Abstract
Targeted gene disruption via Agrobacterium tumefaciens-mediated transformation (ATMT) and homologous recombination is the most common method used to identify and investigate the functions of genes in fungi. However, the gene disruption efficiency of this method is low due to ectopic integration. In this study, a high-efficiency gene disruption strategy based on ATMT and the split-marker method was developed for use in Nomuraea rileyi. The β-glucuronidase (gus) gene was used as a negative selection marker to facilitate the screening of putative transformants. We assessed the efficacy of this gene disruption method using the NrCat1, NrCat4, and NrPex16 genes and found that the targeting efficiency was between 36.2 and 60.7%, whereas the targeting efficiency using linear cassettes was only 1.0–4.2%. The efficiency of negative selection assays was between 64.1 and 82.3%. Randomly selected deletion mutants exhibited a single copy of the hph cassette. Therefore, high-throughput gene disruption could be possible using the split-marker method and the majority of ectopic integration transformants can be eliminated using negative selection markers. This study provides a platform to study the function of genes in N. rileyi.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nomuraea rileyi is an important entomopathogenic fungus that can infect lepidopterous pests, especially Noctuidae species such as Spodoptera litura (Chen et al. 2014), Anticarsia gemmatalis (Palma and Del Valle 2015), Spodoptera frugiperda, Spodoptera exigua, Helicoverpa zea, and Heliothis virescen (Vega-Aquino et al. 2010), making it useful for insect biocontrol.
With the development of high-throughput sequencing technology, the transcriptome and whole genome sequence of N. rileyi have recently become available (Song et al. 2013; Shang et al. 2016). These data provide a basis for research on N. rileyi gene function. At first, double-stranded RNA was used in N. rileyi gene function studies to silence genes (Jiang et al. 2014; Liu et al. 2014; Zhou et al. 2015). However, only transient genetic modifications that are not stably inherited by subsequent generations could be achieved (Shao et al. 2015). Recently, the Agrobacterium tumefaciens-mediated transformation (ATMT) system in N. rileyi was developed by Shao et al. (2015), and several target genes have been disrupted using this system (Li et al. 2016; Song et al. 2016). The conventional gene disruption strategy for filamentous fungi involves the insertion of two homologous recombination sequences (HRS) on either side of the selected marker gene that fully or partially replaces the target gene via the homologous recombination (HR) pathway (Rothstein 1983; Wendland 2003; Weld et al. 2006). However, low frequencies of detected HR events are achieved in filamentous fungi using this method because the introduction of integrated DNA into the host genome results in a high frequency of ectopic integration via the non-homologous end-joining (NHEJ) pathway (Gauthier et al. 2010).
In order to increase the efficiency of gene targeting, the split-marker technique was developed by Fairhead et al. (1996) for use in yeast and was successfully applied to filamentous fungi by Catlett et al. (2003). This technique can be used to combine many transformation systems, such as protoplast-based transformation (Fairhead et al. 1996), biolistic transformation (Kim et al. 2009), electroporation (electropermeabilization) (Liang et al. 2014), and ATMT (Wang et al. 2010). The system involves the construction of two plasmids or DNA fragments, each containing a HRS with two-thirds of the selection marker gene. The two introduced DNA fragments enable a triple-crossover event between the target gene and the two DNA fragments. Previous studies have indicated that the split-marker technique can increase gene targeting efficiency and it has been used in several gene disruption studies in filamentous fungus (Catlett et al. 2003; Jeong et al. 2007; You et al. 2009; Wang et al. 2010; Liang et al. 2014). However, there are no reports of the split-marker method being applied to gene function research in the entomopathogenic fungi N. rileyi.
β-Glucuronidase, encoded by the gus gene, hydrolyzes 5-bromo-4-chloro-3-indulyl glucuronide (X-Glue) to a clear blue color and is widely used as a molecular marker in bacteria, fungi, and plant gene manipulation. The gus gene can be stably inherited in fungi and the expressed phenotypes of transformants show no differences from wild type (WT) (St. Leger et al. 1995). Therefore, this gene has been used as a negative selection marker for gene targeting in Magnaporthe grisea to improve the efficiency of deletion mutant screening (Wang et al. 2009).
In this study, a split-marker technique combined with the ATMT transformation system was established to achieve high-efficiency gene disruption in N. rileyi. The gus gene was introduced as a negative selection marker in the flanking region of the gene disruption vector to facilitate screening of the transformants. This system provides a reliable high-throughput approach for gene disruption in N. rileyi.
Materials and methods
Strains, plasmids, and culture conditions
The N. rileyi WT Nr01 strain was stored at the Engineering Research Center for Fungal Insecticides, Chongqing, China and was cultured as previously described (Song et al. 2013). Escherichia coli DH5α was used to propagate and maintain plasmids following standard procedures. A. tumefaciens AGL-1 laboratory stocks were used to transform N. rileyi. The WT A. tumefaciens AGL-1 was cultured in yeast extract beef (YEB) media with 50 µg mL−1 streptomycin. For plasmid-carrying A. tumefaciens, 50 µg mL−1 of kanamycin was also added to the media. The plasmids pPZP-Hph-Knockout, pPZP-Hph-RNAi and pPZP-Hph-gus were kind gifts from Changwen Shao, PhD, Chongqing University.
Construction of split-marker vectors
The split-marker vectors were derived from pPZP-Hph-Knockout described by Shao et al. (2015). The upstream 2/3hph cassette and the downstream 2/3hph cassette were amplified from the plasmid pPZP-Hph-RNAi using the primers split-marker A–F, A–R, B–F, and B–R (Table 1). Both the PCR products and the pPZP-Hph-Knockout plasmid were purified and digested with XhoI and XbaI. The two digested PCR fragments were ligated with the purified pPZP-Hph-Knockout backbone. The two new plasmids were named pPZP-split-marker-A and pPZP-split-marker-B (Fig. 1).
Multistep construction of vectors for split-marker gene replacement. The vector pPZP-Hph-Knockout was dual-enzyme digested with XhoI and XbaI. The two 2/3hph cassette fragments were amplified from the vector pPZP-Hph-RNAi using the primers split-marker A–F, split-marker A–R, split-marker B–F, and split-marker B–R. The two fragments were ligated with the pPZP-Hph-Knockout backbone and named pPZP-split-marker-A and pPZP-split-marker-B, respectively. The promoter Ptrpc was amplified from the vector pPZP-Hph-RNAi using the primers Ptrpc-F and Ptrpc-R. The gus gene was amplified from the vector pPZP-Hph-gus using primers gus-F and gus-R. SOE-PCR was performed to ligate Ptrpc and gus. The pPZP-split-marker-B was digested with the enzyme PmeI and ligated with the gus cassettes. The resulting plasmid was named pPZP-split-marker-B-gus. The multiple cloning site (MCS) is the insertion site of the flanking sequence
To conveniently screen the transformants, the gus gene was used as a negative selection marker. The Ptrpc promoter was amplified from plasmid pPZP-Hph-Knockout using the primers Ptrpc-F and Ptrpc-R (Table 1). The gus gene was amplified from the plasmid pPZP-Hph-gus using the primers gus-F and gus-R (Table 1). A splice overlap extension PCR (SOE-PCR) (Ho and Horton 1991) was used to ligate the Ptrpc promoter and the gus gene. The SOE-PCR products and pPZP-split-marker-B plasmid were digested with PmeI and ligated with T4 DNA ligase (Takara Biotechnology Inc. Dalian, China). The new plasmid was designated pPZP-split-marker-B-gus (Fig. 1).
Construction of NrCat1, NrCat4, and NrPex16 gene disruption vectors
A fusion primer and nested integrated PCR (FPNI-PCR) was used to clone the flanking sequence of the target gene NrCat1 as described by Wang et al. (2011). The products of the FPNI-PCR were cloned into the pMD19-T vector (Takara Biotechnology Inc.) and sequenced by Tsingke Biotech Co. Ltd. (Beijing, China). The flanking sequences of NrCat4 and NrPex16 were acquired from the reference genome sequence published by Shang et al. (2016).
The flanking regions of NrCat1, NrCat4, and NrPex16 were amplified using the primers NrCat1LF-F, NrCat1LF-R, NrCat1RF-F, NrCat1RF-R, NrCat4LF-F, NrCat4LF-R, NrCat4RF-F, NrCat4RF-R, NrPex16LF-F, NrPex16LF-R, NrPex16RF-F, and NrPex16RF-R (Table 1). The left flanking region of NrCat1 and the right flanking regions of NrCat4 and NrPex16 were inserted into the pPZP-split-marker-A vector. The right flanking region of NrCat1 and the left flanking regions of NrCat4 and NrPex16 were inserted into the pPZP-split-marker-B-gus vector. The split-marker disruption vectors obtained were named pPZP-split-marker-A-NrCat1, pPZP-split-marker-B-gus-NrCat1, pPZP-split-marker-A-NrCat4, pPZP-split-marker-B-gus-NrCat4, pPZP-split-marker-A-NrPex16, and pPZP-split-marker-B-gus-NrPex16.
The linear cassettes of NrCat1, NrCat4, and NrPex16 were also constructed for use as controls by inserting flanking regions into the pPZP-Hph-Knockout plasmid and were named pPZP-Hph-Knockout-NrCat1, pPZP-Hph-Knockout-NrCat4, and pPZP-Hph-Knockout-NrPex16.
Agrobacterium tumefaciens-mediated transformation (ATMT)
We transformed N. rileyi using the method described by Shao et al. (2015) with a modified blastospore preparation method. The conidia were inoculated on Sabourand maltose agar yeast extract (SMAY) media. The yeast-like blastospores were harvested by flooding with sterile distilled water (Thakre et al. 2011), washed twice with distilled water, and suspended in induction medium (IM) at a concentration of 107 blastospores mL−1. The two A. tumefaciens strains carrying the 2/3hph split-marker cassettes and the blastospore suspension were mixed at a ratio of 1:1:1 for co-transformation. The transformation procedure was performed according to the method of Shao et al. (2015).
Screening for deletion mutants
Gus assay
Transformants were inoculated on SMAY plates containing 450 µg mL−1 hygromycin B and 400 µg mL−1 cephalosporin and incubated for 4–7 days at 25 °C. Resistant transformants were transferred to fresh SMAY plates containing hygromycin B and cephalosporin and cultured for five generations. The transformants were then transferred to fresh SMAY plates without antibiotics and cultured for five generations to determine their morphological stability. Negative screening was performed using a gus assay kit (Huayueyang Biotechnology, Beijing, China). The hygromycin B-resistant transformants that exhibited no gus expression activity were considered to be putative mutants (Fig. 2).
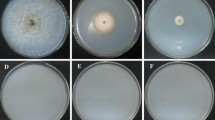
a, b Transformation and integration into the host genome. The transfer DNA of pPZP-split-marker-A and pPZP-split-marker-B-gus were transformed into the N. rileyi Nr01 strain by ATMT. a If the two transfer DNAs overlap and integrate with the host genome by HR as shown, the target sequence of the transformant is disrupted and only hph is expressed. b If the crossover event takes place between the two transfer DNAs and the transfer DNAs integrate into the host genome at a random location by ectopic insertion as shown, the targeted sequence will be not replaced by the hph cassette, and hph and gus will both be expressed. The mutants can initially be screened by negative-selection using the gus assay, and the transformants that do not demonstrate gus activity are further identified by PCR analysis. c Gus assay of putative transformants. Agar plugs are removed from N. rileyi cultures. Column WT, wild type; columns 1–11, putative transformants; columns 2, 3, 6, 7, and 10, gus positive transformants; columns 1, 4, 5, 8, 9, and 11, gus negative transformants
PCR analysis of mutants
Chromosomal DNA was isolated from putative deletion mutants using a genomic DNA isolation kit (Axygen Bio Inc., Union City, CA, USA). To verify mutants, PCR was performed using two pairs of primers, P5 and P6, and P7 and P8 that detected the absence of targeted gene fragments, as shown in Fig. 3a. After sequence verification analyses, those transformants containing amplicon (c) and amplicon (d) sequences were considered to be gene knockout mutants.
PCR verification of deletion mutants. a Predicted DNA structure of target sequences in WT and deletion mutants. P1 and P2 were extracted from the sequence of the hph cassette. P3 and P4 were extracted from the target sequence. P5 and P8 were extracted from the sequence beyond the flanking regions used for HR. P6 and P7 are located in the hph cassette and, therefore, no PCR products are expected for products (a), (c), or (d) for WT. b–d Results of PCR amplification from ΔNrCat1, ΔNrCat4, ΔNrPex16, and WT. Lane 1 shows the positions and sizes of the fragments of Marker III DNA Ladder. Lanes 2–5 show the deletion mutants from which products (a), (b), (c), and (d) were amplified. Lanes 6–9 show the WT from which products (a), (b), (c), and (d) were amplified
Southern blotting
Mycelia were collected from 100 mL of SMY liquid media and 5–10 µg of chromosomal DNA was isolated from each sample using the cetyltrimethylammonium bromide (CTAB) method. The chromosomal DNA was digested with NcoI and separated by electrophoresis on 0.8% agarose gels. The gel was then blotted onto a Hybond-N+ membrane (Amersham Biosciences, Little Chalfont, UK). Part of the left flanking sequence was amplified using the primers NrCat1 Probe-F and NrCat1 Probe-R (Fig. 4). The PCR-amplified product was digoxigenin (DIG)-labeled for use as a specific probe for signal detection. Probe hybridization and immunological detection were performed according to the manufacturer’s protocol (Roche, Mannheim, Germany).
Southern bolt analysis for confirmation of NrCat1 mutants. Genomic DNA samples from N. rileyi Nr01 WT and six ΔNrcat1 samples were digested with NcoI, fractionated on a 0.8% TAE (Tris base, acetic acid, and EDTA) agarose gel, and probed with a 1.1 kb NcoI–NcoI fragment (Nr01-wild-type) and a 2.3 kb NcoI–NcoI fragment (six ΔNrcat1 mutants)
Results
Construction of split-marker vectors and NrCat1, NrCat4, and NrPex16 gene disruption vectors
The modified gene disruption system employing the Hph-split-marker transformation method used in this study is described in Fig. 1. The pPZP-split-marker-A plasmid contained the upstream two-thirds of the hph cassette and the pPZP-split-marker-B plasmid contained the downstream two-thirds of the hph cassette. There was 649 bp of overlap in the center of the hph cassette. SOE-PCR was used to fuse the Ptrpc promoter and the gus gene. The gus cassette was inserted into the pPZP-split-marker-B vector near the right border for use as a negative selection marker. The MCS served as insertion sites for the HR sequences.
The flanking sequences of the NrCat1 gene were identified using FPNI-PCR (Wang et al. 2011). The PCR products ranged in size from 0.37 to 0.8 kb.
Transformation and gene disruption efficiencies were compared between the split-marker and linear deletion cassettes methods. For NrCat1, 1.5 kb of left flanking sequence and 1.3 kb of right flanking sequence were used. For NrCat4, 1.0 kb of left flanking and 1.2 kb of right flanking sequence were used. For NrPex16, 1.7 kb of left flanking sequence and 1.5 kb of right flanking sequence were used. HR sequences were inserted into the pPZP-split-marker-A, pPZP-split-marker-B-gus, and pPZP-Hph-Knockout vectors.
Efficiency of ATMT
Linear deletion cassettes were used to determine the ideal transformation parameters to be applied to ATMT in N. rileyi. The yeast-like blastospores obtained after 72 h were incubated at 25 °C on SMAY media at a concentration of 106 cells per plate, which was typically suitable for transformation. The highest transformation efficiencies were obtained on co-cultured plates incubated for 72–96 h at 26 °C.
Transformation efficiencies of linear deletion cassettes were approximately 11 times higher than split-marker deletion cassettes. A total of 630 hygromycin B-resistant transformants were obtained from 15 plates using linear deletion cassettes with an average of 42 transformants per plate. In contrast, 229 hygromycin B-resistant transformants were obtained from 60 plates using split-marker cassettes with an average of 3.8 transformants per plate.
Screening mutants and gene disruption efficiencies
In our experiments, 127 linear deletion cassettes and 76 split-marker NrCat1 transformants, 96 linear deletion cassettes and 84 split-marker NrCat4 transformants, and 96 linear deletion cassettes and 69 split-marker NrPex16 transformants were selected to verify the efficiency of HR for the two methods. PCR products (a), (c), and (d), but not (b), were obtained from deletion mutants (Fig. 3). In contrast, only PCR product (b) was obtained from the WT strain (Fig. 3). PCR verification results showed that, using the linear deletion cassettes, the HR rates for NrCat1, NrCat4, and NrPex16 were 1.6, 4.2, and 1.0%, respectively. Meanwhile, from the gus assay used for preliminary screening, 42, 62, and 39 putative split-marker transformants of NrCat1, NrCat4, and NrPex16 were obtained, respectively. These transformants grew on media containing hygromycin B, and they had no gus activity (Fig. 2). PCR identification confirmed that the HR rates for the NrCat1, NrCat4, and NrPex16 genes were 81.0, 82.3, and 64.1%, after negative screening (Table 2). These results indicated that the transformation efficiency of the split-marker method was lower, but the HR rates were approximately 21 times higher than those of the linear deletion cassette.
In order to assess copy number and integration events of the split-marker method used in this experiment, six NrCat1 mutants were selected and assessed using Southern blotting (Fig. 4). Only a single copy of the deletion cassette was found in the selected six mutants. No mutant showed evidence of extra ectopic integration.
Discussion
Previously, gene targeting in N. rileyi was achieved via a one-step gene disruption technique developed by Rothstein (1983). However, the gene targeting efficiency of this method was extremely low. For example, Song et al. (2016) demonstrated that gene targeting efficiencies for Nrhog1 and Nrslt2 knockouts were only 2.5 and 6.7%, respectively. In this study, we developed a modified split-marker gene knockout strategy for use in N. rileyi with a much higher gene targeting efficiency than that achieved with conventional linear deletion cassettes. In our experiment, NrCat1, NrCat4, and NrPex16 were knocked out using both the modified split-marker strategy and linear gene deletion cassettes and the results showed that gene targeting efficiencies of split-marker were much higher than those of linear deletion cassettes. We also successfully disrupted two other N. rileyi genes, NrSod1 and NrSod3, using the split-marker method with an integration frequency between 33 and 78% (unpublished data). The split-marker method has also been reported to achieve a high rate of HR in other fungi (Catlett et al. 2003; Jeong et al. 2007; Liang et al. 2014).
Although the split-marker method allows for high-efficiency gene targeting, it had a transformation efficiency rate that was, on average, 11-fold lower than linear deletion cassettes, in our study. Similarly, in Grosmannia clavigera, transformation efficiencies of linear deletion cassettes are approximately 20-fold higher than the split-marker method (Wang et al. 2010). The reason for this is that transformants cannot grow on selective media after integration of incomplete selectable marker genes. However, transformants can grow on selection media when HR occurs between the overlapping regions of the selectable marker gene. As the frequency of ectopic integration decreases, higher frequencies of gene targeting can be achieved. The transformation efficiency also decreased because of the integration of incomplete selectable genes.
Although the split-marker method can achieve higher gene targeting efficiencies, a number of ectopic integration transformants are still obtained. To eliminate ectopic integration transformants, the gus gene was introduced as a negative selection marker. As shown in Fig. 2, split-marker cassettes were able to be integrated through the HR pathway, allowing for replacement of the target gene by hph. Thus, the hph gene was expressed, whereas the gus gene was lost during the integration process. However, the transformants showed both hygromycin B resistance and gus activity when integration occurred via the NHEJ pathway, indicating that the majority of ectopic integration transformants were eliminated using a gus assay (Table 2). Previously, negative selection markers have been used with both linear deletion cassettes and the split-marker method, including studies using the HV-tk gene (Gardiner and Howlett 2004), the GFP gene (Xu et al. 2014), and the neo gene (Liang et al. 2014). However, use of the gus gene is both more convenient and faster compared with these genes.
The described technique, based on split-marker HR cassettes with dual selection and the ATMT system is a simple, reliable, and extremely efficient gene-disruption system for use in N. rileyi. This technique offers several advantages over traditional linear deletion cassettes, including potential use in dual- or even triple-gene knockout studies.
References
Catlett NL, Lee BN, Yoder OC et al (2003) Split-marker recombination for efficient targeted deletion of fungal genes. Fungal Genet Newslett 50:9–11
Chen H, Yin YP, Feng EY et al (2014) Structure and expression of a cysteine proteinase gene from Spodoptera litura and its response to biocontrol fungus Nomuraea rileyi. Insect Mol Biol 23(2):255–268
Fairhead C, Llorente B, Denis F et al (1996) New vectors for combinatorial deletions in yeast chromosomes and for gap-repair cloning using ‘split-marker’ recombination. Yeast 12(14):1439–1457
Gardiner DM, Howlett BJ (2004) Negative selection using thymidine kinase increases the efficiency of recovery of transformants with targeted genes in the filamentous fungus Leptosphaeria maculans. Curr Genet 45(4):249–255
Gauthier GM, Sullivan TD, Gallardo SS et al (2010) SREB, a GATA transcription factor that directs disparate fates in Blastomyces dermatitidis including morphogenesis and siderophore biosynthesis. PLoS Pathog 6(4):e1000846
Ho SN, Horton RM (1991) Method for gene splicing by overlap extension using the polymerase chain reaction. US 5023171 A
Jeong JS, Mitchell TK, Dean RA (2007) The Magnaporthe grisea snodprot1 homolog MSP1 is required for virulence. FEMS Microbiol Lett 273(2):157–165
Jiang SS, Yin YP, Song ZY et al (2014) RacA and Cdc42 regulate polarized growth and microsclerotium formation in the dimorphic fungus Nomuraea rileyi. Res Microbiol 165(3):233–242
Kim MS, Kim SY, Yoon JK et al (2009) An efficient gene-disruption method in Cryptococcus neoformans by double-joint PCR with NAT-split markers. Biochem Biophys Res Commun 390(3):983–988
Li Y, Wang ZK, Liu XE et al (2016) Siderophore biosynthesis but not reductive iron assimilation is essential for the dimorphic fungus Nomuraea rileyi conidiation dimorphism transition resistance to oxidative stress pigmented microsclerotium formation and virulence. Front Microbiol 7(440)
Liang LQ, Li JQ, Cheng L et al (2014) A high efficiency gene disruption strategy using a positive-negative split selection marker and electroporation for Fusarium oxysporum. Microbiol Res 169(11):835–843
Liu JJ, Yin YP, Song ZY et al (2014) NADH: flavin oxidoreductase/NADH oxidase and ROS regulate microsclerotium development in Nomuraea rileyi. World J Microb Biotechnol 30(7):1927–1935
Palma L, Del Valle EE (2015) The fungus Nomuraea rileyi growing on dead larvae of Anticarsia gemmatalis associated with soybean plants (Glycine max) in Esperanza (Argentina). Rev Argent Microbiol 47(3):277–278
Rothstein RJ (1983) One-step gene disruption in yeast. Methods Enzymol 101(6):202–211
Shang YF, Xiao GH, Zheng P et al (2016) Divergent and convergent evolution of fungal pathogenicity. Genome Biol Evol 8(5):1374–1387
Shao CW, Yin YP, Qi ZR et al (2015) Agrobacterium tumefaciens-mediated transformation of the entomopathogenic fungus Nomuraea rileyi. Fungal Genet Biol 83:19–25
Song ZY, Yin YP, Jiang SS et al (2013) Comparative transcriptome analysis of microsclerotia development in Nomuraea rileyi. BMC Genom 14(1):411
Song ZY, Zhong Q, Yin YP et al (2016) The high osmotic response and cell wall integrity pathways cooperate to regulate morphology microsclerotia development and virulence in Metarhizium rileyi. Sci Rep-UK 6:38765
St. Leger RJ, Shimizu S, Joshi L et al (1995) Co-transformation of Metarhizium anisopliae by electroporation or using the gene gun to produce stable GUS transformants. FEMS Microbiol Lett 131(3):289–294
Thakre M, Thakur M, Malik N et al (2011) Mass scale cultivation of entomopathogenic fungus Nomuraea rileyi using agricultural products and agro wastes. J Biopestic 4:176–179
Vega-Aquino P, Blanco CA, Sanchez-Pea SR (2010) Activity of oil-formulated conidia of Nomuraea rileyi and Paecilomyces tenuipes against Spodoptera Heliothis and Helicoverpa larvae and pupae. J Invertebr Pathol 103:145–149
Wang JY, Zhang Z, Du XF et al (2009) Dual screening for targeted gene replacement mutant in Magnaporthe oryzae with GUS as negative marker. Chin J Biotechnol 25(1):129–138 (Chinese)
Wang Y, Diguistini S, Wang T et al (2010) Agrobacterium-meditated gene disruption using split-marker in Grosmannia clavigera a mountain pine beetle associated pathogen. Curr Genet 56(3):297–307
Wang Z, Ye SF, Li JJ et al (2011) Fusion primer and nested integrated PCR (FPNI-PCR): a new high-efficiency strategy for rapid chromosome walking or flanking sequence cloning. BMC Biotechnol 11(1):109
Weld RJ, Plummer KM, Carpenter MA et al (2006) Approaches to functional genomics in filamentous fungi. Cell Res 16:31–44
Wendland J (2003) PCR-based methods facilitate targeted gene manipulations and cloning procedures. Curr Genet 44:115–123
Xu C, Zhang X, Qian Y et al (2014) A high-throughput gene disruption methodology for the entomopathogenic fungus Metarhizium robertsii. PLoS ONE 9(9):e107657
You BJ, Lee MH, Chung KR (2009) Gene-specific disruption in the filamentous fungus Cercospora nicotianae using a split-marker approach. Arch Microbiol 191(7):615–622
Zhou GL, Song ZY, Yin YP et al (2015) Involvement of alternative oxidase in the regulation of hypha growth and microsclerotia formation in Nomuraea rileyi CQNr01. World J Microb Biotechnol 311:1343–1352
Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (Grant No. 31570073).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Su, Y., Wang, Z., Shao, C. et al. An efficient gene disruption method using a positive–negative split-selection marker and Agrobacterium tumefaciens-mediated transformation for Nomuraea rileyi. World J Microbiol Biotechnol 34, 26 (2018). https://doi.org/10.1007/s11274-018-2409-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-018-2409-8