. [4] used classical Doppler ultrasonography for measurement of the left ventricle systolic and diastolic functions among JIA patients. They found diastolic functions to be lower in JIA patients, comparing to controls. Consistent with this study, Koca et al. [25] also showed an impaired diastolic function in active JIA even in the absence of any relevant cardiac symptoms and signs.
Changes in left ventricle functions among JIA patients are considered to be multifactorial. Chronic inflammation resulting in subclinical vasculitis, endothelial damage and dyslipidaemia leading to premature atherosclerosis [26]. Activation of fibroblasts by pro-inflammatory cytokines in chronic inflammatory diseases result with sub-endocardial fibrosis [27]. A 20 % of deaths among patients with rheumatoid arthritis are considered to be secondary to myocardial fibrosis, myocardial damage due to ischemia and inflammation [28, 29]. Affected intermediate and small vessels of myocardium lead to micro-infarcts and ischemia that causes diminished left ventricular functions; consequently, increased filling pressure of the left ventricle causes diastolic dysfunction. Additionally, myocardial dysfunctions can cause decreased systolic functions. If there is a suspicion of myocardial involvement, speckle-tracking echocardiography is a better way to demonstrate it [30, 31].
CV diseases in JIA patients are the second most common cause of mortality. Hence, strict and regular follow-up of CV morphology and functions in JIA patients with a pediatric cardiologist should be done even in the adulthood period.
In previous studies, CV system of JIA patients were assessed especially in terms of systolic and diastolic functions of left ventricle. Impairment of systolic and diastolic functions was frequently demonstrated by some of the studies.
Several mechanisms have been suggested for diastolic dysfunction in children with JIA, including fibrous scarring of the heart muscle, myocarditis or arteritis, nodular granulomatosis, amyloidosis and cardiotoxic therapies used for JIA treatment. Increased fibroblast activity and collagen deposition leading to myocardial fibrosis is likely to affect both ventricles. Myocardial fibrosis and inflammation have been shown in 20 % of patients in autopsy studies [32].
Electrical abnormalities
Conduction system disorders are a significant reason of CV morbidity and mortality in patients with JIA. Data from literature report that conduction disorders and sudden cardiac death are more pronounced in patients with rheumatic disease than healthy population [33, 34]. In patients with rheumatologic disorders, underlying myocarditis, coronary artery disease, vasculitis, myocardial ischemia, pulmonary artery hypertension, as well as arrhythmias are responsible from sudden cardiac death and rhythm disorders.
Long QT dispersion (QTd) is an early sign of ventricular arrhythmias, thus a marker of CV morbidity and mortality. Rheumatoid arthritis (RA) patients were found to have a longer QT dispersion (QTd) compared to general population [35]. QTd is the maximum QT interval (QTmax) minus the minimum QT interval (QTmin) on a simultaneously recorded 12-lead electrocardiography (ECG). Increased QTd and corrected QT dispersion (cQTd) measurements are believed to reflect abnormal repolarization pattern and were found to be more frequent in RA patients than healthy population [20, 36].
Increased QTd could be a useful marker to assess homogeneity of cardiac repolarization and predicts ventricular arrhythmias and mortality [20].
Chronic active disease patterns of rheumatic diseases such as RA, ankylosing spondylitis and systemic lupus erythematosus lead to endothelial dysfunction and atherosclerosis. These endothelial alterations are responsible from the lack of the homogeneity [36–38]. In contrary, Koca et al. found that QTd is similar in patients with JIA and in healthy children. Consequently, the risk of ventricular arrhythmia among JIA patients was not significantly different comparing to healthy children [39].
P wave dispersion (PWD) is used as a determinant of supraventricular arrhythmias [40]. PWD is a good method that is used to be frequently assessed in RA patients to predict atrial fibrillation for a long time [41 ]. PWD was calculated by subtracting minimum P wave duration from maximum P wave duration on standard electrocardiogram [42]. An increase in PWD reflects heterogeneously transmitted signals in atrium and has been reported to be a risk factor for development and recurrence of atrial fibrillation (AF) [43]. In the study by Koca et al. [40], PWD value was found to be similar in JIA patients and healthy children.
Conclusion
Children with JIA remain at higher risk for developing CV disorders than their healthy peers. Subclinical CV involvement begins shortly after the onset of the disease and worsens with disease duration. All cardiac structures may be affected, and the cardiac complications include a variety of clinical manifestations (Table 1). Since CV involvement is associated with poor prognosis, the early detection of subclinical cardiac involvement by standard and speckle-tracking echocardiography together with QTd, PWD measurements in asymptomatic JIA patients is essential for timely treatment and better prognosis.
References
Prakken B, Albani S, Martini A (2011) Juvenile idiopathic arthritis. Lancet 377:2138–2149
Hull RG (1988) Outcome in Juvenile Arthritis. Br J Rheumatol 27:66–71
Thomas E, Symmons DP, Brewster DH, Black RJ, Macfarlane GJ (2003) National study of cause-specific mortality in rheumatoid arthritis, juvenile chronic arthritis, and other rheumatic conditions: a 20 year followup study. J Rheumatol 30:958–965
Oguz D, Ocal B, Ertan Ü, Narin H, Karademir S, Senocak F (2000) Left ventricular diastolic functions in Juvenile Rheumatoid Arthritis. Pediatric Cardiol 21:374–377
Kumar N, Rasheed K, Gallo R, Al-Hales Z, Duran CM (1995) Rheumatic involvement of all four heart valves: preoperative echocardiographic diagnosis and successful surgical management. Eur J Cardiothorac Surg 9:713–714
Turiel M, Sitia S, Atzeni F, Tomasoni L, Gianturco L, Giuffrida M, Colonna VG, Sarzi-Puttini P (2010) The heart in Rheumatoid Arthritis. Autoimmun Rev 9:414–418
Gasparyan AY, Ayvazyan L, Blackmore H, Kitas GD (2011) Writing a narrative biomedical review: considerations for authors, peer reviewers, and editors. Rheumatol Int 31:1409–1417
Brady SR, De Courten B, Reid CM, Cicuttini FM, de Courten MP, Liew D (2009) The role of traditional cardiovascular risk factors among patients with rheumatoid arthritis. J Rheumatol 36:34–40
Coulson EJ, Ng WF, Goff I, Foster HE (2013) Cardiovascular risk in juvenile idiopathic arthritis. Rheumatology 52:1163–1171
Huppertz H, Voigt I, Muller-Scholden J, Sandhage K (2000) Cardiac manifestations in patients with HLA B27-associated juvenile arthritis. Pediatr Cardiol 21:141–147
Goldenberg J, Pessoa AP, Roizenblatt S, Pavoa RMS, Hilario MO, Atra E, Ferraz MB (1990) Cardiac tamponade in juvenile chronic arthritis: report of two cases and review of publications. Ann Rheum Dis 49:549–553
Ozer S, Alehan D, Ozme S, Bakkaloglu A, Söylemezoglu O (1994) Mitral and aortic insufficiency in polyarticular juvenile rheumatoid arthritis. Pediatr Cardiol 15:151–153
Bernstein B, Takahashi M, Hanson V (1984) Cardiac involvement in juvenile rheumatoid arthritis. J Pediatr 85:313–317
Bharti BB, Kumar S, Kapoor A, Agarwal A, Mishra R, Sinha N (2004) Assessment of left ventricular systolic and diastolic function in juvenile rheumatoid arthritis. J Postgrad Med 50:262–265
Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Godenberg J et al (2004) International League of Associations for Rheumatology classification of juvenile idiopathic arthritis. Second revision. Edmonton. J Rheumatol 31:390–392
Alkady EA, Helmy HA, Mohamed-Hussein AA (2012) Assessment of cardiac and pulmonary function in children with juvenile idiopathic arthritis. Rheumatol Int 32:39–46
Sircar D, Ghosh B, Ghosh A, Haldar S (2006) Juvenile idiopathic arthritis. Indian Pediatr 43:429–433
Chen YS, Yang YH, Lin YT, Chiang BL (2004) A patient diagnosed with pauciarticular juvenile rheumatoid arthritis after a mechanical prospective valve replacement due to aortic regurgitation. J Microbial Infect 37:200–202
Lee SJ, Im HY, Schueller WC (2001) HLA-B27 positive juvenile arthritis with cardiac involvement preceding sacroiliac joint changes. Heart 86:19
Goldeli O, Dursun E, Komsuoglu B (1998) Dispersion of ventricular repolarization: a new marker of ventricular arrhythmias in patients with rheumatoid arthritis. J Rheumatol 25:447–450
Svantesson H, Bjorkhem G, Elborgh R (1983) Cardiac involvement in juvenile rheumatoid arthritis: a follow-up study. Acta Paediatr Scand 72:345–350
Miller JJ III, French JW (1977) Myocarditis in juvenile rheumatoid arthritis. Am J Dis Child 131:205–209
Vlahos AP, Alfantaki S, Bechlioulis A, Vakalis K, Michalis LK, Siamopoulou A (2008) Early cardiovascular risk assessment in patients with juvenile idiopathic arthritis. Pediatric Rheumatol 6:1
Gupta M, Rao PS (2004) Cardiac function in juvenile rheumatoid arthritis. Expert’s comments. J Postgrad Med 50(4):266–267
Koca B, Demir T, Kasapcopur O (2015) Use of tissue Doppler and its comparison with other pulse Doppler echocardiography in the evaluation of diastolic functions in patients with active juvenile idiopathic arthritis. Clin Rheumatol 34:1391–1396
Okada T, Shiokawa Y (1975) Cardiac lesions in collagen disease. Jpn Circ J 39:479–484
MacRae VE, Farquharson C, Ahmed SF (2006) The pathophysiology of the growth plate in juvenile idiopathic arthritis. Rheumatology 45:11–19
Birdane A, Korkmaz C, Ata N, Cavuşoğlu Y, Kasifoğlu T, Doğan SM, Gorenek M, Goktekin M, Unalir A, Timuralp B (2007) Tissue Doppler imaging in the evaluation of the left and right ventricular diastolic functions in rheumatoid arthritis. Echocardiogr 24:485–493
Maradit-Kremers H, Nicola PJ, Crowson CS, Ballman KV, Gabriel SE (2005) Cardiovascular death in rheumatoid arthritis: a population-based study. Arthritis Rheum 52:722–732
Pasceri V, Yeh ET (1999) A tale of two diseases: atherosclerosis and rheumatoid arthritis. Circulation 100:2124–2126
Urheim S, Edvarsen T, Torp H, Angelsen B, Smiseth OA (2000) Myocardial strain by Doppler echocardiography: validation of a new method to quantify regional myocardial function. Circulation 102:1158–1164
Maradit-Kremers H, Nicola PJ, Crowson CS, Ballman KV, Gabriel SE (2005) Cardiovascular death in rheumatoid arthritis: a population-based study. Arthritis Rheum 52(3):722–732
Seferovic PM, Ristic AD, Maksimovic R, Simeunovic DS, Ristic GG, Radovanovic G, Seferovic D, Maisch B, Matucci-Cerinic M (2006) Cardiac arrhythmias and conduction disturbances in autoimmune rheumatic diseases. Rheumatology 45:39–42
Urowitz MB, Gladman DD, Abu-Shakra M, Farewell V (1997) Mortality studies in systemic lupus erythematosus. Results from a single center. III. Improved survival over 24 years. J Rheumatol 24:1061–1065
Voskuyl AE (2006) The heart and cardiovascular manifestations in rheumatoid arthritis. Rheumatology (Oxford) 45(Supplement 4):iv4–iv7
Cindas A, Kutsal YG, Tokgozoglu L, Karanfil A (2002) QT dispersion and cardiac involvement in patients with rheumatoid arthritis. Scand J Rheumatol 31:22–26
Yıldırır A, Aksoyek S, Calguneri M, Aytemir K, Kabakci G, Ovunc K, Nazli N, Ozmen F, Oto A, Kes S (2000) QT dispersion as a predictor of arrhythmic events in patients with ankylosing spondylitis. Rheumatology (Oxford) 39(8):875–879
Yavuz B, Atalar E, Karadag O, Tulumen E, Ozer N, Akdogan A, Ertenli I, Kiraz S, Calguneri M, Ozmen F (2007) QT dispersion increases in patients with systemic lupus erythematosus. Clin Rheumatol 26:376–379
Koca B, Kasapcopur O, Bakari S, Celik E, Calay O (2012) QT dispersion and cardiac involvement in patients with juvenile idiopathic arthritis. Rheumatol Int 32:3137–3142
Koca B, Bakari S, Kasapcopur O, Celik E, Oztunc F, Eroglu AG, Saltık L (2012) P wave dispersion in juvenile idiopathic arthritis patients with diastolic dysfunction. Iran J Pediatr 22:512–518
Guler H, Seyfeli E, Sahin G et al (2007) P wave dispersion in patients with rheumatoid arthritis: its relation with clinical and echocardiographic parameters. Rheumatol Int 27(9):813–818
Michelucci A, Bagliani G, Corella A, Pieragnoli P, Porciani MC, Gensini G, Padeletti L (2002) P wave assessment: state of the art update. Card Electrophysiol Rev 6:215–220
Perzanowski C, Ho AT, Jacobson AK (2005) Increased P wave dispersion predicts recurrent atrial fibrillation after cardioversion. J Electrocardiol 38:43–46
Author contributions
OK designed the concept of paper. BK, OK and SS made the literature research and wrote the paper. AA and KB provided critical input in data collection and processing. All of the authors have analyzed the manuscript, finally.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This review was not funded by any person or corporation.
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Video 1
Color flow recording of a mitral and aortic regurgitation jet obtained from a zoomed view in the four chamber (AVI 4767 kb)
Video 2
Color flow recording of an aortic and mitral regurgitation jet obtained from a zoomed view in parasternal long axis chamber (AVI 5067 kb)
Rights and permissions
About this article
Cite this article
Koca, B., Sahin, S., Adrovic, A. et al. Cardiac involvement in juvenile idiopathic arthritis. Rheumatol Int 37, 137–142 (2017). https://doi.org/10.1007/s00296-016-3534-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-016-3534-z