Abstract
Telomere maintenance mechanism is poorly studied in quiescence, a reversible non-proliferative state. We previously described in fission yeast a new mode of repair of telomeres named STEEx, that specifically operates in post-mitotic cells harboring eroded telomeres. This mechanism, promoted by transcription-induced telomeric recombination, prevents cells to exit properly from quiescence, suggesting that STEEx act as an anti-proliferative barrier. Here, we further showed that STEEx are genetically controlled by the Tel1ATM- and Rad3ATR- dependent DDR pathways. We discussed the possibility that STEEx represent a boundary between quiescence and vegetative cycle.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Telomeres consist of tandem repeats of short DNA sequence (TTAGGG) ended by a 3′ single-stranded overhang that is bound by a telomere associated complex, called Shelterin (Xin et al. 2008). The Shelterin protects chromosomes ends from degradation and end-fusion, and prevents the DNA damage response (DDR) to be activated if telomeres are deprotected (de Lange 2005). Telomeres progressively shorten at each replication because of the end replication problem and the 5′-end resection, required to generate the 3′-overhang (Lingner et al. 1995; Gilson and Géli 2007). The Shelterin counteracts telomere erosion by recruiting a specialized reverse transcriptase, called telomerase, that uses its associated RNA to template the addition of telomeric repeats at the 3′ end of chromosomes (Blackburn 2000). In addition, the action of telomerase is thought to limit replication stress induced damaged that occurs at telomeres, known as difficult regions to replicate (Simon et al. 2016; Maestroni et al. 2017b).
Telomere attrition leads to telomere deprotection and activation of the ataxia telangiectasia mutated (ATM) kinase pathway (Arnoult and Karlseder 2015). Tumor suppressor p53 activation by ATM induces a G1 cell cycle arrest via the cyclin-dependent kinase inhibitor p21, leading to replicative senescence (Roake and Artandi 2017). Cellular senescence is defined as a state of irreversible cell cycle arrest despite supply of growth factors, nutrients and oxygen (Campisi and d’Adda di Fagagna 2007). Induction of senescence by telomeres erosion participates to cellular aging by limiting the proliferative capacity of most cells in an organism (van Deursen 2014). When p53 and retinoblastoma protein (Rb) are mutated, cells bypass replicative senescence and continue to grow. Then, fully uncapped telomeres undergo fusions with other telomeric or non-telomeric loci. This stage, named crisis, is characterized by an extreme genome instability and an extensive death of cells among a population (Hayashi et al. 2015).
In contrast to senescence, cellular quiescence is a reversible growth arrest state. Under defined extracellular signals, quiescent adult stem cells are able of restarting proliferation for tissue homeostasis. Indeed, the ability of stem cells to enter and exit quiescence is required to respond to tissue damage resulting from life-threatening challenges (Coller 2011). Contrary to embryonic stem cells, most of adult stem cells exhibit no or low telomerase activity (Hiyama and Hiyama 2007). Thus, in adult stem cells, telomere shortening also occurs during aging, likely at a slower rate than that in normal somatic cells (Saeed and Iqtedar 2013). The consequences of telomeres shortening in adult stem cells may alter their function (Ye et al. 2014).
Eroded telomeres are rearranged in quiescent fission yeast cells
While the mechanisms of telomere maintenance have been investigated in dividing cells, little is known about the stability of telomeres in quiescent cells and how dysfunctional telomeres are processed in non-proliferating cells. We have recently examined the stability of telomeres in quiescent cells using fission yeast as a model (Maestroni et al. 2017a). Schizosaccharomyces pombe is a key model organism since it has a high level of conservation of telomeric proteins with mammalian cells (Miyoshi et al. 2008). Moreover, cells can be easily maintained in quiescence state by nitrogen starvation (Yanagida 2009). As in mammals, telomeres from dividing yeast cells without telomerase activity progressively shorten with each cell division. At late time-point of senescence, most of the cells die or remain arrested.
While we observed that wild-type telomeres are stable in quiescence, we discovered that eroded telomeres were highly rearranged during quiescence in the absence of telomerase (Maestroni et al. 2017a). These rearrangements correspond to the duplication of a subtelomeric region adjacent to telomeric repeats, named STEEx for STE1-Expansion. These telomeric rearrangements depend on homologous recombination (HR), which is initiated at homologous repeated sequence (HRS) within subtelomeres. Furthermore, we established that telomeric transcription, including TERRA, increases in post-mitotic cells with short telomeres and correlates with telomere rearrangements. Importantly, we observed that the HRS contains TERRA transcription start site. Thus, in the absence of DNA replication, we hypothesized that transcription could promote telomeric recombination in quiescence. We demonstrated that, in the absence of Rnase H enzymes that favor the accumulation of RNA-DNA hybrids, STEEx formation was promoted in quiescence, thereby supporting our assumption that telomeric transcription initiates subtelomeric rearrangements. Our results highlighted how non-dividing cells that harbor eroded and unprotected telomeres may circumvent the lack of telomerase. In parallel, we established that rearranged telomeres prevent cells to exit properly from quiescence. Indeed, cells with STEEx either died, failed to re-enter the cell cycle (enlarged cells) or became arrested after they re-entered the cell cycle (micro-colonies). We thus uncovered, in fission yeast, a new mode of telomere repair mechanism, different from previously described (Lue and Yu 2017), specific to post-mitotic cells that is likely promoted by transcription.
In the same way as the Styx forms a boundary between Earth and the Underworld in the Greek mythology, this new “STEEx river” may represent a barrier between quiescence and vegetative cycle when telomeres are critically short. Indeed, subtelomeric rearrangements at eroded telomere may limit the capacity of post-mitotic cells to proliferate, although environmental conditions allow it. Thus, a parallel can be drawn between STEEx in quiescence and replicative senescence. In both cases, the eroded telomeres appear as an anti-proliferative barrier (Fig. 1). As senescence and crisis, we may then consider the STEEx as a structural barrier that prevents cell division. Because stem cells alternate phase of proliferation and quiescence, if a similar mechanism exists in multicellular cells organism, the telomeric rearrangements may restrain the capacity of stem cells to exit quiescence. Consequently, they may participate to organismal aging by limiting the proper tissue renewal and regeneration.
Telomeric rearrangements are an anti-proliferative boundary in quiescence. Fission yeast can be maintained in quiescence by nitrogen starvation, a reversible non-proliferative state. When a source of nitrogen is provided, cells resume division. In the absence of telomerase, successive cell divisions lead to a progressive telomeres attrition (replicative senescence), until telomeres become critically short (red arrow), triggering replicative senescence which is an irreversible non-proliferative state. In contrast, quiescence is a reversible growth arrest state. Post-mitotic fission yeast cells with eroded telomeres (red arrow) undergo telomeric rearrangements in quiescence (Maestroni et al. 2017a). These recombination events, named STEEx, correspond to the duplications of subtelomeric regions that are promoted by telomeric transcription. Rearranged telomeres prevent cells to exit properly from quiescence suggesting that STEEx may represent an anti-proliferative barrier in quiescence that limit cell capacity to divide when telomere are too short
Subtelomeric duplication in quiescence may be controlled by checkpoint response
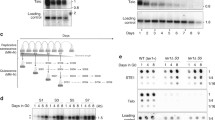
Because the senescence is a biological process that is triggered by DDR, we wondered whether subtelomeric duplications in quiescence are also controlled by checkpoint response. Tel1ATM and Rad3ATR were previously reported to induce an apoptotic-like response in fission yeast quiescent cells. Caffeine treatment, a known inhibitor of the phosphoinositide 3-kinase-like kinases abrogates this phenomenon (Ben Hassine and Arcangioli 2009; Arcangioli and Ben Hassine 2009). Based on these results, we treated telomerase minus cells (deleted for the telomerase RNA, TER1) with 2 mM caffeine in quiescence and monitored STEEx formation. The Fig. 2a shows an uncropped version of a southern blot presented in Fig. 1d of our main manuscript (Maestroni et al. 2017a), including an additional caffeine treatment in quiescence (after 5 and 7 days of senescence). Comparison of STEEx signals, with or without caffeine, at a same day of senescence, showed that caffeine treatment decreases the accumulation of STEEx in quiescence. This observation was further confirmed by dotblot quantification (Fig. 2b). We thus concluded that STEEx formation in quiescence is likely controlled, either by Tel1ATM or Rad3ATR (or both) checkpoint dependent pathways. Next, we monitored the capacity of quiescent cells, either treated with caffeine or untreated, to exit quiescence by micromanipulating these cells on rich medium agar plates. We observed that in presence or absence of caffeine, the percentage of telomerase-minus cells that were not able to form a colony, after 1, 4 or 8 days in quiescence, was similar (Fig. 2C). Among cells that were unable to form a colony, we distinguished cells that tried to exit from quiescence (enlarged cells or micro-colonies) from those that were blocked in quiescence (round cells). We observed that after 8 days of quiescence, the number of round cells was lower in caffeine treated cells than untreated cells. Accordingly, the number of cells that exit quiescence as enlarged cells and micro-colonies was increased. Overall, our observations suggest that in the absence of telomerase, the DDR pathway promotes STEEx formation and prevents cells to exit from quiescence.
Telomeric rearrangements might be controlled by checkpoint response. a Top, relative position of restriction sites in telomeric and subtelomeric regions of S. pombe chromosomes based on pNSU70. Telomeric (Telo) and subtelomeric (STE1) probes used for Southern blot are represented. Bottom, after 1, 3, 5 and 7 days of replicative senescence (S1 to S7), ter1Δ cells with different setting of telomere length were starved from nitrogen and maintained in quiescence for a total of 8 days. Caffeine (2 mM) was added in quiescence in cells that were collected at day 5 and 7 of senescence (S5 and S7). Genomic DNA was digested with EcoRI and Southern blotted. The membrane was hybridized with Telo, STE1 and chromosomic probes. 1, 4, 8 correspond to the number of days in quiescence. A chromosomal probe was used as a loading control. Subtelomeric region duplications (STEEx) are visualized as distinct EcoRI bands when revealed by the STE1 probe as schematized on the right panel. b Genomic DNA from quiescent ter1Δ cells at S5, treated or not with 2 mM caffeine, was spotted onto Hybond-XL membrane and hybridized with the indicated probes. Signal quantification has been performed with “Quantity one” software and STE1/Control ratio was determined. c ter1∆ cells were maintained in quiescence for 1, 4, 6, or 8 days in the presence or not of caffeine, and single cells were micromanipulated and plated onto YES plate allowing them to exit quiescence. The percentage of cells that were not able to form a colony was plotted. Microscopic observation was performed to determine how cells died when they re-enter into cell cycle. We distinguished single round cells, enlarged cells, and micro-colonies (1–20 cells). Round cell died in G0 while enlarged or micro-colony attempted to exit G0
Conclusions
We previously showed that subtelomeric rearrangements occurred at eroded telomeres in post-mitotic fission yeast cells and limited the capacity of cells to exit properly from quiescence. Here, we further show that these telomeric recombination events are likely controlled by the Tel1ATM- and Rad3ATR- dependent DDR pathways. Collectively, our results suggest that STEEx formation and DDR activation cascade are correlated and both contribute to the fate of a quiescent cell. In a same way as short telomeres trigger senescence in dividing cells, STEEx may define a non-proliferative mark of post-mitotic cells. Whether shortening of telomeres occur during quiescence is under investigation. Attrition of telomeres has been observed in brain or skeletal muscle cells regardless of their replicative activity (Daniali et al. 2013; Mamdani et al. 2016) suggesting that other factors than replication, such as oxidative stress, may provoke telomere attrition. Indeed, accumulation of oxidative damage over time may alter the binding of telomeric proteins, trigger DNA repair and cause telomere shortening (Kosmadaki and Gilchrest 2004; Fouquerel et al. 2016; Aeby et al. 2016) representing an active area of research for the next years.
References
Aeby E, Ahmed W, Redon S et al (2016) Peroxiredoxin 1 protects telomeres from oxidative damage and preserves telomeric DNA for extension by telomerase. Cell Rep 17:3107–3114. https://doi.org/10.1016/j.celrep.2016.11.071
Arcangioli B, Ben Hassine S (2009) Unrepaired oxidative DNA damage induces an ATR/ATM apoptotic-like response in quiescent fission yeast. Cell Cycle 8:2326–2331. https://doi.org/10.4161/cc.8.15.9147
Arnoult N, Karlseder J (2015) Complex interactions between the DNA-damage response and mammalian telomeres. Nat Struct Mol Biol 22:859–866. https://doi.org/10.1038/nsmb.3092
Ben Hassine S, Arcangioli B (2009) Tdp1 protects against oxidative DNA damage in non-dividing fission yeast. The EMBO J 28:632–640. https://doi.org/10.1038/emboj.2009.9
Blackburn EH (2000) Telomere states and cell fates. Nature 408:53–56. https://doi.org/10.1038/35040500
Campisi J, d’Adda di Fagagna F (2007) Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol 8:729–740. https://doi.org/10.1038/nrm2233
Coller HA (2011) The essence of quiescence. Science 334:1074–1075. https://doi.org/10.1126/science.1216242
Daniali L, Benetos A, Susser E et al (2013) Telomeres shorten at equivalent rates in somatic tissues of adults. Nat Commun 4:1597. https://doi.org/10.1038/ncomms2602
de Lange T (2005) Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev 19:2100–2110. https://doi.org/10.1101/gad.1346005
Fouquerel E, Lormand J, Bose A et al (2016) Oxidative guanine base damage regulates human telomerase activity. Nat Struct Mol Biol 23:1092–1100. https://doi.org/10.1038/nsmb.3319
Gilson E, Géli V (2007) How telomeres are replicated. Nat Rev Mol Cell Biol 8:825–838. https://doi.org/10.1038/nrm2259
Hayashi MT, Cesare AJ, Rivera T, Karlseder J (2015) Cell death during crisis is mediated by mitotic telomere deprotection. Nature 522:492–496. https://doi.org/10.1038/nature14513
Hiyama E, Hiyama K (2007) Telomere and telomerase in stem cells. Br J Cancer 96:1020–1024. https://doi.org/10.1038/sj.bjc.6603671
Kosmadaki MG, Gilchrest BA (2004) The role of telomeres in skin aging/photoaging. Micron 35:155–159. https://doi.org/10.1016/j.micron.2003.11.002
Lingner J, Cooper JP, Cech TR (1995) Telomerase and DNA end replication: no longer a lagging strand problem? Science 269:1533–1534
Lue NF, Yu EY (2017) Telomere recombination pathways: tales of several unhappy marriages. Curr Genet 63:401–409. https://doi.org/10.1007/s00294-016-0653-8
Maestroni L, Audry J, Matmati S et al (2017a) Eroded telomeres are rearranged in quiescent fission yeast cells through duplications of subtelomeric sequences. Nat Commun 8:1684. https://doi.org/10.1038/s41467-017-01894-6
Maestroni L, Matmati S, Coulon S (2017b) Solving the Telomere Replication Problem. Genes (Basel) 8:55. https://doi.org/10.3390/genes8020055
Mamdani F, Rollins B, Morgan L et al (2016) Variable telomere length across post-mortem human brain regions and specific reduction in the hippocampus of majordepressive disorder. 5:e636–e636. https://doi.org/10.1038/tp.2015.134
Miyoshi T, Kanoh J, Saito M, Ishikawa F (2008) Fission yeast Pot1-Tpp1 protects telomeres and regulates telomere length. Science 320:1341–1344. https://doi.org/10.1126/science.1154819
Roake CM, Artandi SE (2017) Control of cellular aging, tissue function, and cancer by p53 downstream of telomeres. Cold Spring Harb Perspect Med. https://doi.org/10.1101/cshperspect.a026088
Saeed H, Iqtedar M (2013) Stem cell function and maintenance—ends that matter: role of telomeres and telomerase. J Biosci 38:641–649
Simon MN, Churikov D, Géli V (2016) Replication stress as a source of telomere recombination during replicative senescence in Saccharomyces cerevisiae. FEMS Yeast Res 16:fow085. https://doi.org/10.1093/femsyr/fow085
van Deursen JM (2014) The role of senescent cells in ageing. Nature 509:439–446. https://doi.org/10.1038/nature13193
Xin H, Liu D, Songyang Z (2008) The telosome/shelterin complex and its functions. Genome Biol 9:232. https://doi.org/10.1186/gb-2008-9-9-232
Yanagida M (2009) Cellular quiescence: are controlling genes conserved? Trends Cell Biol 19:705–715. https://doi.org/10.1016/j.tcb.2009.09.006
Ye J, Renault VM, Jamet K, Gilson E (2014) Transcriptional outcome of telomere signalling. Nat Rev Genet 15:491–503. https://doi.org/10.1038/nrg3743
Acknowledgements
This project was funded by the “Agence Nationale de la Recherche” (ANR-QuiescenceDNA). LM is supported by the ANR-QuiescenceDNA. VG laboratory is supported by the “Ligue Nationale Contre le Cancer” (LNCC) (Equipe labélisée). SC is supported by the “Association pour la Recherche contre le Cancer” (ARC) and the Cancéropôle PACA.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by M. Kupiec.
Rights and permissions
About this article
Cite this article
Maestroni, L., Géli, V. & Coulon, S. STEEx, a boundary between the world of quiescence and the vegetative cycle. Curr Genet 64, 901–905 (2018). https://doi.org/10.1007/s00294-018-0808-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-018-0808-x