Abstract
The Aspergillus nidulans amdS selection marker was used for the identification of multicopy T-DNA insertions in Agrobacterium-mediated transformation of Asp. awamori. The selection of transformants on agar plates containing acetamide as sole nitrogen source and hygromycin resulted in a six-fold decrease in the transformation frequency, compared with the transformation frequency obtained after hygromycin selection alone. However, it was found that 47% of the transformants obtained after hygromycin and acetamide double selection contained multiple T-DNA integrations. Furthermore, it was found that the multicopy transformants could easily be identified based on their growth rate on agar plates containing acetamide medium. Based on these data, it can be concluded that the amdS marker can also be used as a selection marker in Agrobacterium-mediated transformation of Asp. awamori and that it is a very useful marker to identify those transformants containing multiple T-DNA integrations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Agrobacterium tumefaciens, a plant pathogen, is widely used for the transformation of plants. Agr. tumefaciens has the unique ability to transfer DNA (so-called transferred DNA or T-DNA), which is located between two inverted repeats, known as the left border (LB) and right border (RB), to its host. The transferred DNA is transported into the host as a single-stranded DNA molecule. Once inside the host, the DNA is targeted to the nucleus where it randomly integrates into the host genome (for reviews, see Gelvin 2000; Zupan and Zambryski 1995). Agr. tumefaciens is able to transfer its DNA not only to plants, but also to other organisms, including yeast, filamentous fungi and human cells (Bundock et al. 1995; de Groot et al. 1998; Kunik et al. 2001). The number of fungi that has been shown to be transformed by Agr. tumefaciens is rapidly increasing and Agrobacterium-mediated transformation (AMT) has been shown to be a good alternative to protoplast-based transformation methods (Amey et al. 2002; de Groot et al. 1998; Fitzgerald et al. 2003; Meyer et al. 2003). To date, only a limited number of selection markers have been used, including the auxotrophic pyrG marker and dominant selection markers based on resistance to the antibiotics hygromycin, geneticin, or phleomycin (de Groot et al. 1998; Gouka et al. 1999; Pardo et al. 2002; Vijn and Govers 2003). Analysis of the T-DNA integration patterns of transformants obtained from various fungi has revealed that the T-DNA integrates at random and predominantly as a single copy (Abuodeh et al. 2000; Bundock et al. 2002; Covert et al. 2001; Degefu and Hanif 2003; Leclerque et al. 2003; Vijn and Govers 2003), indicating that fungal AMT can be used for insertional mutagenesis and for the generation of DNA-insertion mutant banks.
To optimize AMT for various fungi, several variables [e.g. the influence of co-cultivation period and temperature, the Agrobacterium/spore ratio, the addition or omission of the vir gene inducer acetosyringone (AS) in the Agrobacterium pre-culture] have been found to influence transformation frequency (Abuodeh et al. 2000; Combier et al. 2003; Leclerque et al. 2003; Malonek and Meinhardt 2001; Michielse et al. 2004b; Mullins et al. 2001; Rho et al. 2001). Furthermore, it has been shown that these environmental conditions also have an influence on T-DNA copy number. For example, the addition of AS to the Agrobacterium pre-culture can result in a decrease (or an increase) in single-copy T-DNA integration (Combier et al. 2003; Mullins et al. 2001; Rho et al. 2001). Prolongation of the co-cultivation period was shown to lead to multiple T-DNA integrations in Magnaporthe grisea (Rho et al. 2001). However, the length of the co-cultivation period had no influence on T-DNA copy number in Fusarium oxysporum (Mullins et al. 2001). Applying AMT for the generation of mutant banks, single-copy T-DNA integration is preferred as it enables linkage of an observed phenotype with a single alteration in the genome. For heterologous and homologous protein production in filamentous fungi, it has been shown that the introduction of multiple copies of the gene of interest results in an increase in protein production (Verdoes et al. 1995). Therefore, it would be beneficial to be able to generate and identify multicopy transformants obtained with AMT. Gouka et al. (1999) demonstrated the use of AMT in Aspergillus awamori for the generation of transformants carrying multiple copies of the gene of interest, using a binary vector carrying these multiple copies on its T-DNA.
A selection marker which is frequently used in protoplast-based transformation methods to generate transformants carrying multiple copies of the introduced DNA is the Asp. nidulans amdS selection marker (Hanegraaf et al. 1991; Kelly and Hynes 1985; Penttila et al. 1987; Rodriguez and Yoder 1987). In this study, we demonstrate the use of the Asp. nidulans amdS selection marker and AMT in Asp. awamori to generate and identify multicopy T-DNA transformants.
Materials and methods
Strains, plasmids and growth conditions
Asp. awamori CBS115.52 (CBS, The Netherlands) was used as a recipient strain for transformation. Agr. tumefaciens LBA1100 (pAL1100ΔT-DNA, Δtra, Δocc; Beijersbergen et al. 1992), carrying pUR5750AmdS (this study, Fig. 2a) or pSDMAmdSΔBB (Michielse, Arentshorst, Ram and van den Hondel, submitted for publication; Fig. 2b) was used for AMT and was grown in LB medium (Sambrook et al. 1989) containing spectinomycin (250 μg/ml) and kanamycin (100 μg/ml) at 28°C. Introduction of plasmids into LBA1100 was performed as described by Mattanovich et al. (1989). Escherichia coli XL1-Blue (Stratagene) was used for construction of pUR5750AmdS and was grown in LB medium containing kanamycin (25 μg/ml) at 37°C. The binary vector pUR5750AmdS, containing both the hygromycin and amdS selection markers, was constructed by inserting an XbaI fragment of p3SR2 (Corrick et al. 1987), corresponding to the amdS gene and its promoter and terminator sequences, into pUR5750 (de Groot et al. 1998) previously digested with XbaI.
AMT in Asp. awamori
AMT was performed as described by de Groot et al. (1998), with minor adjustments (Michielse et al. 2004b). Transformants were either selected on agar plates containing MM (Punt and van den Hondel 1992) supplemented with 100 μg/ml hygromycin or on agar plates containing acetamide as a sole nitrogen source (Kelly and Hynes 1985) with or without 100 μg/ml hygromycin, as indicated. All plates were supplemented with 200 μM cefotaxim to inhibit the growth of Agr. tumefaciens.
DNA isolation and Southern analysis
Fungal chromosomal DNA isolation and Southern analysis were performed as described by Kolar et al. (1988) and Michielse et al. (2004a), respectively. Chromosomal DNA was digested with BglII and probed with a 3.1-kb XhoI–HindIII fragment of pAN7.1 (Punt et al. 1987), corresponding to the hygromycin cassette, or with a 2.6-kb XbaI fragment of p3SR2 (Corrick et al. 1987), corresponding to the amdS expression cassette.
Results and discussion
The use of the dominant Asp. nidulans amdS selection marker (Hynes and Pateman 1970) to identify multicopy T-DNA transformants of Asp. awamori obtained with AMT was assessed. Asp. awamori was transformed with Agrobacterium strain LBA1100 carrying the binary vector pUR5750AmdS, which contains both the hygromycin and amdS expression cassettes on its T-DNA. Asp. awamori was also transformed with Agrobacterium strain LBA1100 carrying the binary vector pSDMAmdSΔBB, which contains only the amdS expression cassette on its T-DNA. Depending upon the binary vector used, transformants were selected on agar plates containing medium supplemented with hygromycin, hygromycin and acetamide, or only acetamide.
Selection of transformants obtained with Agr. tumefaciens carrying pUR5750AmdS on medium containing only hygromycin yielded the highest transformation efficiency (Table 1). Double selection of the transformants obtained with the same binary vector on acetamide medium containing hygromycin resulted in a six-fold decrease in the transformation frequency (Table 1). The lower transformation efficiency is most likely due to more stringent selection conditions to obtain transformants. It is known that the use of the amdS marker requires high expression levels of the amdS gene to allow growth on medium containing acetamide. High expression levels can be the result of either multicopy integration or integration at a certain locus, which allows efficient transcription (Hanegraaf et al. 1991; Kelly and Hynes 1985; Penttila et al. 1987; Rodriguez and Yoder 1987). The selection of transformants obtained with the binary vector which carried only the amdS expression cassette between the T-DNA borders (pSDMAmdSΔBB), using acetamide-containing agar plates, resulted in a low transformation frequency (Table 1). About 20 co-cultivations were performed, yielding only three transformants. Some background growth of Asp. awamori under these selection conditions was observed. It is likely that Asp. awamori contains a low level of endogenous acetamidase activity, which caused this background growth and complicated the identification of transformants. These putative transformants grew very slowly and each remained a fluffy colony of white mycelium with very few spores. It is possible that the identification of putative amdS positive transformants may be facilitated by performing the co-cultivation on black filter paper rather than on white nitrocellulose membranes, as both are equally efficient (Covert et al. 2001). Alternatively, the background growth might be reduced if fungal and bacterial cells are recovered from the filters after co-cultivation and subsequently spread onto selection media. This method has been shown to reduce the background growth of Beauveria bassiana in AMT (Leclerque et al. 2003). Since variation in co-cultivation conditions has been shown to influence transformation frequency and T-DNA copy number (Abuodeh et al. 2000; Combier et al. 2003; Leclerque et al. 2003; Malonek and Meinhardt 2001; Michielse et al. 2004b; Mullins et al. 2001; Rho et al. 2001), it might be possible to increase the transformation frequency obtained by AMT in Asp. awamori based on the amdS selection marker by using different co-cultivation conditions.
It should be noted that the addition of AS to induce expression of the virulence genes of Agr. tumefaciens during co-cultivation is an absolute requirement to obtain transformants. Furthermore, all transformants obtained were mitotically stable (data not shown).
All transformants obtained were tested for their ability to grow on agar plates containing acetamide or hygromycin. A total of 25 [pUR5750AmdS] transformants, of which ten were obtained after selection on minimal medium with hygromycin (designated pUR5750AmdS-H) and 15 obtained after selection on acetamide medium supplemented with hygromycin (designated pUR5750AmdS-HA), were replica-plated onto agar plates containing either minimal medium supplemented with hygromycin or acetamide medium. All 25 transformants were able to grow on minimal medium containing hygromycin and no growth differences between the transformants were observed under these conditions (Fig. 1a,c). However, growth differences between the transformants were observed on acetamide medium (Fig. 1b,d). The ten pUR5750AmdS-H transformants remained small on acetamide medium and, even after prolonged incubation, these colonies did not grow further and did not sporulate. Seven of the 15 pUR5750AmdS-HA transformants (Fig. 1d, numbers 1, 2, 3, 6, 7, 9, 15; identified in caption) grew much faster on acetamide medium compared with the pUR5750AmdS-H transformants, suggesting that these transformants may contain more than one T-DNA copy. Also, the three [pSDMAmdSΔBB] transformants were able to grow on agar plates containing acetamide and no major growth differences between the three transformants were observed (data not shown).
Growth phenotype of transformants grown on hygromycin and acetamide selection plates. a, b Ten pUR5750AmdS transformants were obtained after selection on hygromycin-containing agar plates (top row, from left to right: numbers 1–5; bottom row: numbers 6–10) and replica-plated onto hygromycin-containing agar plates (a) or acetamide-containing agar plates (b). c, d pUR5750AmdS transformants obtained after selection on acetamide- and hygromycin-containing agar plates (top row, from left to right: numbers 1–5; middle row: numbers 6–10; bottom row: numbers 11–16) and replica-plated onto hygromycin-containing agar plates (c) or acetamide-containing agar plates (d)
Southern analysis was performed to determine the T-DNA copy number. Chromosomal DNA was digested with BglII and probed with hygromycin (hph) and amdS genes to determine single and multicopy T-DNA integration events. A single T-DNA integration event was expected to result in the detection of one fragment when probed with the hph gene (≥4,674 bp) and in two fragments (≥4,563 bp, ≥820 bp) when probed with the amdS gene (Fig. 2a). Based on previous studies, we know that 0–30% of the transformants contain more than one T-DNA copy when selected on medium containing hygromycin (Michielse et al. 2004a; Michielse, unpublished data). In this study, we found that all ten pUR5750AmdS-H transformants contained a single T-DNA integration. The Southern analysis result of a representative pUR5750AmdS-H transformant is shown in Fig. 2c,d (lane 1).
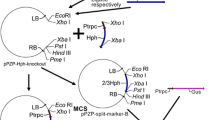
a Schematic representation of pUR5750AmdS T-DNA. LB Left border, RB right border. B BglII restriction site, Pgpd Asp. nidulans glyceraldehyde-3-phosphate dehydrogenase promoter, hph hygromycin resistance gene, TtrpC Asp. nidulans trpC terminator, amdS Asp. nidulans acetamidase enzyme, nptII neomycin/kanamycin resistance gene. b Schematic representation of pSDMAmdSΔBB T-DNA. c, d Southern analysis of [pUR5750AmdS] and [pSDMAmdSΔBB] transformants. Chromosomal DNA was digested with BglII and hybridized with a hygromycin (hph) probe (c) and amdS probe (d). Lane 1 pUR5750AmdS H-1 lane 2 pUR5750AmdS HA-1, lane 3 pUR5750AmdS HA-3, lane 4 pUR5750AmdS HA-2 lane 5 pSDMAmdSΔBB 6
Of the 15 pUR5750AmdS-HA transformants, eight single, four double and three triple T-DNA integrations were found. The double T-DNA integration occurred as inverted repeats at either the LB repeat (see Fig. 2c,d; 9,348-bp fragment in c, two times in d, lane 2, fragments of ≥4,563 bp and ≥820 bp) or the RB repeat (see Fig. 2c,d; two times in c, ≥4,674-bp fragments, two times in d, lane 3, 9,126-bp and 820-bp fragments). In three of the 15 transformants, multiple fragments were found for the hph and the amdS probes, indicating a triple T-DNA integration as either tandem or inverted repeats (Fig. 2c,d, lane 4).
The transformants obtained using [pSDMAmdSΔBB] were also analyzed by Southern analysis to determine the T-DNA copy number (Fig. 2d, lane 5). With this vector, a single T-DNA integration would be expected to result in the detection of two fragments (Fig. 2a; ≥1,940 bp, ≥875 bp). This pattern was observed in two of the three transformants obtained (data not shown). Southern analysis of the third transformant revealed the presence of four hybridizing bands. Fragments of 1,750 bp and 2,815 bp were observed (Fig. 2d, lane 5), indicating inverted and tandem integration, respectively. Furthermore, two fragments of 8.5 kb and 8 kb (two times ≥1,940 bp) were observed. This hybridization pattern indicates that this transformant contains three T-DNA copies which are linked.
Based on the Southern analysis, the pUR5750AmdS-HA1, HA2, HA3, HA6, HA7, HA14 and HA15 transformants were identified as multicopy transformants. Six out of these seven transformants grew well on acetamide plates, indicating a good correlation between the Southern data and the growth phenotype. pUR5750AmdS-HA transformant 9 grew and sporulated well on agar plates containing acetamide medium. However, its sporulating phenotype was different from the other transformants (Fig. 1d). Southern analysis revealed that this transformant contained a single T-DNA copy (data not shown). It could be that, in this transformant, the location of the T-DNA integration into the genome resulted in this phenotype. Conversely, pUR5750AmdS-HA transformant 14 was identified as a multicopy transformant, based on Southern analysis (data not shown). However, on agar plates containing acetamide, this transformant did not grow better than the single copy pUR5750AmdS-H transformants (Fig. 1). This could be due to the location of the T-DNA integration into the genome, such that the amdS gene is not expressed highly enough to result in better growth of this transformant than the wild-type Asp. awamori strain on agar plates containing acetamide.
Based on our results, it can be concluded that the amdS marker can be used as a selection marker for AMT in Asp. awamori and that with this marker growth on agar plates containing acetamide correlates with the T-DNA copy number enabling the identification of multicopy T-DNA transformants.
References
Abuodeh RO, Orbach MJ, Mandel MA, Das A, Galgiani JN (2000) Genetic transformation of Coccidioides immitis facilitated by Agrobacterium tumefaciens. J Infect Dis 181:2106–2110
Amey RC, Athey-Pollard A, Burns C, Mills PR, Bailey A, Foster GD (2002) PEG-mediated and Agrobacterium-mediated transformation in the mycopathogen Verticillium fungicola. Mycol Res 106:4–11
Beijersbergen AG, Den Dulk A, Schilperoort RA, Hooykaas PJ (1992) Conjugative transfer by the virulence system of Agrobacterium tumefaciens. Science 256:1324–1327
Bundock P, Dulk-Ras A, Beijersbergen A, Hooykaas PJ (1995) Trans-kingdom T-DNA transfer from Agrobacterium tumefaciens to Saccharomyces cerevisiae. EMBO J 14:3206–3214
Bundock P, Attikum H van, Dulk-Ras A, Hooykaas PJ (2002) Insertional mutagenesis in yeasts using T-DNA from Agrobacterium tumefaciens. Yeast 19:529–536
Combier JP, Melayah D, Raffier C, Gay G, Marmeisse R (2003) Agrobacterium tumefaciens-mediated transformation as a tool for insertional mutagenesis in the symbiotic ectomycorrhizal fungus Hebeloma cylindrosporum. FEMS Microbiol Lett 220:141–148
Corrick CM, Twomey AP, Hynes MJ (1987) The nucleotide sequence of the amdS gene of Aspergillus nidulans and the molecular characterization of 5′ mutations. Gene 53:63–71
Covert SF, Kapoor P, Lee M, Briley A, Nairn CJ (2001) Agrobacterium-mediated transformation of Fusarium circinatum. Mycol Res 105:259–264
Degefu Y, Hanif M (2003) Agrobacterium tumefaciens-mediated transformation of Helminthosporium turcicum, the maize leaf-blight fungus. Arch Microbiol 108:279–284
Fitzgerald AM, Mudge AM, Gleave AP, Plummer KM (2003) Agrobacterium and PEG-mediated transformation of the phytopathogen Venturia inaequalis. Mycol Res 107:803–810
Gelvin SB (2000) Agrobacterium and plant genes involved in T-DNA transfer and integration. Annu Rev Plant Physiol Plant Mol Biol 51:223–256
Gouka RJ, Gerk C, Hooykaas PJ, Bundock P, Musters W, Verrips CT, Groot MJ de (1999) Transformation of Aspergillus awamoriby Agrobacterium tumefaciens-mediated homologous recombination. Nat Biotechnol 17:598–601
Groot MJ de, Bundock P, Hooykaas PJ, Beijersbergen AG (1998) Agrobacterium tumefaciens-mediated transformation of filamentous fungi. Nat Biotechnol 16:839–842
Hanegraaf PP, Punt PJ, Hondel CA van den, Dekker J, Yap W, Verseveld HW van, Stouthamer AH (1991) Construction and physiological characterization of glyceraldehyde-3-phosphate dehydrogenase overproducing transformants of Aspergillus nidulans. Appl Microbiol Biotechnol 34:765–771
Hynes MJ, Pateman JA (1970) The genetic analysis of regulation of amidase synthesis in Aspergillus nidulans. I. Mutants able to utilize acrylamide. Mol Gen Genet 108:97–106
Kelly JM, Hynes MJ (1985) Transformation of Aspergillus niger by the amdS gene of Aspergillus nidulans. EMBO J 4:475–479
Kolar M, Punt PJ, Hondel CA van den, Schwab H (1988) Transformation of Penicillium chrysogenum using dominant selection markers and expression of an Escherichia coli lacZ fusion gene. Gene 62:127–134
Kunik T, Tzfira T, Kapulnik Y, Gafni Y, Dingwall C, Citovsky V (2001) Genetic transformation of HeLa cells by Agrobacterium. Proc Natl Acad Sci USA 98:1871–1876
Leclerque A, Wan H, Abschutz A, Chen S, Mitina GV, Zimmermann G, Schairer HU (2003) Agrobacterium-mediated insertional mutagenesis (AIM) of the entomopathogenic fungus Beauveria bassiana. Curr Genet (in press)
Malonek S, Meinhardt F (2001) Agrobacterium tumefaciens-mediated genetic transformation of the phytopathogenic ascomycete Calonectria morganii. Curr Genet 40:152–155
Mattanovich D, Ruker F, Machado AC, Laimer M, Regner F, Steinkellner H, Himmler G, Katinger H (1989) Efficient transformation of Agrobacterium spp. by electroporation. Nucleic Acids Res 17:6747
Meyer V, Mueller D, Strowig T, Stahl U (2003) Comparison of different transformation methods for Aspergillus giganteus. Curr Genet 43:371–377
Michielse CB, Ram AFJ, Hooykaas PJJ, Hondel CAMJJ van den (2004a) Agrobacterium-mediated transformation of Aspergillus awamori in the absence of full length VirD2, VirC2 and VirE2 leads to the insertion of aberrant T-DNA structures. J Bacteriol (in press)
Michielse CB, Ram AFJ, Hooykaas PJJ, Hondel CAMJJ van den (2004b) Role of bacterial virulence proteins in Agrobacterium-mediated transformation of Aspergillus awamori. Fungal Genet Biol (in press)
Mullins ED, Chen X, Romaine P, Raina R, Geiser DM, Kang S (2001) Agrobacterium-mediated transformation of Fusarium oxysporum: an efficient tool for insertional mutagenesis and gene transfer. Phytopathology 91:173–180
Pardo AG, Hanif M, Raudaskoski M, Gorfer M (2002) Genetic transformation of ectomycorrhizal fungi mediated by Agrobacterium tumefaciens. Mycol Res 106:132–137
Penttila M, Nevalainen H, Ratto M, Salminen E, Knowles J (1987) A versatile transformation system for the cellulolytic filamentous fungus Trichoderma reesei. Gene 61:155–164
Punt PJ, Hondel CA van den (1992) Transformation of filamentous fungi based on hygromycin B and phleomycin resistance markers. Methods Enzymol 216:447–457
Punt PJ, Oliver RP, Dingemanse MA, Pouwels PH, Hondel CA van den (1987) Transformation of Aspergillus based on the hygromycin B resistance marker from Escherichia coli. Gene 56:117–124
Rho HS, Kang S, Lee YH (2001) Agrobacterium tumefaciens-mediated transformation of the plant pathogenic fungus, Magnaporthe grisea. Mol Cells 12:407–411
Rodriguez RJ, Yoder OC (1987) Selectable genes for transformation of the fungal plant pathogen Glomerella cingulata f.sp. phaseoli (Colletotrichum lindemuthianum). Gene 54:73–81
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
Verdoes JC, Punt PJ, Hondel CAM van den (1995) Molecular genetic strain improvement for the overproduction of fungal proteins by filamentous fungi. Appl Microbiol Biotechnol 43:195–205
Vijn I, Govers F (2003) Agrobacterium tumefaciens mediated transformation of the oomycete plant pathogen Phytophthora infestans. Mol Plant Pathol 4:459–467
Zupan JR, Zambryski P (1995) Transfer of T-DNA from Agrobacterium to the plant cell. Plant Physiol 107:1041–1047
Acknowledgements
We would like to thank Simon Flitter and Patricia vanKuyk for critically reading this manuscript. This work was supported by Unilever Research, The Netherlands.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by U. Kück
Rights and permissions
About this article
Cite this article
Michielse, C.B., Ram, A.F.J. & van den Hondel, C.A.M.J.J. The Aspergillus nidulans amdS gene as a marker for the identification of multicopy T-DNA integration events in Agrobacterium-mediated transformation of Aspergillus awamori . Curr Genet 45, 399–403 (2004). https://doi.org/10.1007/s00294-004-0500-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-004-0500-1