Abstract
Paraffin oil was encapsulated in a urea–formaldehyde polymer shell by in situ polymerization. The effect of modifying the fabrication parameters, specifically the emulsifier, the core material concentration, the stirring rate, and the pH, on the resulting microcapsules was characterized by FTIR, SEM, particle size analysis and TGA. The stiffness and the mechanical stability during mixing of the microcapsules were also evaluated. It was found that the ethylene maleic anhydride copolymer (EMA)-based microcapsules are smaller, harder and have an increase in yield of 15 % or more compared to the polyvinyl alcohol (PVA)-based microcapsules. Both EMA- and PVA-based microcapsules have good thermal stability up to 400 °C. Smaller EMA-based microcapsules require a higher force, up to 0.96 N, to be 80 % deformed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microencapsulation is used for storing functional core materials and is used in many different fields such as pharmaceuticals [1, 2], food additives [3], coatings [4, 5], electronic ink [6, 7], textiles [8], biotechnology [9] and energy storage [10]. For example, core materials which have high heat of fusion can be used for energy storage. Commonly, phase change materials (PCM) are used to absorb and release heat [11]. PCM can be used in many different types of applications such as thermoregulating textile materials [12], heat transfer [13], energy conservation in buildings [14] and packaging [15]. They have to be environmentally safe and have a melting temperature close to room temperature. Encapsulation of PCM can be used to prevent unwanted reactions with the environment and control volume differences as the phase change occurs. Urea–formaldehyde (U/F) and melamine–formaldehyde (M/F) resins are often used for encapsulating PCM [16–18].
Paraffin wax, a PCM candidate with high heat of fusion and low melting temperature, was encapsulated by Fallahi et al. [19] using M/F as the shell. They used the in situ polymerization process which resulted in spherical microcapsules with a rough surface. Mayya et al. [20] encapsulated paraffin oil with gelatin and Arabic gum by complex coacervation and found that the encapsulation yield increased from 35 to 70 % after adding small quantities of an oppositely charged surfactant to the polyelectrolyte. Bhattacharyya et al. [21] have shown that the yield of the paraffin oil microcapsules can be increased when using a cationic surfactant during the coacervation of gelatin and Arabic gum. Mao et al. [22] obtained uniform and spherical U/F microcapsules with paraffin oil as a core material by in situ polymerization without using surfactant. Currently, there is no information on the effect of the fabrication parameters of microencapsulated paraffin oil on the microcapsules properties in the literature.
In this work, the effect of the following parameters: (1) emulsifier type, (2) paraffin oil concentration (3) stirring rate and (4) pH, on the diameter, morphology, hardness, thermal and mechanical stability of the microcapsules is characterized.
Experimental
Materials
Urea (U), 37 wt% formaldehyde (F), ammonium chloride, hydrochloric acid, sodium hydroxide, polyvinyl alcohol (PVA) (M w 89,000–98,000, 99+ % hydrolyzed and the measured viscosity of 2.5 wt% PVA is 2.83 mPa s), ethylene maleic anhydride copolymer (EMA) (M w 100,000–500,000 and the measured viscosity of 2.5 wt% EMA is 3.99 mPa s), and resorcinol were from Sigma Aldrich. Epoxy resin (828) was from Miller-Stephenson, USA. Epoxy resin (D.E.R. 732) was from Fluka. Mineral turpentine oil (MTO) (commercial grade) was from Emichem company, EAU. Paraffin oil, was from BDH. All chemicals and materials were used as received without purification.
Synthesis of microcapsules
Encapsulation of paraffin oil was performed by in situ polymerization of oil in water emulsion following the method in Brown et al. [23]. The effect of emulsifiers (PVA and EMA), core material volume percent (7, 9.2, 13.2, 18.6 %), stirring rates (400, 550, 700 and 1000 rpm) and pH (3, 3.5 and 4) values on the microcapsule diameter, shell wall thickness and shell morphology will be elucidated.
Analysis of microcapsules
The chemical structure of the microcapsules was analyzed using a Fourier transform infrared (FTIR) spectrophotometer (Perkin Elmer 400) in the range of 400–4000 cm−1. A particle size analyzer (Mastersizer 2000, Malvern) along with a scanning electron microscope (SEM)) (Nova NanoSEM 450) was used to determine the microcapsules diameter. The surface morphology and shell thickness of the microcapsules were determined by SEM. Shell thickness was determined by evaluating ruptured microcapsules. The microcapsules were flash frozen by immersing them in liquid nitrogen, mounted on adhesive tape, and ruptured with a razor blade. After coating with gold, the ruptured microcapsules were evaluated using the SEM. Examples of the SEM images are shown in Figs. 2 and 3. The microcapsules were also analyzed using a thermogravimetric analyzer (Pyris 6) in a nitrogen environment using 3 mg sample weight. The heating rate was 10 °C/min between 30 and 500 °C.
The microcapsules were tested on a Texture Analyzer TA-2Xi (Stable Micro Systems, Godalming, UK) and software Texture Expert version 1.16. The Texture Analyzer utilizes a probe moving vertically at a constant velocity. The microcapsules were placed on a measuring plate in a single layer and the probe was lowered at 0.5 µm/s. The microcapsules were compressed until they ruptured.
The mechanical stability of the capsules during mixing with a host material was examined by varying the mixing speed, the host viscosity, and the mixing time. The microcapsules were added to epoxy resin (D.E.R. 732) and subjected to the mixing stresses generated during different mechanical stirring rates (100, 200, 300, 400 and 500 rpm) for 30 min. The viscosity of the host epoxy was modified by adding different volumes of mineral turpentine oil (MTO) to the epoxy resin. Each mixture of epoxy, MTO and microcapsules was stirred at 200 rpm for 15 min. Finally, long-term durability was evaluated by increasing the length of stirring of the epoxy with microcapsules at 200 rpm. All samples were examined using FlowCam particle analysis to determine the number of unbroken microcapsules. This was accomplished by pumping 1 ml of the sample through a glass flow chamber (FC 2000 X4) at a rate of 0.5 ml/L for 2 min and recording the flow at 5 frames per second.
Results and discussion
Microcapsule chemical structure
During encapsulation, urea and formaldehyde react to form methylol-ureas, which then condense upon acidification resulting in a crosslinked polymer shell which encapsulates the core material [24]. The core material, in this case paraffin oil, is suspended as droplets in an aqueous bath. During the beginning of the polymerization process the polymer that has formed is rich in polar groups resulting in a high hydrophilicity, which gradually decreases during polymerization [25]. Eventually, a hydrophobic polymer is formed that deposits on the emulsified oil droplets (core material) forming spherical capsules.
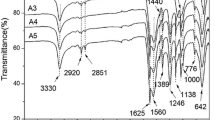
The chemical structure of the microcapsules (the sample with 18.6 vol% core material in Table 1) was characterized by FTIR as shown in Fig. 1. The spectrum of the microcapsules contains peaks at 3330 cm−1 (O–H and N–H stretching), 1620 cm−1 (C=O stretching) and 1540 cm−1 (N–H bending), indicating the formation of poly(urea–formaldehyde). These peaks match closely with the spectrum of the polymerization reaction between urea and formaldehyde [26]. Figure 1 includes curves for the filled microcapsules, the core material and the shell material structures. The spectrum of the filled microcapsules contains the peaks seen in both of the individual spectra of the shell and the core material, which demonstrates that the core material was successfully encapsulated in the urea–formaldehyde shell.
Effects of emulsifier type and core material volume
The morphology, diameter and shell thickness of the microcapsules with EMA and PVA emulsifiers were investigated using SEM as shown in Figs. 2 and 3, respectively. Table 1 shows the process parameters and the resulting yield, microcapsules diameter and shell wall thickness.
Using EMA as an emulsifier results in more regular and spherical microcapsules. The SEM images of EMA microcapsules are shown in Fig. 2. Microcapsules fabricated with PVA result in approximately 30 % more broken microcapsules as compared to EMA-based microcapsules and are shown in Fig. 3. This difference might be attributed to the thinner shell of the PVA-based microcapsules as compared to the EMA-based microcapsules. Table 1 provides the yield, shell wall thickness and diameter of EMA-based and PVA-based microcapsules.
For EMA-based microcapsules, as the volume percent of core material in the aqueous bath increases the average diameter of the final microcapsules increases while the yield decreases. Changing the emulsifier to PVA results in no significant effect on the average diameter of the microcapsules, but the yield is reduced approximately 15 %. However, the EMA-based microcapsules have a smaller average diameter as compared to PVA-based microcapsules. This may be attributed to the higher (by 17 %) viscosity of EMA compared to PVA. The high viscosity of the emulsifier reduces the mobility of the dispersing material (core material) and increases its homogeneity in the bath solution which results in additional dispersion of the oil droplets in the shear field caused by the stirring action. These smaller oil droplets then form smaller microcapsules.
The shell wall thickness of the microcapsule depends on the type of the emulsifier and the amount of the core material used during the fabrication of the microcapsules. Microcapsules that are fabricated with EMA or PVA as the emulsifier show a decreasing shell wall thickness as the volume percent of the core material in the process is increased. In addition, microcapsules that are fabricated with PVA have thinner shell walls than EMA microcapsules. The higher viscosity of EMA facilitates the deposition of the poly(urea–formaldehyde) particles on the core material droplets [23, 27] leading to thicker shell formation. Therefore, the smaller yield of the PVA-based microcapsules can be attributed to breakage of the thinner PVA-based microcapsules shell. In addition, as the volume percent of the core material used during fabrication of PVA microcapsules is increased, the stickiness of the microcapsules is also increased. The large microcapsule diameter and thinner shell wall facilitate the diffusion of the core material through the shell wall. This results in microcapsules that clump together and are difficult to separate.
Figures 4 and 5 show examples of particle diameter distributions of the EMA- and PVA-based microcapsules with 18.6 vol% of paraffin oil. The diameter of the microcapsules is distributed over a range of 100–600 µm with a mean of 210–255 µm when EMA was used as the emulsifier. The diameter variation is due to the turbulence around the stirrer blade. Fluid near the blade has higher turbulence and results in smaller microcapsules, while regions away from the blade produce larger microcapsules [28]. When PVA was used as the emulsifier, the maximum diameter distributed increased to 700 µm with the mean diameter ranging from 280 to 300 µm. The particle diameter analysis agrees well with the observations obtained from SEM. Some small particles, ranging from 20 to 80 µm are also formed during the fabrication process. They account for less than 1 % of the volume. These particles are urea–formaldehyde polymer without paraffin oil, effectively dust particles.
Effect of stirring rate
Variation of the stirring rate significantly affects the diameter of the microcapsules [23]. Lower stirring rates result in larger microcapsules and increasing the stirring rate can change the diameter of the microcapsules up to 52 % under the conditions used in this study. Figure 6a shows microcapsules fabricated using EMA at a low stirring rate (400 RPM) which results in average microcapsule diameters of 390 μm and tabulated in Table 2. As the stirring rate is increased the interfacial area is increased and results in improved homogeneity of the reaction medium which leads to the formation of more uniform diameter microcapsule. The particle diameter distribution of microcapsules at different stirring rates is shown in Fig. 7. The mean diameter at each stirring rate is as follows: 340 µm at 400 rpm, 246.5 µm at 700 rpm and 161.9 µm at 1000 rpm. Therefore, the mean diameter of microcapsules is strongly controlled by the rate of stirring and is relatively unaffected by the amount of the core material. A large change in the amount of the core material (paraffin oil) only leads to an 18 % change in the diameter of the microcapsule.
Effect of pH
EMA-based microcapsules fabricated using different pH values are shown in Fig. 8. No significant change is observed for the morphology of the microcapsules in the SEM micrographs for microcapsules formed at a pH of 3 (Fig. 8a), a pH of 3.5 (Fig. 2a), and a pH of 4 (Fig. 8b). As the acidity during the fabrication of the microcapsule decreases, the diameter of the microcapsule increases. The shell thickness of the microcapsules ranges from 1.57 to 2.33 µm with an average of 2.07 at a pH of 3. At a pH of 4, the shell wall ranges from 1.62 to 2.66 with an average of 1.84 µm as shown in Fig. 8A, B. The shell thickness increases at lower pH values since the rate of increasing viscosity of the emulsion solution and the rate of polymerization are accelerated by decreasing the pH [23, 27].
Thermal stability of microcapsules
The thermal stability of microcapsules plays an important role in the survivability of the microcapsules when they are embedded in a host material or during use. Figure 9 shows the TGA curves for microcapsules that were fabricated using EMA and PVA with different amounts of paraffin oil (18.6, 13.2, 9.2 and 7 vol%). At temperatures between 30 and 250 °C, the weight loss of the microcapsules is very small. This small weight loss is attributed to the evaporation of the residual water and elimination of free formaldehyde [29]. As can be seen in Fig. 10, the type of emulsifier as well as the amount of paraffin oil used during the fabrication of the microcapsule does not have a significant effect on the thermal stability of the microcapsules at temperatures below 250 °C.
Left TGA of EMA- and PVA-based microcapsules fabricated with 18.6, 13.2, 9.2 and 7 % by volume of paraffin oil at 550 rpm and pH 3.5 and symbolized as EMA18.6, EMA13.2, EMA9.2 and EMA7 for EMA-based microcapsules and PVA18.6, PVA13.2, PVA9.2 and PVA7 for PVA-based microcapsules, respectively. Right zoom-in in the range 250–450 °C
At higher temperatures, in the range of 250–420 °C, the different emulsifiers and core material become important. Within this range, the shell wall degrades and the core material evaporates. The weight loss at higher temperatures depends on the rigidity of the microcapsules’ shell. The EMA-based microcapsules have higher thermal stability than the PVA-based microcapsules. This can be attributed to a higher average molecular weight of EMA [30] and crosslink density of EMA-based microcapsules. The EMA-based microcapsules undergo an extensive decomposition above 420 °C. On the other hand, the decomposition temperature of microcapsules fabricated with PVA is 370 °C as can be seen in Fig. 9.
Figure 10 shows similar TGA curves for EMA-based microcapsules fabricated at stirring rates of 400, 700 and 1000 rpm as well as at different pH values of 3 and 4. There is a slight change in the decomposition temperatures in the range of 400 to 430 °C when the stirring rate and pH are varied. This change in the decomposition temperature is attributed to the variation of the diameter of the microcapsules, i.e., larger microcapsules decompose completely at lower temperatures due to its lower thermal stability.
Deformation of microcapsules (stiffness)
The stiffness of the microcapsules was characterized by measuring the force required to deform the microcapsules using a Texture Analyser. Compression of the microcapsules using a texture analyser is advantageous since it is rapid, accurate, reproducible and requires only a small number of capsules. Figure 11 shows the relation between the diameter of the microcapsule versus the applied force (at 80 % deformation). The stiffness of the microcapsule increases as the microcapsule diameter decreases, i.e., smaller microcapsules are stiffer than larger microcapsules [31] for both EMA and PVA microcapsules. In addition, the microcapsules formed using EMA emulsifier are stiffer than microcapsules prepared using PVA. This is expected since the crosslink density of the EMA-based microcapsules is greater than that of the PVA-based microcapsules [32]. However, the stiffness depends not only on the crosslinking density but also the flexibility of the shell and the core volume fraction. The core volume fraction for PVA microcapsules is approximately 7 % for microcapsules with an average diameter of 270 microns. In an equivalent EMA-based microcapsule, the core volume fraction is approximately 18.6 %. This difference in core volume fraction can lead to flexibility difference between the microcapsules.
Figures 12 and 13 represent the force as a function of the deformation of the EMA-based microcapsules obtained by different stirring rates (400, 550, 700 and 1000 rpm) and pH values (3, 3.5, and 4). As previously mentioned, increasing the stirring rates and decreasing the pH values lead to smaller microcapsules and a thicker shell wall. The force required to deform the microcapsules significantly increases upon increasing the stirring rate (Fig. 12) and decreasing the pH (Fig. 13), i.e., the smaller and thicker the microcapsules are, the stiffer they become. Therefore, the morphological properties and diameter of the microcapsules play an important role in the strength of the microcapsules [25].
Mechanical stability of EMA-based microcapsules
Microcapsules require sufficient mechanical stability to prevent them from breaking during mixing with the matrix material, which is typically a viscous resin. Figure 14a depicts the effect of different mixing rates on the durability of the microcapsules over 30 min. Figure 14b shows the durability of the microcapsules at a constant mixing rate of 200 rpm over variable time periods, and Fig. 14c shows the effect of different viscosities of the matrix material at a mixing rate of 200 rpm for 15 min.
The microcapsules are resistant to damage up to 200 rpm. Mixing speeds over 200 rpm result in an increase in broken microcapsules. Figure 14a shows that at 500 rpm, about 70 % of the microcapsules are broken. Lower mixing speeds generally result in fewer broken microcapsules, but slow mixing over long time periods will also result in more broken microcapsules. Figure 14b shows that longer time periods result in more broken microcapsules, for example, at 200 rpm, almost 40 % of the microcapsules were broken after 4 h of continuous mixing. In general, it is easier to incorporate the microcapsules into matrix materials that have lower viscosities. In matrix materials that have viscosities greater than 50 mPa s, there is more resistance to the mobility of the microcapsules and the survivability of the microcapsules significantly decreases as can be seen in Fig. 14c.
Conclusion
Paraffin oil was successfully encapsulated by in situ polymerization of urea–formaldehyde shell using two different emulsifiers, EMA and PVA. Using EMA creates more regular spherical-shaped microcapsules with a smaller diameter than microcapsules fabricated with PVA. The EMA-based microcapsule diameter was around 160 µm in diameter at 1000 rpm and increased in diameter as the stirring rate was decreased. In general, the microcapsules have good thermal stabilities, up to 250 °C, and hence have long shelf life at room temperature. Smaller microcapsules resist the breaking during their compression better than larger microcapsules. The breaking of the microcapsules during mixing in a host material is dependent on not only the mixing speed, but also the mixing duration. These results will play an important role in understanding the modification of microcapsules for industrial applications.
A smaller ratio of the core material (paraffin oil) results in tougher microcapsules. Microcapsules produced with EMA are generally better than microcapsules produced with PVA. Using EMA as the emulsifier resulted in an increased yield of microcapsules by at least 15 % and produced smaller, harder and more thermally stable microcapsules than that of PVA.
Improvement of the fabrication parameters results in higher yields, longer lasting and stronger microcapsules that will improve the longevity and manufacturability of the microcapsules.
References
Biju SS, Saisivam S, Maria NS, Rajan G, Mishra PR (2004) Dual coated erodible microcapsules for modified release of diclofenac sodium. Eur J Pharm Biopharm 58:61–67
Yutaka U, Kenichi H, Kageyosi S, Kazunori A, Yoshikazu T, Mutsuo S (2001) Improved survival and ammonia metabolism by intraperitoneal transplantation of microencapsulated hepatocytes in totally hepatectomized rats. Surgery 130:513–520
Yufera M, Fernandez-Diaz C, Pascual E (2005) Food microparticles for larval fish prepared by internal gelation. Aquaculture 248:253–262
Giraud S, Bourbigot S, Rochery M, Vroman I, Tighzert L, Delobel R (2002) Microencapsulation of phosphate: application to flame retarded coated cotton. Polym Degrad Stab 77:285–297
Krishnan S, Bhosale R, Singhal RS (2005) Microencapsulation of cardamom oleoresin: evaluation of blends of gum arabic, maltodextrin and a modified starch as wall materials. Carbohydr Polym 61:95–102
Kim CA, Joung MJ, Ahn SD, Kim GH, Kang SY, You IK, Oh J, Myoung HJ, Baek KH, Suh KS (2005) Microcapsules as an electronic ink to fabricate color electrophoretic displays. Synth Met 151:181–185
Guo HL, Zhao XP (2004) Preparation of a kind of red encapsulated electrophoretic ink. Opt Mater 26:297–300
Nelson G (2002) Application of microencapsulation in textiles. Int J Pharm 242:55–62
Sukhorukov G, Fery A, Mohwald H (2005) Intelligent micro- and nanocapsules. Prog Polym Sci 30:885–897
Palanikkumaran M, Gupta KK, Agrawal AK, Jassal M (2010) Effect of emulsion preparation method on microencapsulation of n-octadecane using melamine-formaldehyde pre-polymers. Ind J Fibre Text Res 35:101–106
Farid MM, Khudhair AM, Amar M, Razack SKA, Al-Hallaj S (2004) A review on phase change energy storage: materials and application. Energy Convers Manag 45:1597–1615
Shin Y, Yoo D, Son K (2005) Development of thermoregulating textile materials with microencapsulated phase change materials (PCM) II: preparation and application of PCM microcapsules. J Appl Polym Sci 96:2005–2010
Choi J, Lee JG, Kim HO, Yang H (2001) Preparation of microcapsules containing phase change materials as heat transfer media by in situ polymerization. J Ind Eng Chem 7:358–362
Hawlader MNA, Uddin MS, Khin MM (2003) Microencapsulation phase change materials: thermal-energy storage system. Appl Energy 74:195–202
Hohnston JH, Dodds M (2011) The development of a flexible re-useable thermal buffering and insulating liner for packing temperature sensitive products. Appita 64:153–157
Hoshi Y, Hayashy T (1983) US Patent 4409156 (to Fuji Photo Film Co., Ltd., Kanagawa, Japan)
Thies C (1987) Microencapsulation: polymer science and engineering encyclopedia. Willey, New York
Zhang Z, Sun G (2002) Mechanical strength of microcapsules made of different wall materials. Int J Pharm 242:307–311
Fallahi E, Barmar M, Kish MH (2010) Preparation of phase-change material microcapsules with paraffin or camel fat cores: application to fabrics. Iran Polym J 19:277–286
Mayya KS, Bhattacharra A, Argillier JF (2003) Microencapsulation by complex coacervation: influence of surfactant. Polym Int 52:644–647
Bhattacharra A, Argillier JF (2005) Microencapsulation by complex coacervation: effect of cationic surfactant. J Surf Sci Technol 21:161–168
Mao J, Yang H, Zhou X (2012) In-situ polymerization of uniform poly(urea–formaldehyde) microcapsules containing paraffins under the high-speed agitation without emulsifier. Polym Bull 69:649–660
Brown EN, Kessler MR, Sottos NR, White SR (2003) In situ poly(urea–formaldehyde) microencapsulation of dicyclopentadiene. J Microencapsul 20:719–730
Park SJ, Shin YS, Lee JR (2001) Preparation and characterization of microspheres containing lemon oil. J Colloid Interface Sci 241:502–508
Suryanarayana C, Rao KC, Kumar D (2008) Preparation and characterization of microcapsules containing linseed oil and its use in self-healing coatings. Prog Org Coat 63:72–78
Spectral Database for Organic Compounds (SDBS), organized by National Institute of Advanced Industrial Science and Technology (AIST), Japan (2014). http://riodb01.ibase.aist.go.jp/sdbs/cgi-bin/direct_frame_top.cgi
Mehdiabadi S, Nehzat MS, Bagheri R (1998) Correlating viscosity in urea–formaldehyde polymerization. J Appl Polym Sci 69:631–636
Jadhav RS, Hundiwale DG, Mahulikar PP (2011) Synthesis and characterization of phenol–formaldehyde microcapsules containing linseed oil and its use in epoxy for self-healing and anticorrosive coating. J Appl Poly Sci 119:2911–2916
Zhang XX, Tao XM, Yick KL, Wang XC (2004) Structure and thermal stability of microencapsulated phase-change materials. Colloid Polym Sci 282:330–336
Boh B, Knez E, Staresinic M (2005) Microencapsulation of higher hydrocarbon phase change materials by in situ polymerization. J Microencapsul 22:715–735
Hu J, Chen H, Zhang Z (2009) Mechanical properties of melamine formaldehyde microcapsules for self-healing materials. Mater Chem Phys 118:63–70
Chao HY (1992) Polyurea and polyurea-epoxy microcapsules, Google Patents (Patent #CA1300987C)
Acknowledgments
This publication was made possible by NPRP Grant 4-800-2-297 from the Qatar National Research Fund (a member of Qatar Foundation). The statements made herein are solely the responsibility of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fayyad, E.M., Almaadeed, M.A. & Jones, A. Preparation and characterization of urea–formaldehyde microcapsules filled with paraffin oil. Polym. Bull. 73, 631–646 (2016). https://doi.org/10.1007/s00289-015-1518-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-015-1518-x