Abstract
A genome-wide association study of gout in European populations identified 12 genetic variants strongly associated with risk of gout, but it is unknown whether these variants are also associated with gout risk in Chinese populations. A total of 145 patients with gout and 310 healthy control patients were recruited for a case–control association study. Twelve SNPs of CLNK and ZNF518B gene were genotyped, and association analysis was performed. Odds ratios (ORs) with 95 % confidence intervals (CIs) were used to assess the association. Overall, we found four risk alleles for gout in patients: the allele “G” of rs2041215 and rs1686947 in the CLNK gene by dominant model (OR 1.66; 95 % CI 1.04–2.63; p = 0.031) (OR 2.19; 95 % CI 1.38–3.46; p = 0.001) and additive model (OR 1.39; 95 % CI 1.00–1.93; p = 0.049) (OR 1.67; 95 % CI 1.19–2.32; p = 0.003), respectively, and the allele “A” of rs10938799 and rs10016022 in ZNF518B gene by recessive model (OR 4.66; 95 % CI 1.44–15.09; p = 0.008) (OR 4.54; 95 % CI 1.23–16.76; p = 0.020). Further haplotype analysis showed that the TCATTCTGA haplotype of CLNK was more frequent among patients with gout (adjusted OR 0.48; 95 % CI 0.24–0.95; p = 0.036). Additionally, polymorphisms of rs2041215, rs10938799, and rs17467273 were also correlated with clinical pathological parameters. This study provides evidence for gout susceptibility genes, CLNK and ZNF518B, in a Chinese population, which may have potential as diagnostic and prognostic marker for gout patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gout, a common form of inflammatory arthritis, is caused by urate crystal precipitates in the joint via an inflammatory reaction. It is increasingly assumed that gout occurs when serum uric acid (SUA) levels exceed the physiological saturation threshold for uric acid [1, 2]. Gout is characterized by persistent pain, nerve compression, and joint destruction and deformities, if left untreated [3]. With general changes in lifestyle and the rise of obesity, epidemiological data clearly demonstrate that the incidence and prevalence of gout are on the rise [4]. One to two percent of adults were affected by gout in developed countries in 2011, with the disease being more prevalent in men [5]. The prevalence rate of gout is also on the rise in China. In the Shandong coastal cities of Eastern China, reports showed that the overall gout prevalence increased to 1.14 % (1.94 % in men and 0.42 % in women) in 2008, from 0.1 % 10 years earlier [6].
An elevated concentration of SUA, or hyperuricemia (HUA), is a pivotal “danger signal” for gout and may also be a risk factor for a number of comorbidities including cardiovascular disease and mortality, hypertension, diabetes, obesity, hyperlipidemia, and metabolic syndrome [7–9]. In addition, elevated SUA levels can result in chronic kidney disease, by triggering chronic interstitial nephritis and the formation of urinary tract stones composed of uric acid [10]. Understanding the control of uric acid homeostasis is, thus, critical to improving the management and the treatment of patients with not only HUA and gout, but also a number of other diseases, which are linked to elevated SUA levels.
Although the specific pathogenesis of gout is still unclear, there is mounting evidence that both environmental and genetic factors play crucial roles in the etiology of HUA and gout. Observational studies have shown that dietary factors (animal purines, alcohol, and fructose), obesity, metabolic syndrome, hypertension, diuretic use, and chronic kidney disease may be clinically relevant risk factors for HUA and gout [11, 12]. However, epidemiological studies of genes affecting SUA levels have been performed and manifested that SUA levels are highly impacted by hereditary factors. Previous genome-wide association studies (GWAS) identified susceptibility genes, that affected UA levels, located in or near 12 genes and six regions, including hURAT1, SLC2A9, ABCG2, SLC17A1, SLC17A3, and GCKR, especially in populations of European descent [13–15].
In an attempt to discriminate other genes affecting gout, we performed a comprehensive association analysis between gout and 12 susceptible SNPs in the CLNK gene and ZNF518B gene that were previously reported to involve in the risk of gout [16]. The study sheds light on the association between common SNPs and gout risk in the Chinese population.
Materials and methods
Study participants
We recruited a total of 145 patients diagnosed with gout from 2011 to 2013 among Han Chinese. All the subjects were treated by the Affiliated Hospital of Tibet University for Nationalities and Xianyang Central Hospital. All the patients were recently diagnosed and histologically confirmed to suffer from gout according to the American College of Rheumatology classification criteria (1977) and had no history of cancer, infection, nephropathy, or other autoimmune diseases. All cases were verified, and patients were recruited without age, sex, or disease stage restriction. Moreover, patients did not receive systemic inflammatory treatment including drug control treatment before the blood samples used in this study were obtained.
A number of 310 healthy unrelated individuals were recruited randomly as sample, and the participants were Han Chinese living in Xi’an city and nearby. All of the chosen subjects were from the Medical Center in the Affiliated Hospital of Tibet University for Nationalities. To reduce the potential environmental and therapeutic factors impacting the variation of complex human diseases, we performed detailed recruitment and set exclusion criteria to exclude subjects with chronic disease and conditions involving vital organs (brain, liver, heart, and lung) and more advanced cardiovascular, metabolic, or endocrine diseases.
Clinical data and demographic
At recruitment, each subject gave written informed consent, and was interviewed by a nurse to collect detailed information including region, ethnicity, gender, age, education status, smoking status, alcohol use, occupational radiation exposure, family history of cancer, and other lifestyle factors. The use of samples was approved by the Human Research Committee of the Affiliated Hospital of Tibet University for Nationalities for Approval of Research Involving Human Subjects.
SNP selection and genotyping
Using HapMap database, candidate SNPs in the CLNK and ZNF518B gene with minor allele frequencies (MAFs) >5 % in Asian were identified in previously published polymorphisms associated with gout, resulting in 12 genotyped SNPs. The phenol–chloroform extraction method was performed to extract genomic DNA from whole blood [17]. DNA concentration was measured by spectrometry (DU530 UV/VIS spectrophotometer, Beckman Instruments, Fullerton, CA, USA). Sequenom MassARRAY Assay Design 3.0 software was used to design multiplexed SNP MassEXTEND assay, and SNP genotyping was performed utilizing the Sequenom MassARRAY RS1000 recommended by the manufacturer [18]. Sequenom Typer 4.0 software was used to perform data management and analyses [18, 19].
Statistical analysis
We used Microsoft Excel and SPSS 16.0 (SPSS, Chicago, IL, USA) to perform statistical analyses. In this study, all p values were two-sided, and p ≤ 0.05 was considered as achieving the threshold of statistical significance. Observed genotype frequencies were compared with expected frequencies to test for deviations from Hardy–Weinberg equilibrium (HWE). Chi-squared test/Fisher’s exact test was used to calculate the allele and genotype frequencies of cases and controls [20]. Odds ratios (ORs) and 95 % confidence intervals (CIs) were used for unconditional logistic regression analysis with adjustment for age and gender [21]. The possibility of sex differences as a source of population substructure was evaluated by a genotype test for each SNP in male and female, and the number of significant results at the 5 % level was compared with the number expected by the Chi-squared test [20].
Three genetic models (dominant, recessive, and additive) were performed using PLINK software (http://pngu.mgh.harvard.edu/purcell/plink/) to estimate ORs for SNP main effects. We determined p values for trend by entering the variable as a single term in the model (i.e., one degree-of-freedom) and testing using the Wald’s test. For SNP main effects analysis, we used ordinal variables coded as the number of variant alleles, zero, one, or two, assuming a log-additive genetic model. ORs and 95 % CIs were calculated by unconditional logistic regression analyses adjusted for age and sex [21, 22].
Finally, the Haploview software package (version 4.2) and SHEsis software platform (http://www.nhgg.org/analysis/) were used for estimate the pairwise linkage disequilibrium (LD), haplotype construction, and genetic association at polymorphism loci [23, 24].
Results
A number of 145 cases and 310 controls were enrolled in our study. The demographic and clinical variables in gout cases and control are shown in Table 1. The genotyping rate of 12 SNPs was 98.5 %, and all 12 SNPs were in Hardy–Weinberg equilibrium in control subjects (p > 0.01) (Table 2). We compared the differences in frequency distributions of alleles between cases and controls by Chi-squared test and found that two significant SNPs were associated with gout risk in the CLNK gene at a 5 % level (rs2041215 p = 0.033, OR 1.36; 95 % CI 1.02–1.82 and rs16869474 p = 0.002, OR 1.57; 95 % CI 1.17–2.10). To reduce the potential of spurious findings due to multiple testing, a strict Bonferroni correction analysis was applied; we found that rs16869474 (p < 0.05) satisfied the threshold between CLNK SNPs and risk of gout (Table 2).
Comparisons of the SNP genotypes and the risk of gout are listed in Table 3. We identified four significant SNP genotypes associated with the risk of gout. They were genotype “AA” of rs10938799 (OR 4.63; 95 % CI 1.41–15.20; p = 0.011), genotype “AA” of rs10016022 (OR 4.51; 95 % CI 1.21–16.82; p = 0.025), genotype “GT” of rs2041215 (OR 1.64; 95 % CI 1.01–2.66; p = 0.049), and genotype “GC” and “CC” of rs16869474 (OR 2.18; 95 % CI 1.34–3.53; p = 0.002) (OR 2.22; 95 % CI 1.06–4.64; p = 0.035).
The minor allele of each SNP was assumed a risk allele compared to the wild-type allele. Minor allele frequency (MAF) in cases and controls are listed in Table 4. We performed logistic tests to analyze further model association. rs10938799 and rs10016022 were observed to be associated with gout risk by recessive model analyses (OR 4.66; 95 % CI 1.44–15.09; p = 0.008 and OR 4.54; 95 % CI 1.23–16.76; p = 0.020, respectively). We also observed two susceptibility SNPs additionally: rs2041215, dominant model analyses (OR 1.66; 95 % CI 1.04–2.63; p = 0.031) and additive model analyses (OR 1.39; 95 % CI 1.00–1.93; p = 0.049); and rs16869474 dominant model analyses (OR 2.19; 95 % CI 1.38–3.46; p = 0.001) and additive model analyses (OR 1.67; 95 % CI 1.19–2.32; p = 0.003).
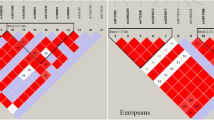
Furthermore, the candidate SNPs in the CLNK and ZNF518B genes showed strong linkage (Figs. 1, 2). The results for the association between the CLNK haplotype and the risk of gout are listed in Table 5. Haplotype “TCATTCTGA” was found to be associated with a decreased risk of gout (OR 0.48; 95 % CI 0.24–0.95; p = 0.036). We have not found any association between ZNF518B haplotype and the risk of gout.
Finally, correlations of clinicopathological parameters and prominent SNPs in patients with gout are shown in Supplementary Table. For rs2041215, a significant difference exists in total cholesterol (TC) as the genotype changes; for rs10938799 and rs10016022, a difference is seen in urea concentrations. We did not find any statistically significant associations between the clinicopathological parameters examined and rs16869474 genotypes in gout patients and control subjects.
Discussion
In this case–control study, we identified 12 previously reported gout risk loci identified through GWAS in European populations. Four susceptibility locis from chromosomes 4(rs10938799, rs10016022, rs2041215, and rs1686947) were statistically significantly associated with an increased risk of gout. We also observed that a haplotype “TCATTCTGA” of CLNK was associated with a 52 % reduction in the risk of gout. These results suggest that some gout risk variants identified in European population are also associated with risk in Han Chinese populations.
CLNK, a gene mapped in chromosome 4p16.1, is also termed MIST, which is expressed in several cell types, including T cells, natural killer cells, and mast cells, and its expression seems to be strictly dependent on sustained exposure to cytokines such as interleukin (IL)-2 and IL-3. Thus, CLNK may be involved in a cross-talk mechanism between cytokine receptor and immunoreceptor signaling [25, 26]. We have not found any evidence for the role of heredity between CLNK and gout susceptibility in previous studies. In the present study, nine CLNK SNPs (rs7667644, rs10033825, rs6819820, rs17467273, rs13109939, rs13125670, rs2041215, rs16869474, and rs12641877) were genotyped, and rs2041215 and rs1686947 were identified to be associated with gout risk. Carriers of the rs2041215 G allele exhibited a statistically significant increased 1.36-, 1.66-, and 1.39-fold gout susceptibility by the allele model, dominant model, and additive model, respectively. As for rs1686947, the risk was 1.57-, 2.19-, and 1.67-fold, respectively. Simultaneously, genotype “GT” of rs2041215 predicted an increased 1.64-fold gout risk, and genotype “GC” and “CC” of rs10016022 increased gout risk 2.18- and 2.22-fold. One of the most critical characteristics of gout and SUA is to stimulate the inflammatory response. Therefore, these findings indicate that CLNK may play a critical role in arthritis caused by gout. More samples and functional test are required to confirm our result. In subsequent genotype–phenotype analysis (Supplementary Table), TC presented prominent difference as the genotype changed, which may be the reason for the relationship between HUA and gout and levels of plasma lipids (cholesterol) that has been observed in a number of investigations [27].
ZNF518B, which is located in 4p16.1, has been reported in several researches. Observational studies have shown that the WDR1-ZNF518B intergenic region is implicated in a complex mechanism that regulates SCL2A9 function, which may potentially contribute to the SLC2A9-mediated effect on gender differences in human UA concentration levels [28]. However, study based on this ZNF518B gene is rare. As part of this study, the allele “A” of rs10938799 and “A” rs10016022 exhibited increased gout risk. Hence, ZNF518B gene may play an essential function in UA transport metabolism and further affect gout. Subsequently, we performed a detailed genotype–phenotype analysis among gout patient and controls (Supplementary Table). A significant difference exists in the concentration of urea as the genotype changes, which could be explained by the renal dysfunction caused by HUA and gout.
Our study is the first report on association between the SNPs CLNK and ZNF518B and gout risk. Meanwhile, GWAS is a powerful research strategy that uses SNPs as markers to identify susceptibility genes of many complex diseases. Furthermore, MassARRAY SNP genotyping method provided the reliability of these data as a broad method in the post-GWAS area. Finally, a haplotype-based association approach is an increasingly accepted approach for genetic association studies.
This study has some potential limitations. Firstly, the subgroup analyses testing for age or gender, and gender-specific significant variants were not performed because of the limited sample size. Secondly, the ethnicity of study participants was limited to the Han Chinese population. Hence, whether current research is applicable to other ethnicities still needs to be validated and therefore further meta-analysis is required to confirm our findings. Thirdly, when selecting SNPs, we achieved SNPs with MAF higher than 5 % in HapMap CHB (Chinese Han Bejing) population to ensure that the statistical power was large enough for analyzing data. This approach will leave out some significant SNPs that have been reported in other studies, and therefore, large sample size will be convincing. Finally, the function genetic variants and mechanisms underpinning this association will require additional studies including fine mapping and laboratory studies.
To sum up, we have confirmed that two genes previously reported in Europeans are associated with risk of gout in Han Chinese population for the first time, which may provide new data for screening of gout in Han population and shed light on the new candidate genes and new ideas for the study of subsequent occurrence mechanism of gout.
References
Robinson PC, Horsburgh S (2014) Gout: joints and beyond, epidemiology, clinical features, treatment and co-morbidities. Maturitas 78(4):245–251
Roddy E, Doherty M (2010) Epidemiology of gout. Arthritis Res Ther 12(6):223
Wang SM, Aranda GA Jr, Gao S, Patel BV (2013) Benefit restrictions and gout treatment. J Manag Care Pharm JMCP 19(9):773–782
Sautner J (2014) Diagnosis and management of gout in Austria : survey of current practice considering the EULAR recommendations. Zeitschrift fur Rheumatologie. PubMed PMID: 24763908. Diagnose und Therapiestandard der Gicht in Osterreich : Umfrage unter Berucksichtigung der EULAR-Empfehlungen
Bardin T, Richette P (2011) The epidemiology and genetic of gout. Presse medicale. Epidemiologie et genetique de la goutte 40(9 Pt 1):830–835
Miao Z, Li C, Chen Y, Zhao S, Wang Y, Wang Z et al (2008) Dietary and lifestyle changes associated with high prevalence of hyperuricemia and gout in the Shandong coastal cities of Eastern China. J Rheumatol 35(9):1859–1864
Sakiyama M, Matsuo H, Shimizu S, Nakashima H, Nakayama A, Chiba T et al (2014) A common variant of organic anion transporter 4 (OAT4/SLC22A11) gene is associated with renal underexcretion type gout. Drug Metab Pharmacokinet 29(2):208–210
Vitale A, Cantarini L, Rigante D, Bardelli M, Galeazzi M (2014) Anakinra treatment in patients with gout and type 2 diabetes. Clin Rheumatol [Epub ahead of print]
Stamp LK, Chapman PT (2013) Gout and its comorbidities: implications for therapy. Rheumatology 52(1):34–44
Zhou L, Liu L, Liu X, Chen P, Zhang Y, Wu Y et al (2014) Systematic review and meta-analysis of the clinical efficacy and adverse effects of chinese herbal decoction for the treatment of gout. PLoS One 9(1):e85008
Roddy E, Choi HK (2014) Epidemiology of gout. Rheum Dis Clin North Am 40(2):155–175
Stark K, Reinhard W, Neureuther K, Wiedmann S, Sedlacek K, Baessler A et al (2008) Association of common polymorphisms in GLUT9 gene with gout but not with coronary artery disease in a large case–control study. PLoS One 3(4):e1948
Graessler J, Graessler A, Unger S, Kopprasch S, Tausche AK, Kuhlisch E et al (2006) Association of the human urate transporter 1 with reduced renal uric acid excretion and hyperuricemia in a German Caucasian population. Arthritis Rheum 54(1):292–300
Kolz M, Johnson T, Sanna S, Teumer A, Vitart V, Perola M et al (2009) Meta-analysis of 28,141 individuals identifies common variants within five new loci that influence uric acid concentrations. PLoS Genet 5(6):e1000504
Wang J, Liu S, Wang B, Miao Z, Han L, Chu N et al (2012) Association between gout and polymorphisms in GCKR in male Han Chinese. Hum Genet 131(7):1261–1265
Kottgen A, Albrecht E, Teumer A, Vitart V, Krumsiek J, Hundertmark C et al (2013) Genome-wide association analyses identify 18 new loci associated with serum urate concentrations. Nat Genet. 45(2):145–154
Kochl S, Niederstatter H, Parson W (2005) DNA extraction and quantitation of forensic samples using the phenol–chloroform method and real-time PCR. Methods Mol Biol 297:13–30
Gabriel S, Ziaugra L, Tabbaa D (2009) SNP genotyping using the Sequenom MassARRAY iPLEX platform. In: Current protocols in human genetics, Chapter 2, Unit 2.12
Thomas RK, Baker AC, Debiasi RM, Winckler W, Laframboise T, Lin WM et al (2007) High-throughput oncogene mutation profiling in human cancer. Nat Genet 39(3):347–351
Adamec C (1964) [Example of the use of the nonparametric test. test X2 for comparison of 2 independent examples. Ceskoslovenske zdravotnictvi. 12:613–619. PubMed PMID: 14246305. Epub 1964/12/01. P r’iklad pou zit’i neparametrick’eho testu. text x2 pro srovn’an’i dvou nez’avisl’ych v’yb eru. cze
Bland JM, Altman DG (2000) Statistics notes. The odds ratio. BMJ 320(7247):1468
Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D et al (2007) PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81(3):559–575
Barrett JC, Fry B, Maller J, Daly MJ (2005) Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21(2):263–265
Shi YY, He L (2005) SHEsis a powerful software platform for analyses of linkage disequilibrium, haplotype construction, and genetic association at polymorphism loci. Cell Res 15(2):97–98
Goitsuka R, Kanazashi H, Sasanuma H, Fujimura YI, Hidaka Y, Tatsuno A et al (2000) A BASH/SLP-76-related adaptor protein MIST/Clnk involved in IgE receptor-mediated mast cell degranulation. Int Immunol 12(4):573–580
Cao MY, Davidson D, Yu J, Latour S, Veillette A (1999) Clnk, a novel SLP-76—related adaptor molecule expressed in cytokine-stimulated hemopoietic cells. J Exp Med 190(10):1527–1534
Darlington L, Scott J (1972) Plasma lipid levels in gout. Ann Rheum Dis 31(6):487
Wei WH, Guo Y, Kindt AS, Merriman TR, Semple CA, Wang K et al (2014) Abundant local interactions in the 4p16. 1 region suggest functional mechanisms underlying SLC2A9 associations with human serum uric acid. Hum Mol Genet 23(19):5061–5068
Acknowledgments
This work is supported by the Social Science Foundation of Chinese Ministry of Education (No. 12YJA850011), the Key Program of Natural Science Foundation of Xizang (Tibet) Autonomous Region (201122, 2014), and Major Training Program of Tibet University for Nationalities (No. 13myZP06). We would also like to thank BioScience Writers (BSW) for the assistance in the preparation of this manuscript.
Conflict of interest
The authors have no conflicts of interest to report.
Ethical standard
The use of samples was approved by the Human Research Committee of the Affiliated Hospital of Tibet University for Nationalities for Approval of Research Involving Human Subjects.
Author information
Authors and Affiliations
Corresponding author
Additional information
Tian-bo Jin and Yongchao Ren are joint first author.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jin, Tb., Ren, Y., Shi, X. et al. Genetic variations in the CLNK gene and ZNF518B gene are associated with gout in case–control sample sets. Rheumatol Int 35, 1141–1147 (2015). https://doi.org/10.1007/s00296-015-3215-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-015-3215-3