Abstract
Polymerization of propylene was carried out by using MgCl2-supported TiCl4 catalyst in conjunction with triethylaluminium (TEA) as cocatalyst. The effect of polymerization temperature on polymerization of propylene was investigated. The catalyst activity was influenced by the polymerization temperature significantly and the maximum activity of the catalyst was obtained at 40 °C. With increasing the polymerization temperature, the molecular weight of polypropylene (PP) drastically decreased, while the polydispersity index (PDI) increased. The effect of the two-stepwise polymerization procedure on the molecular weight and molecular weight distribution of PP was studied and the broad PDI of PP was obtained. It was also found that the PDI of PP could be controlled for propylene polymerization through regulation of polymerization temperature. Among the whole experimental cases, the M w of PP was controlled from 14.5 × 104 to 75.2 × 104 g/mol and the PDI could be controlled from 4.7 to 10.2.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The world market for polypropylene (PP) was currently over 30 × 106 metric tons/year, and more than 90% world production of PP was produced by Ziegler–Natta catalyst system [1, 2]. Most of the PP produced based on the market needs was manufactured using traditional Ziegler–Natta catalysts which typically comprise TiCl4 supported on MgCl2 through various chemical modifications [1, 2].
In propylene polymerization by MgCl2-supported Ti-based catalysts, hydrogen (H2) was known to be the most efficient industrial regulator of the molecular weight of the propylene polymerization as chain transfer agent [3]. The use of H2 as molecular weight regulator was based on the hydrogenolysis of metal to polymer bonds and the rate of chain transfer to H2 was substantially higher than chain transfer to olefin or alkylaluminium [3, 4].
The properties of PP were influenced by the polymerization condition significantly, such as polymerization time [5], temperature [6], H2 feed ratio [3, 7], catalyst and cocatalyst concentration or their feed ratio [8]. As reported by Kojoh et al. [9], the catalyst activity and molecular weight decreased with the increasing polymerization temperature, while the molecular weight distribution was broadened for polymerization of propylene under MgCl2-supported TiCl4 catalyst system.
Molecular weight and molecular weight distribution were important factors in determining the physical, mechanical, and rheological properties of polymers [10]. The molecular weight controls the mechanical properties of polymers and molecular weight distribution mainly controls the rheological properties [11]. The molecular weight was controlled by H2 [7], temperature [6], and polymerization time [5], etc, while the molecular weight distribution was controlled by the methods of physical blending of the polymers with different average molecular weight [12], cascade reactor process [13], and mixing or hybrid of different catalysts [14]. However, the methods of control molecular weight distribution more or less have some defects such as gelation, high cost and complex manipulation, etc [12]. Therefore, it is necessary and interesting to seek a single polymerization system or condition to control the molecular weight and molecular weight distribution of PP.
Therefore, the aim of the present work was to study the effect of polymerization temperature on catalyst activity, isospecificity, molecular weight and molecular weight distribution of PP by using MgCl2-supported Ti-based catalyst system. The two-stepwise polymerization procedure was used to control the molecular weight distribution of PP.
Experimental
Materials
The MgCl2-supported Ti-based catalyst (internal donor: DiBP) and external donor (cyclohexyldimethoxymethylsilane (CHDMMS)) were provided by Samsung Total Petrochemicals Co., Ltd., Korea. Polymerization grade propylene and triethylaluminium (TEA, 1.0 M solution in n-hexane) were provided by Korea Petrochemical Ind. Co., Ltd., Korea. n-Hexane was distilled from sodium/benzophenone under nitrogen prior to use.
Polymerization procedure
The polymerization was carried out in a 300 mL glass reactor equipped with a magnetic stirring bar. The reactor was back-filled three times with nitrogen and charged with the required amount of n-hexane. At the stipulated temperature of 40 °C the reaction solution was vigorously stirred under 1 atm of propylene for the desired period of time after which the cocatalyst (TEA) was added to the reactor. After cocatalyst was added, the external donor and catalyst (suspension in n-hexane) were injected, respectively, and then the polymerization started with a continuous feed of propylene and H2 (V H:V P = 0.05). The pressure was kept constant throughout the polymerization through use of a bubbler and the reaction temperature was controlled by using a water bath. With regards to the two-stepwise polymerization temperature system, the polymerization was started at a lower temperature for 1 h, and then the polymerization temperature was changed to a higher temperature for 1 h polymerization. After a desired time, the polymerization was terminated by adding 10% HCl–methanol solution, and then the mixture was poured into 500 mL of methanol to precipitate the polymer and followed by drying under vacuum at 60 °C to its constant weight. Based on the weight of the obtained polymer, the catalyst activity (kg-polymer/mol-Ti h) was calculated.
Characterization
The polymer product was fractionated by extraction with boiling n-heptane for 8 h to determine the isotactic index (I.I.) and the I.I. values reported for each samples were the weight percentage of n-heptane insoluble polymer.
The molecular weight and PDI of n-heptane insoluble PP fractions were determined by gel permeation chromatography (GPC) measurement with 1,2,4-trichlorobenzene as solvent at 135 °C using a PL-220 (Polymer Laboratories) equipped with a refractive-index detector.
Calculation of theoretical PDI
Two hypothetical polymers (A and B polymer) with different molecular weights were used to describe the calculation process. The calculation of theoretical PDI was described as following: (1) to unify the total amount of polymer fraction in GPC curves (Fig. 1a, AreaA = AreaB); (2) to decide the related amount of A and B fraction to the combined GPC curve, e.g. the yield of A is 2 g and B is 1 g, when the polymerization system is the mixture of A and B at 1:1 feed ratio, the obtained polymer will contain 1 g A polymer and 0.5 g B polymer in theory; (3) to combine the curve of A and B according to the following process:
where y A and y B are y-axis of GPC curves obtained from A and B polymer (Fig. 1a). A A and A B are related amount of A and B polymer in the final polymer. The y Combined is the y-axis of the theoretical GPC curve. The combined GPC curve was shown in Fig. 1b. The theoretical PDI was calculated from the combined GPC curve [15].
Results and discussion
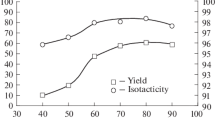
The polymerization of propylene was carried out by using MgCl2-supported TiCl4 catalyst in conjunction with TEA as cocatalyst. The polymerization behavior was examined for various polymerization temperature, 10 °C, 25 °C, 40 °C, 55 °C, and two-stepwise polymerization temperature. H2 was usually used in the industrial polymerization process to regulate the molecular weight of the polymer and external donor was used to improve the isospecificity of obtained PP. Therefore, the polymerization was carried out in the presence of H2 and alkoxysilane compound. The effect of polymerization temperature on experimental results was given in Table 1.
With regards to the polymerization among the experimental cases, the maximum value of catalyst activity was obtained at 40 °C. The catalyst activity increased with increasing polymerization temperature from 10 °C to 40 °C. However, the catalyst activity slightly decreased at polymerization temperature higher than 40 °C. The decrease of catalyst activity may be ascribed to the low solubility of propylene monomer at higher polymerization temperature [16]. In addition, the theoretical catalyst activity of two-stepwise polymerization system was calculated according to the following equation and the results were summarized in Table 1.
where A1 and A2 are the activity of first and second step polymerization, respectively. The difference between calculated and experimental results more or less observed. The yield of PP did not increase with increasing polymerization temperature linearly, because the heating time of a desired temperature was different after the first hour polymerization. With regards to the two-stepwise polymerization temperature systems, the catalyst activity was in the range from 2.0 to 3.6 kg-polymer/g-Ti h.
As can be seen from Table 1, the I.I. value of obtained PP roughly decreased with the increase of polymerization temperature. It was well known that the internal donor could be extracted by the alkylaluminium cocatalyst from the solid catalyst during polymerization and the effectiveness of the extraction process increased with increasing polymerization temperature [17–19]. Thus, it was understandable that the I.I. value decreased with the increase of polymerization temperature. The I.I. value of higher than 95 wt% was obtained in the whole range of this study.
The molecular weight distribution curves of the n-heptane insoluble fractions of the samples obtained at different polymerization temperature with MgCl2-supported TiCl4 catalysts were shown in Fig. 2.
The molecular weight of obtained PP decreased with the increase of polymerization temperature, while the PDI value increased slightly. The decreased molecular weight could correspond to the strong chain transfer reaction at higher polymerization temperature [6]. In addition, the decreased molecular weight and increased PDI were reported by Kojoh et al. [9] also. However, the opposite results were observed by Chadwick et al. [20] where the molecular weight increased with the increasing polymerization temperature for propylene polymerization by using MgCl2-supported TiCl4 catalyst (internal donor: DiBP) in presence of external donor. With regards to the one-stepwise polymerization temperature system, the weight-average molecular weight (M w) of obtained PP was in the range from 14.5 × 104 to 75.2 × 104 g/mol and the PDI value was in the range from 4.7 to 5.6 for one-stepwise polymerization temperature system.
As described before, the PDI slightly increased with the increasing polymerization temperature. In order to produce broad molecular weight distribution of PP, a two-stepwise polymerization temperature was introduced, while the higher molecular weight fraction was obtained at lower polymerization temperature and the lower molecular weight fraction was obtained at higher polymerization temperature. Additionally, with regards to the two-stepwise polymerization temperature systems, the theoretical PDI values were calculated from the combined molecular weight distribution curve of PP obtained by the two different temperatures. The calculation was based on ideal conditions (polymerization yield linear increased with polymerization time and without delay during the temperature change). The ratio of combined molecular weight distribution curve was determined by the ratio of polymer yield of the two different polymerization temperatures respectively. The experimental and theoretical GPC curves were given in Fig. 3.
In the whole range of this study the unimodal molecular weight distribution curves were obtained even at the broad temperature change (10 → 55 °C). Among the experimental cases, the experimental molecular weight distribution curve slightly shifted to the higher molecular weight part related to the theoretical curve, although the shift was not so clear for the small change in polymerization temperature, e.g. Fig. 3a and d. It was found that the experimental molecular weight was higher than the theoretical one. The reason could correspond to the PP yield not linear increased with increasing polymerization temperature, and more PP product was obtained at lower temperature than the theoretical one [21]. As can be seen in Table 1, the PDI value of PP obtained by two-stepwise polymerization temperature system was higher than the PP obtained by polymerization at the single polymerization temperature. We suggest that the obtained PP of broad molecular weight distribution including two parts; (1) high molecular weight fraction and (2) low molecular weight fraction. The high molecular weight fraction was obtained at lower polymerization temperature (lower chain transfer reaction), while the strong chain transfer reaction occurred with the increasing polymerization temperature lead to decrease the molecular weight of obtained PP. Thus, the broad molecular weight distribution PP was obtained by a single catalyst and reactor. The highest PDI (PDI = 10.2) of PP was obtained at the broad temperature change (10 → 55 °C). In comparison with traditional physical blending method, the two-stepwise polymerization procedure could save more cost. With regard to Ziegler–Natta and metallocene hybrid catalyst, they suffer from many drawbacks. In particular, alkylaluminium cocatalyst, which is used to activate Zeigler-Natta catalyst, has a serious detrimental effect on metallocene catalysts. In this research, it could be found that the PDI value of obtained PP could be controlled through a single reactor and single catalyst system. Among the two-stepwise polymerization temperature systems, the PDI value was in the range from 5.7 to 10.2 by regulating the polymerization temperature.
Conclusions
The catalyst activity in the propylene polymerization was influenced by the polymerization temperature significantly. The maximum activity of the catalyst was obtained at 40 °C. The catalyst activity increased with increasing polymerization temperature from 10 °C to 40 °C. The polymerization temperature higher than 40 °C resulted in lower catalyst activity. With increasing the polymerization temperature, the molecular weight of PP drastically decreased, while the T m and X c, as well as PDI increased. In addition, the PDI of PP could be broadened by introduction of two-stepwise polymerization temperature system. Among the whole experimental cases, the unimodal molecular weight distribution curves were obtained. It showed that the molecular weight and molecular weight distribution of PP could be controlled and predicted by changing the polymerization temperature. By regulating the polymerization temperature, the M w of PP was in the range from 14.5 × 104 to 75.2 × 104 g/mol and the PDI value was in the range from 4.7 to 10.2.
References
Kissin YV (2008) In: Alkene polymerization reactions with transition metal catalysts, chap 1. Elsevier, Amsterdam, pp 1–34
Kashiwa N (2004) The discovery and progress of MgCl2-supported TiCl4 catalysts. J Polym Sci Polym Chem 42:1–8
Lee DH, Jeong YT (1993) Propene polymerization with Mg(OEt)2-supported TiCl4 catalyst—4 Effects of hydrogen. Eur Polym J 29:883–888
Kissin YV, Rishina LA, Vizen EI (2002) Hydrogen effects in propylene polymerization reactions with titanium-based Ziegler–Natta catalyst. II. Mechanism of the chain transfer reaction. J Polym Sci A 40:1899–1911
Abedi S, Hosseinzadeh M, Kazemzadeh MA, Daftari-Besheli M (2006) Effect of Polymerization time on the molecular weight and molecular weight distribution of polypropylene. J Appl Polym Sci 100:368–371
Kojoh SI, Kioka M, Kashiwa N, Itoh M, Mizuno A (1995) A study of chain-end structures of polypropylene prepared with MgCl2-supported titanium catalyst. Polymer 36:5015–5018
Soga K, Shiono T (1982) Effect of hydrogen on the molecular weight of polypropylene with Ziegler–Natta catalysts. Polym Bull 8:261–268
Keii T, Doi Y, Suzuki E, Tamura M, Murata M, Soga K (1984) Propene polymerization with a magnesium chloride-supported Ziegler catalyst, 2 Molecular weight distribution. Makromol Chem 185:1537–1557
Kojoh SI, Kioka M, Kashiwa N (1999) The influences of cocatalyst on propylene polymerization at high temperature with a MgCl2-supported TiCl4 catalyst system. Eur Polym J 35:751–755
Ahn TO, Hong SC, Kim JH, Lee DH (1998) Control of molecular weight distribution in propylene polymerization with Ziegler–Natta/metallocene catalyst mixtures. J Appl Polym Sci 67:2213–2222
Zucchini U, Checchin G (1983) Control of molecular-weight distribution in polyolefins synthesized with Ziegler–Natta catalytic systems. Adv Polym Sci 51:101–153
Heidemeyer P, Pfeiffer J (2002) Special requirements on compounding technology for bimodal polyolefins and their industrial application. Macromol Symp 181:167–176
Alt FP, Bohm LL, Enderle HF, Berthold J (2001) Bimodal polyethylene-interplay of catalyst and process. Macromol Symp 163:135–144
Lopez-Linares F, Barrios AD, Ortega H, Matos JO, Joskowicz P, Agrifoglio G (2000) Control of molecular weight distribution for polyethylene catalyzed over Ziegler–Natta/metallocene hybrid and mixed catalysts. J Mol Catal A 159:203–213
Zhang HX, Lee YJ, Park JR, Lee DH, Yoon KB (2010) Control of molecular weight distribution for polypropylene obtained by a commercial Ziegler–Natta catalyst: effect of a cocatalyst and hydrogen. J Appl Polym Sci 120:101–108
Li J, Tekie Z, Mizan TI, Morsi BI, Maier EE, Singh CPP (1996) Gas-liquid mass transfer in a slurry reactor operating under olefinic polymerization process conditions. Chem Eng Sci 51:549–559
Sacchi MC, Forlini F, Tritto I, Locatelli P, Morini G, Noristi L, Albizzati E (1996) Polymerization stereochemistry with Ziegler–Natta catalysts containing dialkylpropane diethers: a tool for understanding internal/external donor relationships. Macromolecules 29:3341–3345
Matsuoka H, Liu B, Nakaani H, Nishiyama I, Terano M (2002) Active sites deterioration of MgCl2-supported catalyst induced by the electron donor extraction by alkylaluminium. Polym Int 51:781–784
Yang CB, Hsu CC (1995) Propene polymerization with MgCl2-supported TiCl4/dioctylphthalate catalyst. II. Effect of polymerization conditions on the microstructure of isotactic polymer. J Appl Polym Sci 58:1237–1243
Chadwick JC, Morini G, Balbontin G, Sudmeijer O (1998) Effect of polymerization temperature on the microtacticity of isotactic poly(propylene) prepared using heterogeneous (MgCl2-supported) Ziegler–Natta catalysts. Macromol Chem Phys 199:1873–1878
Kissin YV (2003) Multicenter nature of titanium-based Ziegler–Natta catalysts: comparison of ethylene and propylene polymerization reactions. J Polym Sci A 41:1745–1758
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Hx., Lee, Yj., Park, Jr. et al. Control of molecular weight distribution for polypropylene obtained by commercial Ziegler–Natta catalyst: effect of temperature. Polym. Bull. 67, 1519–1527 (2011). https://doi.org/10.1007/s00289-011-0472-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-011-0472-5