Abstract
Blend microspheres of chitosan and polyurethane (PU) have been prepared by water-in-oil emulsion cross-linking method and used to encapsulate two water-soluble and having widely different plasma half-life cardiovascular drugs, viz., isoxsuprine hydrochloride and calcium dobesilate. The blend miscibility of the polymers was confirmed by differential scanning calorimetry at >60 wt% of PU. The microspheres were characterized by scanning electron microscopy to understand the morphology of the drug-loaded microspheres. Chemical interactions between drug molecules and the carrier polymers have been investigated by Fourier transform spectroscopy. XRD measurements on placebo matrices, drug-loaded formulations and nascent drugs indicated their uniform dispersion in the polymer matrix. In vitro release experiments performed in both acidic pH of 1.2 and alkaline pH of 7.4 increased the release time of both the drugs in the media employed. Kinetics of drug release was analyzed by empirical equation, suggesting the deviation from Fickian transport to non-Fickian trend.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polymer blends are an important class of engineering materials having a wide spectrum of applications, since their physical properties can be tailored by varying the relative concentrations of individual polymeric components. One of the important parameters determining the quality of blends is the degree of compatibility that has been widely studied using a number of techniques [1–4]. As a consequence of technical and commercial importance, the use of polymer blend systems in various scientific disciplines is growing rapidly [5–9]. Among the well-known biopolymers, chitosan (CS), i.e., ((1→4) 2-amino-2-deoxy, β-d-glucan), a naturally occurring and the second most abundant organic material, next to cellulose, is obtained by alkaline deacetylation of chitin [10]. CS has been widely used in drug delivery applications [11, 12] due to its outstanding properties such as non-toxicity, biocompatibility, mucus adhesion and biodegradability as well as it can be easily broken down into harmless products (amino sugars) that are easily absorbed by the body system. The CS-based micro/nanoparticles have been extensively studied over the past few decades to deliver drugs and genes, since the reactive amino and hydroxyl groups of CS can be utilized to chemically modify its structure [13–15].
Polyurethanes (PUs) are a class of biomedical polymers that are widely used in making heart valves [16], aortic grafts [17], pacing leads insulation [18], indwelling catheters [19], intra-aortic balloons [20], etc., mainly due to their attractive physical properties and good biocompatibility. Besides such traditional applications, development of biodegradable PUs for novel biomedical applications [21], including ligament reconstruction prostheses [22], temporary scaffolds [23, 24], controlled release (CR) systems of the active ingredients [25, 26], etc., have been investigated extensively. The majority of PUs are considered to be the most attractive biodegradable polymers, since their biodegradability can be achieved by incorporating labile and hydrolysable moieties into the main polymer backbone [27]. To fulfill this goal, polyols containing hydrolysable bonds are employed as soft segments for PUs like those of hydroxyl-terminated oligomers of polycaprolactone and polylactides [28] in addition to several different novel PUs developed before [29, 30] for drug delivery applications. The PU used in this work is of aliphatic nature, whose chemical structure is ROC(O)N(H)R′ where R and R′ are alkyl or aryl groups.
Even though CS has been used as a carrier for drugs, [31–36] in our ongoing efforts, however, we have developed few important blend biomaterials as drug delivery devices [11]. Different methods have been used to prepare the microspheres of CS [37, 38] but, the selection of any if such method depends on factors like particle size requirement, thermal and chemical stability of the active agent, reproducibility of in vitro release kinetic profiles, stability of the final product and its residual toxicity. Kumbar et al. [37] used the emulsion cross-linking method to prepare CS microspheres to encapsulate diclofenac sodium using three different cross-linking agents, viz., glutaraldehyde, sulfuric acid or heat treatment. Among these, glutaraldehyde cross-linked microspheres exhibited the slowest release profiles, but burst release was observed for heat-treated cross-linked microspheres. Ionic gelation method was also used [12] to produce CS microspheres using tripolyphosphate (TPP), but TPP/CS microspheres have poor mechanical strength thus, limiting their applications in drug delivery. Following these approaches, in the present study, we have prepared the blend microspheres by emulsion cross-linking method using glutaraldehyde (GA) as a cross-linker. Other studies include pH-sensitive chitosan-N,N′-dimethylacrylamide semi-interpenetrating network microspheres [38] cross-linked with GA for the CR of chlorothiazide, temperature-sensitive semi-interpenetrating (semi-IPN) microsphere [39] of sodium alginate and N-isopropylacrylamide for the CR of 5-fluorouracil and GA cross-linked hydrogel microspheres of CS and hydroxypropyl cellulose for the CR of chlorothiazide [40].
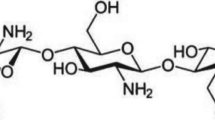
In continuation of these studies, we report here the preparation of blend microspheres of CS with PU for investigating the release properties of two typical water-soluble cardiovascular drugs, viz., isoxsuprine hydrochloride (ISX) and calcium dobesilate (CD) that have widely different chemical structures and plasma half-life. Of these, ISX, which is p-hydroxy-N-(1-methyl-2-phenoxyethyl) norephedrine hydrochloride (MW = 338 g/mol; plasma half-life = 1.25 h) is an active peripheral and cerebral vasodilator; it has the direct relaxant effect on smooth muscular tissue of the blood vessels and uterus. On the other hand, CD (MW = 418 g/mol; plasma half-life = 5 h) is a calcium 2,5-dihydroxy-benzenesulfonate, used in the treatment of chronic venous insufficiency and diabetic retinopathy. Both the drugs have completely different chemical structures (see Fig. 1) and CR formulations of these drugs are not available in the literature. In pursuit of our further efforts, we have developed the CR devices for these drugs using the blends of CS with PU prepared by emulsion cross-linking method. The formulations have been characterized by X-ray diffraction (XRD), differential scanning calorimetry (DSC) and scanning electronic microscopy (SEM). Fourier transform infrared spectroscopy (FTIR) was used to understand the chemical interactions of the drugs with the carrier matrix. The in vitro release studies have been performed in acidic (pH = 1.2) and alkaline (pH = 7.4) media to suggest the formulations as oral delivery devices. Furthermore, the release profiles of the drugs have been investigated in terms of the blend compositions and variations in the nature of the drug molecules.
Experimental section
Materials
ISX and CD drugs were received as gift samples from a drug company (Dharwad). CS of medium molecular weight (viscosity = 200–800 cP) and aliphatic PU (88 % solution in water) were purchased from Sigma Aldrich Chemicals, Milwaukee, WI, USA. Analytical reagent grade glutaraldehyde solution 25 % (v/v), petroleum ether, Span-80 and liquid paraffin oil were all purchased from s.d. fine Chemicals, Mumbai, India. Water used was of high purity grade after double distillation and deionization.
Preparation of CS/PU membranes
Dilute solutions of 1 % (w/v) CS and PU in 2 % acetic acid were prepared separately in two different conical flasks. Different blend compositions, viz., 20/80, 40/60, 60/40 and 80/20 of CS/PU were prepared by mixing appropriate quantities of stock solutions of CS and PU. Blend solutions prepared were cast as films onto a clean glass plate, dried initially at ambient temperature (25 °C) and then in a vacuum oven maintained at 40 °C for 48 h. The films containing different amounts of CS were designated as CS-20, CS-40, CS-60 and CS-80, while the symbol CS was used for pure chitosan.
Preparation of CS/PU microspheres
Microspheres of CS/PU blends were prepared by water-in-oil (w/o) emulsion cross-linking method [41]. Briefly, 20 mL of 2 % (w/v) of both CS and PU dissolved in 2 % acetic acid were continuously stirred until attainment of a homogeneous solution. Different amounts of ISX were dissolved in the above polymer blend solution and emulsified slowly into light liquid paraffin (100 g, w/w) containing 1 % (w/w) Span-80 with constant stirring at 600 rpm speed using Eurostar high-speed stirrer (IKA Labortechnik, Germany) for about 15 min. To this w/o emulsion, 5 mL of glutaraldehyde (GA) as a cross-linking agent containing 0.5 mL of 0.1 N HCl was added slowly and stirred for 3 h. The hardened microspheres were filtered, washed with petroleum ether and subsequently with water to remove the unreacted GA as well as any adhered Span-80. The method of CD loading is exactly similar to that of ISX.
Many studies have been reported earlier [42, 43] to evaluate the safety of GA, which is proven to be non-carcinogenic and safe if present in trace amount. This was further confirmed by the Brady’s test [44] that was found to be negative. Solid microspheres obtained were vacuum dried at 40 °C for 24 h and stored in a desiccator until further use. Similar protocol was used to prepare all other formulations. Therefore, we have excluded drug loading procedure for CD. The compositions of various formulations are given in Table 1.
Fourier transform infrared (FTIR) spectra
Stability of the drug in the polymer after encapsulation was evaluated by FTIR in case of (a) placebo CS/PU microspheres, (b) ISX-loaded CS/PU microspheres, (c) pristine ISX, (d) CD-loaded CS/PU microspheres and (e) pristine CD using Nicolet (Model Impact 410, Milwaukee, WI, USA) in the wavelength region between 500 and 4,000 cm−1. Microspheres were crushed to make the potassium bromide (KBr) pellets under a hydraulic pressure of 600 kg/cm2.
Differential scanning calorimetric (DSC) study
Differential scanning calorimetric (DSC) experiments were performed on pristine CS and blends of CS/PU to study their compatibility. DSC (DSC Q20, TA, USA) was carried out on (a) placebo CS/PU microspheres, (b) ISX-loaded CS/PU microspheres, (c) pristine ISX, (d) CD-loaded CS/PU microspheres and (e) pristine CD. Samples were heated from 25° to 400 °C at the heating rate of 10 °C/min in a nitrogen atmosphere.
X-ray diffraction (XRD) studies
Crystallinity of the drugs after encapsulation was evaluated by X-ray diffraction (XRD) measurements recorded for (a) placebo CS/PU microspheres, (b) ISX-loaded CS/PU microspheres, (c) pristine ISX, (d) CD-loaded CS/PU microspheres and (e) pristine CD using X-ray diffractometer (x-Pert, Philips, UK). Scanning was done at ambient temperature (25 °C) by varying the angle, 2θ up to 50°.
Scanning electron microscopic (SEM) analysis
Some representative samples of the microspheres were taken on a copper stub and sputtered with gold coating of 10 nm thickness for about 2 min. The gold-coated microspheres were mounted on SEM instrument (JEOL model JSM-840A, Japan) to record the spectra.
Drug loading
Estimation of drug concentration was done as per the protocol adopted before [45]. Particles of known weight (~10 mg) were ground to get the powder using an agate mortar, extracted with 50 mL of 7.4 pH buffer solution and sonicated for 30 min (UP 400 s, Dr. Hielscher, GmBH, Germany). The solution was centrifuged (Jouan, MR23i, France) to remove polymeric debris and washed twice to extract the drug. The solution was centrifuged to remove the suspended polymer particles and the clear supernatant liquid was diluted with buffer solution. Drug was assayed using UV–VIS spectrophotometer (Model Anthelie, Secomam, France) at the λ max of 219 and 198 nm for ISX and CD, respectively. The % encapsulation efficiency (EE) was calculated as described before [45].
In vitro drug release
In vitro drug release was carried out at 37 °C in a tablet dissolution tester (LabIndia, Mumbai, India) at 100 rpm speed. Drug release from the microspheres was studied in gastric acidic fluid (1.2 pH) for the initial 2 h followed by intestinal alkaline (7.4 pH) fluid until the attainment of equilibrium. At regular intervals of time, aliquot samples were withdrawn from the dissolution baskets and analyzed for drugs using UV–Vis spectrophotometer (Secomam, Anthelie, France) at the fixed λ max of 219 and 198 nm for ISX and CD, respectively. The utilized solvent was replenished each time by adding 5 mL of fresh solvent. Triplicate data were collected, but curves were drawn through the average points, maintaining the standard deviations within ±3 % for all formulations.
Results and discussion
Fourier transform infrared spectra
FTIR of (a) placebo CS/PU microspheres, (b) ISX-loaded CS/PU microspheres, (c) pristine ISX, (d) CD-loaded CS/PU microspheres and (e) pristine CD are displayed in Fig. 2. FTIR of placebo CS/PU matrix has shown a characteristic –NH stretching peak of CS and urethane appeared at 3,443 cm−1. The peaks observed at 2,925 and 2,856 cm−1 are due to aliphatic –CH stretching vibrations of both CS and PU. A band at 1,640 cm−1 is due to the presence of imine group formed from the reaction between hydroxyl groups of GA with –NH2 groups of CS, thus confirming the cross-linking of CS by GA.
FTIR of the pristine ISX showed characteristic –OH and –NH bands, respectively, at 3,380 and 3,326 cm−1. Two peaks observed at 2,968 and 3,136 cm−1 are due to aliphatic and aromatic –CH stretching vibrations, while –CH3 bending vibrations are observed at 1,397 cm−1. A band appearing at 1,096 cm−1 indicates the –CO stretching vibration. FTIR spectra of ISX-loaded CS/PU microspheres showed characteristic peaks of ISX in addition to peaks for placebo CS/PU matrix, confirming the absence of chemical interactions between the blend polymers and the drug.
In case of pristine CD, a characteristic –OH peak is observed at 3,431 cm−1. The –CH aromatic stretching vibrations are observed at 3,155 and 822 cm−1, while –SO and –CO stretching bands are observed at 1,361 and 1,023 cm−1, respectively. FTIR spectrum of the CD-loaded CS/PU microspheres does not contain any new peak. The absence of additional peak confirms the absence of any chemical interactions between the polymer matrix and the drug thus, suggesting the chemical stability of drugs in the blend matrix, which can also be seen in the in vitro release profiles.
Differential scanning calorimetric study
One of the most commonly used methods for estimating polymer–polymer compatibility is to determine the glass-transition temperature (T g) of the blend system and to compare it with the T g of the component polymers. Presence of a single T g in a blend can be used to assess the complete miscibility of polymers. In this study, DSC was used to estimate the T g to investigate the miscibility of CS and PU blends. Figure 3 displays the DSC thermograms of CS and CS/PU blends, wherein the T g of CS is observed at 87 °C. Luo et al. [46], observed the T g value for CS at 101 °C, which confirms the present data. However, a slight deviation is observed in the T g of CS, which may be due to the difference in the degree of deacetylation. The CS/PU blends of compositions of 60–100 % have shown a systematic trend of T g, but such a trend of T g is absent in the blends of compositions of 20 and 40 %. The systematic trend of T g observed between 60 and 100 % of CS indicates the blend compatibility.
Figure 4 shows the DSC thermograms of (a) placebo microspheres, (b) ISX-loaded CS/PU microspheres, (c) pristine ISX, (d) CD-loaded CS/PU microspheres and (e) pristine CD. In case of placebo microspheres, an endothermic transition is observed around 100 °C due to T g of the polymer matrix, while an exothermic transition is observed around 270 °C due to polymer degradation. For pristine ISX, an endothermic peak is observed at 234 °C, but for pristine CD, an endothermic peak is observed at 354 °C. However, both ISX-loaded and CD-loaded microspheres exhibit identical trends as that of placebo, but no characteristic peaks of the respective drugs are observed, indicating that the drugs are molecularly dispersed in the blend polymer matrix.
X-ray diffraction studies
XRD patterns are used to investigate the crystallinity of drugs in the cross-linked microspheres. X-ray diffractograms of (a) placebo microspheres, (b) ISX-loaded CS/PU microspheres, (c) pristine ISX, (d) CD-loaded CS/PU microspheres and (e) pristine CD are presented in Fig. 5. Diffraction patterns of ISX have shown many peaks in the 2θ region of 9°–25° due to its crystalline nature. The diffraction patterns of CD have many peaks in the 2θ region of 12°–27°, indicating its crystalline nature. However, these peaks have disappeared in ISX- or CD-loaded microspheres, but only the peaks observed in placebo matrix are seen, confirming that drugs are dispersed molecularly in the polymer matrix and crystallinity of drugs is not observed in the drug-loaded microspheres.
Scanning electron microscopic analysis
Typical SEM pictures of placebo, ISX- and CD-loaded microspheres of 90:10 of CS:PU blend matrix taken at 1,000×, 500× and 500× magnifications displayed in Fig. 6a–c, respectively, suggest the spherical nature. However, smooth surfaces are observed with agglomerations of particles. Particularly, in case of ISX-loaded particles (Fig. 6b) as well as to some extent in CD-loaded particles (Fig. 6c), we can observe a slight peeling of the outer surface of the microspheres due to high cross-link density at lower PU composition of the blend matrix. However, this effect is not prevalent in case of placebo microspheres.
Encapsulation efficiency
The % EE of formulations varied from 35 to 62 and shows a decreasing trend with increasing composition of PU in the blend microspheres. To study the effect of PU composition on EE, microspheres were prepared with 10, 20 and 30 % w/w of PU (formulations F1, F2, and F3) that offered systematically decreasing trends of EE values of 51, 48, and 35 %, respectively. This may be due to increase in the composition of PU, since the viscosity of blend microspheres also decreases and size of the microspheres is reduced. Therefore, free volume in the blend matrix would decrease giving low values of % EE. However, with increasing PU content of the blend matrix, cross-link density decreases, making the delivery matrix less rigid, thus increasing the leaching of drug particles from the polymer matrix, thereby giving the low values of EE.
The % EE values and in vitro release trends of both the drugs not only depend on process parameters, but also on the nature of drugs (solubility, molecular weight, chemical structure, etc.). Calcium dobesilate is more water soluble than isoxsuprine and hence, a decrease in % EE values in case of calcium dobesilate may be due to its leaching effect from the matrix during the formulation step. Overall, it is evident that EE values are affected greatly by the process variables as reported before [41].
In vitro drug release
Drug release from the blend matrix generally follows several types of mechanisms, viz., (a) release from the surface of microspheres, (b) diffusion through the swollen rubbery blend matrix and (c) release due to polymer erosion. In case of release from the surface, adsorbed drug particles will instantaneously dissolve in the presence of the release medium. Thus, the drug encapsulated in the surface layer of the microspheres follows surface erosion mechanism, leading to initial burst effect. To understand the drug release kinetics from the microspheres in stomach as well as in the intestinal conditions, the in vitro release experiments were performed in gastric media (1.2 pH) for the initial 2 h followed by alkaline (7.4 pH) media.
Figure 7 displays the effect of blend ratio on the cumulative release of ISX at 37 °C. The initial burst release is observed in all the formulations by extending the slow release up to 10 h. Notice that plain CS (0 w/w % of PU) and formulation F1 (10 w/w % of PU) have shown a maximum of 35 % drug release within 1 h, while F2 (20 w/w % of PU) has shown about 20 % release. Formulation F3 (30 w/w % of PU) exhibited a minimum initial drug release of 10 % in 1 h. Thus, lower concentration of PU in the blend matrix seems to be the optimum condition for the release of less water-soluble drug such as ISX. Hence, we felt no need to study the effect of the blend composition on CD. The observed initial rapid release may be accounted for direct exposure of the polymer matrix to the dissolution media, facilitating a quick release of the drug from the surface of the microspheres. Such an observed initial burst release would be helpful in achieving the therapeutic plasma concentration of the drug in a short time and thereafter releasing it at a constant rate for a longer time [47–49]. From Fig. 7, it is observed that, in case of pristine CS, the release of ISX is faster than in the blend microspheres and formulation F1 shows a faster release rate than either F2 or F3. This could be because of the fact that at higher composition of chitosan, hydrophilicity of the matrix would increase, resulting in the formation of pores on the surface of the microspheres, which accelerates the drug release.
Release kinetics from the microspheres also depends on the molecular weight of drugs displayed in Fig. 8. The formulation encapsulated with a lower molecular weight (MW = 301) ISX was released much faster than the formulation encapsulated with higher molecular weight (MW = 418) CD, i.e., formulation F1 released faster than F4. However, no formulation has released 100 % of drug which may be due to the presence of weak interaction (hydrogen bonding) between the drugs and the polymer matrix, since both the drugs contain hydroxyl groups (see Fig. 1) and polymers contain imide and urethane groups. It may be noted that variations in blend compositions was studied only in case of ISX, which showed optimum release at 10 % of PU in the blend. To reduce the number of in vitro release experiments, the optimized blend composition (10 % PU) was considered in the case of CD to study its in vitro release pattern.
To investigate the nature of release mechanism through the matrices of this study, the initial linear portion (<50 %) of the cumulative release vs. time curves was analyzed using an empirical equation [50, 51] by the method of least-squares analysis:
Here, M t /M ∞ represents the fractional drug release at time, t; k is a kinetic parameter characterizing the drug–polymer system and n is an empirical parameter characterizing the release mechanism. Using the regression analysis, values of n and k were obtained for all the formulations at 95 % confidence limit; these data along with the estimated correlation coefficients (r) are also included in Table 1. If the value of n = 0.43, the drug diffuses and releases out of the polymer matrix following the Fickian diffusion; if n > 0.43, anomalous or non-Fickian transport occurs, while for n = 0.85, non-Fickian or more commonly called case II transport is operative. If the values of n vary between 0.43 and 0.83, the transport follows anomalous type trend [51]. In this work, n values for all the microspheres range from 0.14 to 0.54, indicating that the dug release follows Fickian to non-Fickian transport.
Conclusions
This work reports on the successful preparation of blend microspheres of CS and PU by the water-in-oil emulsion cross-linking method using GA as a cross-linker. Two widely different types of water-soluble cardiovascular drugs in terms of plasma half-life and chemical structures, viz., ISX and CD have been loaded into the blend matrix to investigate their controlled release characteristics in acidic and alkaline pH conditions to suggest these formulations as oral dosage forms. The blend released nearly 60 % of the drug in about 10 h. The effects of chemical structure and molecular weight of the drugs as well as their slight variations in solubility have been investigated in relation to the dissociation media of different pH values at 37 °C. The drug release followed the Fickian to non-Fickian transport mechanism.
References
Liu Y, Messmer MC (2003) Structures and segregation of polystyrene/poly(methyl methacrylate) blends studied by sum-frequency (SF) spectroscopy. J Phys Chem B 107:9774
Zhang X, Kale DM, Jenekhe SA (2002) Electroluminescence of multicomponent conjugated polymers. 2. Photophysics and enhancement of electroluminescence from blends of polyquinolines. Macromolecules 35:382
Radhakrishnan J, Tanigaki N, Kaito A (1999) Electronic energy transfer in compatible blends of poly(di-n-hexylsilane) and poly(methyl-n-propylsilane). Polymer 40:1381
Kurkuri MD, Nayak JN, Aralaguppi MI, Naidu BVK, Aminabhavi TM (2005) Sorption/diffusion of aqueous mixtures of 1,4-dioxane/tetrahydrofuran through blend membranes of poly(vinyl alcohol) and sodium alginate: their compatibility and pervaporation separation studies. J Appl Polym Sci 98:178
Siepmann F, Siepmann J, Walther M, MacRae RJ, Bodmeier R (2008) Polymer blends for controlled release coatings. J Control Release 125:1
Karavas E, Georgarakis E, Bikiaris D (2006) Application of PVP/HPMC miscible blends with enhanced mucoadhesive properties for adjusting drug release in predictable pulsatile chronotherapeutics. Eur J Pharm Biopharm 64:115
Kerres J, Ullrich A, Meier F, Häring T (1999) Synthesis and characterization of novel acid–base polymer blends for application in membrane fuel cells. Solid State Ionics 125:243
Manea C, Mulder M (2002) Characterization of polymer blends of polyethersulfone/sulfonated polysulfone and polyethersulfone/sulfonated polyetheretherketone for direct methanol fuel cell applications. J Membr Sci 206:443
Kalyani S, Smitha B, Sridhar S, Krishnaiah A (2006) Blend membranes of sodium alginate and hydroxyethylcellulose for pervaporation-based enrichment of t-butyl alcohol. Carbohydr Polym 64:425
Bergera J, Reista M, Mayera JM, Feltb O, Peppasc NA, Gurny R (2004) Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. J Pharm Biopharm 57:19
Aminabhavi TM, Rudzinski WE (2010) Chitosan as a carrier for targeted delivery of small interfering RNA. Int J Pharm 399:1
Al-Qadi S, Grenha A, Carrión-Recio D, Seijo B, Remuñán-López C (2012) Microencapsulated chitosan nanoparticles for pulmonary protein delivery: in vivo evaluation of insulin-loaded formulations. J Control Release 157:383
Park JH, Saravanakumar G, Kim K, Kwon IC (2010) Targeted delivery of low molecular drugs using chitosan and its derivatives. Adv Drug Del Rev 62:28
Kim TH, Jiang HL, Jere D, Park IK, Cho MH, Nah JW, Choi YJ, Akaike T, Cho CS (2007) Chemical modification of chitosan as a gene carrier in vitro and in vivo. Progr Polym Sci 32:726
Prabaharan M (2008) Chitosan derivatives as promising materials for controlled drug delivery. J Biomater Appl 23:5
Kidane AG, Burriesci G, Edirisinghe M, Ghanbari H, Bonhoeffer P, Seifalian AM (2009) A novel nanocomposite polymer for development of synthetic heart valve leaflets. Acta Biomater 5:2409
Xue L, Greisler HPJ (2003) Biomaterials in the development and future of vascular grafts. Vasc Surg 37:472
Rosenheck S, Sharon Z, Leibowitz D (2000) Artifacts recorded through failing bipolar polyurethane insulated permanent pacing leads. Europace 2:60
Lawrence EL, Turner IG (2005) Materials for urinary catheters: a review of their history and development in the UK. Med Eng Phys 27:443
Yang MJ, Den XY, Zhang Z, Julien M, Pelletier F, Desaulniers D, Cossette R, Teijeira FJ, Laroche G, Guidoin R (1997) Are intraaortic balloons suitable for reuse? A survey study of 112 used intraaortic balloons. Artif Organs 21:121
Ghosh B, Urban MW (2009) Self-repairing oxetane-substituted chitosan polyurethane networks. Science 323:1458
Gisselfalt K, Edberg B, Flodin P (2002) Synthesis and properties of degradable poly(urethane urea)s to be used for ligament reconstructions. Biomacromolecules 3:951
Zhang CH, Wen XJ, Vyavahare NR, Boland T (2008) Synthesis and characterization of biodegradable elastomeric polyurethane scaffolds fabricated by the inkjet technique. Biomaterials 29:3781
Adhikari R, Gunatillake PA, Griffiths I, Tatai L, Wickramaratna M, Houshyar S, Moore T, Mayadunne RTM, Field J, Mcgee M, Carbone T (2008) Biodegradable injectable polyurethanes: synthesis and evaluation for orthopaedic applications. Biomaterials 29:3762
Kang WM, Cheng BW, Li QX, Zuo FF (2010) Novel antibacterial nanofibers of chitosan and polyurethane prepared by electrospinning. Adv Mat Res 150–151:1452
Lijuan Z, Lunquan Y, Mingming D, Jiehua L, Hong T, Zhigao W, Qiang F (2011) Synthesis and characterization of pH-sensitive biodegradable polyurethane for potential drug delivery applications. Macromolecules 44:857
Okada M (2002) Chemical syntheses of biodegradable polymers. Prog Polym Sci 27:87
Zhang JN, Wu MY, Yang JJ, Wu QY, Jin ZL (2009) Anionic poly (lactic acid)-polyurethane micelles as potential biodegradable drug delivery carriers. Colloids Surf A 337:200
Shelke NB, Sairam M, Halligudi SB, Aminabhavi TM (2007) Development of transdermal drug-delivery films with castor-oil-based polyurethanes. J Appl Polym Sci 103:779
Shelke NB, Aminabhavi TM (2007) Synthesis and characterization of methoxypolyethyleneglycol and lauric acid grafted novel polyurethanes for controlled release of nifedipine. J Appl Polym Sci 105:2155
Costa-Júnior ES, Barbosa-Stancioli EF, Mansur AAP, Vasconcelos WL, Mansur HS (2009) Preparation and characterization of chitosan/poly(vinyl alcohol) chemically crosslinked blends for biomedical applications. Carbohydr Polym 76:472
Torre PMDL, Enobakhare, Torrado YG, Torrado S (2003) Release of amoxicillin from polyionic complexes of chitosan and poly(acrylic acid). Study of polymer/polymer and polymer/drug interactions within the network structure. Biomaterials 24:1499
Nafee N, Taetz S, Schneider M, Schaefer UF, Lehr CM (2007) Chitosan-coated PLGA nanoparticles for DNA/RNA delivery: effect of the formulation parameters on complexation and transfection of antisense oligonucleotides. Nanomed Nanotechnol Biol Med 3:173
Alves NM, Mano JF (2008) Chitosan derivatives obtained by chemical modifications for biomedical and environmental applications. Int J Biolog Macromol 43:401
Jayakumar R, Prabaharan M, Nair SV, Tokura S, Tamura H, Selvamurugan N (2010) Novel carboxymethyl derivatives of chitin and chitosan materials and their biomedical applications. Progr Mater Sci 55:675
Kim IY, Seo SJ, Moon HS, Yoo MK, Park IY, Kim BC, Cho CS (2008) Chitosan and its derivatives for tissue engineering applications. Biotechnol Adv 26:1
Kumbar SG, Kulkarni AR, Aminabhavi TM (2002) Crosslinked chitosan microspheres for encapsulation of diclofenac sodium: effect of crosslinking agent. J Microencapsul 19:173
Ramesh Babu V, Hosamani KM, Aminabhavi TM (2008) Preparation and in-vitro release of chlorothiazide novel pH-sensitive chitosan-N,N'-dimethylacrylamide semi-interpenetrating network microspheres. Carbohydr Polym 71:208
Reddy KM, Ramesh Babu V, Krishna Rao KSV, Subha MCS, Chowdoji Rao K, Sairam M, Aminabhavi TM (2008) Temperature sensitive semi-IPN microspheres from sodium alginate and N-isopropylacrylamide for controlled release of 5-fluorouracil. J Appl Polym Sci 107:2820–2829
Rokhade AP, Kulkarni PV, Mallikarjuna NN, Aminabhavi TM (2009) Preparation and characterization of novel semi-interpenetrating polymer network hydrogel microspheres of chitosan and hydroxypropyl cellulose for controlled release of chlorothiazide. J Microencapsul 26:27–36
Sullad AG, Manjeshwar LS, Aminabhavi TM (2010) Polymeric blend microspheres for controlled release of theophylline. J Appl Polym Sci 117:1361–1370
NTP (1999) Toxicology and carcinogenesis studies of glutaraldehyde (CAS No. 111-30-8) in F344/N rats and B6C3F1mice (inhalation studies). Natl Toxicol Progr Tech Rep Ser 490:1
Dhawan S, Singla AK, Sinha VR (2004) Evaluation of mucoadhesive properties of chitosan microspheres prepared by different methods. AAPS Pharm Sci Tech 5:1–7
Kajjari PB, Manjeshwar LS, Aminabhavi TM (2011) Semi-interpenetrating polymer network hydrogel blend microspheres of gelatin and hydroxyethyl cellulose for controlled release of theophylline. Ind Eng Chem Res 50:7833–7840
Sullad AG, Manjeshwar LS, Aminabhavi TM (2010) Controlled release of theophylline from interpenetrating blend microspheres of poly(vinyl alcohol) and methyl cellulose. J Appl Polym Sci 116:1226–1235
Luo K, Yin J, Khutoryanskaya OV, Khutoryanskiy VV (2008) Mucoadhesive and elastic films based on blends of chitosan and hydroxyethylcellulose. Macromol Biosci 8:184–192
He P, Davis SS, Illum L (1999) Chitosan microspheres prepared by spray drying. Int J Pharm 187:53–65
Al-Helw AA, Al-Angary AA, Mahrous GM, Al-Dardari MM (1998) Preparation and evaluation of sustained release cross-linked chitosan microspheres containing phenobarbitone. J Microencapsul 15:373–382
Rokhade AP, Shelke NB, Patil SA, Aminabhavi TM (2007) Novel interpenetrating polymer network microspheres of chitosan and methylcellulose for controlled release of theophylline. Carbohydr Polym 69:678–687
Ritger PL, Peppas NA (1987) A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J Control Release 5:37–42
Sullad AG, Manjeshwar LS, Aminabhavi TM (2010) Novel pH-sensitive hydrogels prepared from the blends of poly(vinyl alcohol with acrylic acid-graft-guar gum matrixes for isoniazid delivery. Ind Eng Chem Res 49:7323–7329
Acknowledgments
Miss. A. G. Sullad and Prof. L. S. Manjeshwar thank the University Grants Commission (UGC), New Delhi, India (KU/SCH/UGC/RFSMS/2008-09). Prof. T. M. Aminabhavi thanks the All India Council for Technical Education [AICTE, F.No. 1-51/RIFD/EF(13)/2011-12], New Delhi, India for the award of Emeritus Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sullad, A.G., Manjeshwar, L.S. & Aminabhavi, T.M. Blend microspheres of chitosan and polyurethane for controlled release of water-soluble antihypertensitive drugs. Polym. Bull. 72, 265–280 (2015). https://doi.org/10.1007/s00289-014-1271-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-014-1271-6