Abstract
Antagonistic activity of strains from Bacillus species has made them among the preferred agricultural biological control agents against phytopathogenic fungi. These microorganisms’ success is mostly based on the production of antagonistic secondary metabolites, mainly those of the non-ribosomal cyclic lipopeptides (CLPs) nature, which can affect phytopathogens directly (iturins and fengycins) or indirectly (surfactins and fengycins). However, abiotic factors in the target site can influence the behavior of the biocontrol traits, but to date, few studies attempting to decipher this kind of interaction have been conducted. This work aimed to evaluate the effect of temperature and culture medium on growth, antagonistic activity against Fusarium oxysporum f. sp. physali (Foph), and the profile of CLPs produced by Bacillus velezensis Bs006. The data showed that measured traits in Bs006 varied with temperature and medium interaction. The concentration of CLPs, as well as the antagonistic activity against Foph, was increased as the nutritional wealth, temperature, and time of incubation increased. The concentration of fengycins and iturins was higher than surfactins at high temperatures. However, a bacteriostatic effect was detected with a combination of Landy medium and 15 °C, which prevented both the biosynthesis of CLPs and the antagonistic activity. The results of this work highlight the importance of abiotic conditions of the target site where a biocontrol agent will be applied to stay active and develop its full antagonistic potential. This response by Bs006 could partly explain the variability of its biocontrol efficacy in the Foph-golden berry pathosystem.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The modern push for alternatives to chemical control of plant disease comes from two primary sources, the need for sustainable agroecosystems and the desire for food free of chemical residues. Plant growth-promoting rhizobacteria (PGPR) are an important cluster of beneficial, root-colonizing bacteria thriving in the plant rhizosphere and bulk soil [1]. Several commercial bioproducts have been developed from rhizobacteria, many from the genus Bacillus [2]. This genus forms endospores, which are resistant to chemical exposure, radiation, desiccation, and nutritional deficit [3].
Bacillus velezensis (formerly Bacillus amyloliquefaciens subsp. plantarum) is an ideal example of this genus, as it utilizes three forms of antagonistic activity against phytopathogens. Firstly, this bacterium produces a number of non-ribosomal cyclic lipopeptides (CLPs) and polyketides (PKs), with a number of effects [4]. Secondly, this bacterium aids in the induction of systemic resistance (ISR) in the host plant [5, 6]. Finally, this bacterium produces siderophores, which give it an advantage when competing for resources [7].
Environmental factors such as temperature, pH, and available nutrients influence the physiology and growth of beneficial microorganisms introduced to the soil, which can limit root colonization and the expression of microbial suppression traits [8]. Particularly, the biosynthesis of antagonistic secondary metabolites by rhizobacteria is related to the general metabolic state of the cell [9], which in turn depends on these factors [8]. Although these interactions have not been widely studied, it is known that biosynthesis of CLPs and their isoforms vary with the strain of Bacillus, the growing conditions, and the components of the culture media [10,11,12]. In turn, differential production of homologous compounds and the isoforms impact both the physicochemical features of the CLPs and their interactions with target microorganisms [13]. Carbon and nitrogen sources can affect both type and yield of CLPs, and therefore the antifungal activity by Bacillus spp. [13, 14]. The presence of amino acids as nitrogen sources in the culture media can also influence the biosynthesis of surfactin by Bacillus [15]. Considering that to be successful, the beneficial bacterium needs to adapt to the range of environmental conditions of the target site to express its modes of action, and the active ingredients of eco-friendly biopesticides could be based on living cells and secondary metabolites of beneficial Bacillus and substrates that stimulate both the growth of the bacterium and the production of antagonistic compounds [16, 17].
The rhizobacterium B. velezensis Bs006 has shown high biocontrol potential against Fusarium oxysporum f. sp. physali (Foph), the causal agent of vascular wilt disease (Fusarium wilt ~ FW) in golden berry (Physalis peruviana). However, factors such as the absence of native microflora in autoclaved soil, the relationship between densities of Bs006 and Foph, and high concentrations of Bs006 supernatant were established as some biotic factors that can influence the biocontrol efficacy by the rhizobacterium [18], but no studies have been conducted to determine the influence of abiotic factors.
Since little is known about the impact of abiotic factors on growth and biocontrol activity of Bacillus strains, the effect of temperature and its interaction with the nutritional level of the culture media on the growth of B. velezensis Bs006, cyclic lipopeptides production, and its antagonistic activity against Foph were studied. The knowledge generated in this regard could help explain the variability of biocontrol activity by Bs006 in the Foph-golden berry pathosystem and design strategies for enhancing the efficacy of this biological control agent.
Materials and Methods
Preparation of Microbial Inocula
F. oxysporum f. sp. physali strain Map5 (hereinafter referred to as Foph) was used as the target pathogen, while B. velezensis strain Bs006 was the biocontrol agent. Both are native microorganisms, isolated from golden berry orchards in Colombia [18], and were provided from the microorganisms-germplasm bank collection of AGROSAVIA. The procedures for preservation and production of the inoculum of both microorganisms were previously described by Moreno-Velandia et al. (2019) [18]. Briefly, microconidia of Foph were produced by growing the fungus in sterile PDB medium (Difco®) (50 mL in Erlenmeyer-250 mL) for 7 days (shaking 125 rpm, 30 °C, photoperiod 12L:12D), and then suspension of microconidia was prepared in sterile distilled water (SDW) and adjusted at 1 × 105 microconidia mL−1 by counting in a Neubauer chamber. The inoculum of the rhizobacterium consisted of cell suspension in SDW prepared from a subculture in Luria–Bertani Agar medium (LBA) (30 °C, 24 h). Scraped biomass from solid medium was centrifuged, washed twice with SDW, and then re-suspended in SDW. Cell density of Bs006 was adjusted using an Optizen spectrophotometer (Mecasys Co, Banseok-dong, South Korea) at 600 nm wavelength (OD600 = 0.5 ~ 2.49 × 108 cfu mL−1), according to a previously standardized calibration curve.
Effect of Temperature on Growth of Bs006 and CLPs Production
Fermentations of Bs006 were carried out to measure the impact of incubation temperature on its growth, cyclic lipopeptides produced during 72 h of incubation, and antagonistic activity on Luria–Bertani Broth medium (LB), commonly used in laboratory for Bacillus spp. growth. Landy medium [19] was previously used for production of bacillomycin and mycosubtilin [20, 21] with modifications; and the medium used previously for production of plantazolicin and amylocyclicin [22, 23] which we named here PZN. The content of each culture medium was as follows: LB: tryptone 10 g/L; yeast extract 5 g/L; NaCl 10 g/L; pH 7. Landy: glucose 20 g/L; glutamic acid 5 g/L; MgSO4 0.5 g/L; KCl 0.5 g/L; K2HPO4 1 g/L; yeast extract 1 g/L; Fe2(SO4)3 1.2 mg/L; MnSO4 0.4 mg/L; CuSO4 1.6 mg/L; pH 7. PZN: soy peptone 40 g/L; maltodextrin 40 g/L; KH2PO4 1.8 g/L; K2HPO4 4.5 g/L; MgSO4·7H2O 0.3 g/L; Kelly solution 0.2 mL/L; pH 7. Kelly solution contains EDTA 50 mg/L; ZnSO4·7H2O 1 g/L; MnCl2·4H2O 2.5 g/L; CaCl·2H2O 7.34 g/L; CoCl·6H2O 0.5 g/L; (NH4)2MoO4·4H2O 0.5 g/L; FeSO4·7H2O 5.0 g/L; CuSO4·5H2O 0.2 g/L; pH was adjusted to 6 with NaOH solution.
Bioassay Under Liquid Fermentation
Liquid cultures of Bs006 (30 mL × three Erlenmeyer flasks-125 mL × three culture media) were incubated at 15, 25 , and 30 °C with continuous shaking (125 rpm). Initial concentration of Bs006 in the broth media was adjusted to 5 × 106 cfu mL−1. Bacterial growth was measured by optical density (OD600nm) at 24, 48, and 72 h of incubation. Supernatant samples free of bacterium were taken to quantify cyclic lipopeptides (CLPs) from iturin, fengycin, and surfactin families, through UPLC-ESI-MS following the procedure described previously [24].
Bioassay Under Solid Conditions
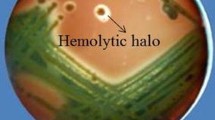
Dual confrontation test between Bs006 and Foph was performed in the solid media LB, PZN, and Landy in Petri dishes-90 mm incubated at 15, 25, and 30 °C under dark conditions. 10 μL of Foph suspension [5 × 105 microconidia mL−1] were dropped in the center of a Petri dish. 24 h later 10 μL of Bs006 cell suspension [1 × 108 cfu mL−1] were inoculated at two opposite points at 1 cm from the edge of the Petri dish. Solid media with Foph or Bs006 inoculum were used as controls. Two diameters of Foph colony growth, toward and away from colonies of the bacterium, were measured at 7 days of incubation. The antagonistic efficacy by Bs006 against Foph was calculated using the equation: E = [(C−T)/C] × 100, where C represents the diameter of Foph growing alone and T represents the diameter of Foph growing in the presence of Bs006 treatment. Moreover, two agar cylinders samples (plugs of 5 mm diameter × 5 mm high) from the inhibition zones were taken to verify the presence of CLPs. Agar samples were immersed in 500 μL of acetonitrile (50%) and formic acid (0.1%) solution overnight. The samples were vortexed vigorously for 2 min and then centrifuged at 12,000 rpm for 10 min. The collected supernatant was filtered by sterile filters (0.22 µm), stored in glass vials, and directly injected to the UPLC-ESI-MS equipment.
Experimental Arrangement and Data Processing
Experimental unit in the bioassay under liquid conditions was an Erlenmeyer flask (125 mL), while in the solid conditions trial, the experimental unit was a Petri dish. Both bioassays were arranged under a completely randomized design with a factorial structure. There were three and five replicates in each bioassay, respectively. The culture medium and the temperature were the experimental factors, three levels each.
Statistical analysis was performed with SAS software, version 9.4. Data were submitted to normality (Shapiro–Wilk α = 0.05) and homoscedasticity (Bartlett α = 0.05) tests followed by two-way ANOVA and post hoc Tukey’s test (α = 0.05).
Results
Growth of Bs006 at 15, 25, and 30 °C in Three Culture Media
Overall, regardless of the media and the incubation time, it was observed that increased temperatures stimulated the growth of Bs006, with the lowest population levels at 15 °C, and higher at 25 and 30 °C (2.8 × 109 cfu mL−1 and 2.4 × 109 cfu mL−1, respectively) at 72 h of incubation. However, a significant effect was detected in the interaction between temperature and medium on the growth of Bs006 (F4, 18 = 101.94, 630.68, and 182.08; P < 0.0001 for 24, 48, and 72 h samples, respectively). For instance, Bs006 did not grow in the combination of Landy medium and 15 °C, while the population of the bacterium was 9 × 108 cfu mL−1 and 1.8 × 109 cfu mL−1 under LB and PZN media, respectively. Moreover, the growth curves of Bs006 showed a similar trend under 30 °C in all three culture media, but not at temperatures of 15 and 25 °C. Thus, the population of Bs006 reached the stationary phase in the lapse of 24–48 h of incubation in LB medium at 15 °C and after this time began to decline. However, the growth of the bacterium was slower on PZN than LB at 15 °C until 48 h and was higher after this time of incubation. The growth curve of Bs006 showed an earlier exponential phase under 25 °C (Fig. 1), and the population was significantly higher (P < 0.05) in LB and PZN media (8.2 × 108 and 1.08 × 109 cfu mL−1, respectively) compared to the population in Landy medium (2.2 × 108 cfu mL−1). Moreover, the lapse of the stationary phase at 25 °C was shorter in LB than PZN and Landy. On the other hand, the growth of Bs006 reached the stationary phase at 24 h of incubation in all three culture media incubated at 30 °C and remained that way until the end of the fermentation (Fig. 1).
Production of CLPs by Bs006 in Liquid Media
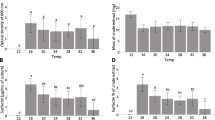
A significant interaction between temperature, culture medium, and time of incubation indicated that the biosynthesis of iturins (F8, 54 = 3.79; P < 0.0014), fengycins (F8, 54 = 5.55; P < 0.0001), and surfactins (F8, 54 = 5.14; P < 0.0001) by Bs006 was influenced by these abiotic conditions. Overall, the production of the three CLPs families was increased by higher temperatures and longer times of incubation in PZN and Landy media. However, the concentration of iturins and fengycins was similar in LB from 24 to 72 h, under all evaluated temperatures, while the concentration of surfactins varied largely with the temperature and time of incubation (Fig. 2).
Concentration of cyclic lipopeptides (surfactins, iturins, and fengycins) detected in liquid cultures of B. velezensis Bs006 on LB, PZN, and Landy media at 15, 25, and 30 °C after 24, 48, and 72 h of incubation. Columns within each CLP family with the same letter are not significantly different according to the Tukey's test (α = 0.05). Data are the mean from three biological replicates and the bars on the columns represent the standard deviation
The concentration of CLPs was reduced in PZN and LB media at 15 °C and was not produced in Landy medium, which can be related to the inactivation of Bs006 growth at this temperature of incubation. It was confirmed that the effect of incubation of Bs006 under Landy and 15 °C combination was bacteriostatic, since at the fifth day after incubation, the experimental units were moved to 25 °C conditions, resulting in evident growth of Bs006 after 24 h of incubation.
The highest concentration of surfactins was found in LB medium on which it was observed a similar concentration (13.1–14.7 µg mL−1) from 24 to 72 h at 25 °C, while the concentration was significantly increased at 15 °C (5.2–13.2 µg mL−1), as time of incubation increased. By contrast, the concentration of surfactins tended to decline from 11.6 to 0.84 µg mL−1 at 30 °C, over time (Fig. 2). However, the concentration of surfactins was the lowest compared to the concentration of iturins and fengycins in all tested media.
The concentration of fengycins was the highest in the three media. This group of CLPs was produced in the highest concentration (300 µg mL−1) in PZN medium, followed by Landy (123.5 µg mL−1) and LB (75.5 µg mL−1). The concentration of fengycins significantly increased in PZN medium as time of incubation and temperature increased, while the concentration was similar in Landy at 25 and 30 °C. The concentration of fengycins was not significantly different among temperatures in LB, but the highest concentration was found at 25 °C (Fig. 2). Regardless of culture media and incubation temperature, Bs006 produced up to 20 times more fengycins and up to five times more iturins than surfactins.
Lastly, biosynthesis of iturins and fengycins by Bs006 followed a similar pattern, although the concentration of iturins was higher than surfactins and lower than fengycins. The highest concentration of iturins was detected in PZN incubated at 25 and 30 °C for 72 h (49.5 and 60.8 µg mL−1, respectively). The concentration of iturins was not significantly different in Landy medium at 72 h of incubation, at 25 and 30 °C, up to 35.9 µg mL−1. Finally, the concentration of iturins in LB was similar under all temperatures and times of incubation (Fig. 2).
Antagonistic Activity of Bs006 Against Foph
It was observed that antagonistic activity of Bs006 against Foph was affected by the culture medium as well as the temperature of incubation (Fig. 3). Consistent with the results described above, the temperature vs medium interaction was significant (F4, 18 = 13.60; P < 0.0001). Accordingly, Bs006 did not grow under Landy agar and 15 °C combination, in which the growth of Foph was not reduced (Fig. 4, Supplementary material Figures S1 and S2). Bs006 did grow on LBA at 15 °C and reduced the growth of Foph by 22%. The efficacy of antagonism by Bs006 against Foph was lower under this treatment along with Landy at 25 and 30 °C combinations compared to all other combinations, where the antibiosis zones were more evident. Finally, the antagonistic activity of Bs006 against Foph was from 49 to 55% on PZN medium with no significant differences among temperatures (Fig. 3).
Growth reduction of F. oxysporum f. sp. physali colony by the antagonistic effect of B. velezensis Bs006 under three temperatures of incubation (15, 25, and 30 °C) on three solid media (LB, PZN, and Landy). Columns with the same letter are not significantly different according to the Tukey's test (α = 0.05). Bars on the columns represent standard deviation of data (n = 3)
Production of CLPs in Inhibition Zones
Consistent with previous analysis by this research group, CLPs from Bs006 were detected in the antibiosis zones. Here, it was observed that the concentration of CLPs was similar in the Petri dishes with and without Foph inoculum (Fig. 5), indicating that the pathogen has no effect on the biosynthesis of CLPs by the bacterium. However, the culture medium and the temperature significantly affected the concentration of iturins (F2, 36 = 67.57; P < 0.0001 for medium, and F2, 36 = 16.23; P < 0.0001 for temperature), fengycins (F2, 36 = 72.70; P < 0.0001 for medium, and F2, 36 = 12.55; P < 0.0001 for temperature), and surfactins (F2, 36 = 13.04; P < 0.0001 for medium, and F2, 36 = 3.28; P = 0.0492 for temperature) produced by Bs006. Consistent with the results from liquid media, the highest concentrations of iturins and fengycins were detected on PZN solid medium, while the concentration of CLPs was similar in LB and Landy solid media. Regarding the nature of the CLPs, in contrast with liquid media, in all solid media the concentration of iturins was the highest, followed by fengycins and lastly surfactins (Fig. 5).
Concentration of cyclic lipopeptides detected on the antibiosis halos between B. velezensis Bs006 and F. oxysporum f. sp. physali strain Map5 incubated at 15, 25, and 30 °C. Columns within each CLP family with the same letter are not significantly different according to the Tukey's test (α = 0.05). Bars on the columns represent the standard deviation of data (n = 3)
It was observed that the concentration of CLPs increased as temperature increased. Thus, concentration of iturins was significantly higher in PZN at 30 °C (37.2 µg mL−1) than 25 °C (29.8 µg mL−1) and 15 °C (22.9 µg mL−1). Concentration of fengycins (8.44–10.1 µg mL−1) was similar under the three tested temperatures on PZN solid medium. The concentration of iturins ranged from 2.42 to 13.49 µg mL−1, while fengycins ranged from 1.92 to 3.74 µg.mL−1 on LB and Landy solid media (Fig. 5). However, no CLPs were produced in Landy incubated at 15 °C concomitantly with the absence of growth of Bs006.
Discussion
Previous unpublished works have shown that application of the rhizobacterium B. velezensis Bs006 to the soil resulted in high variability of golden berry-Fusarium wilt reduction under greenhouse conditions, in which the air temperature ranged between 10 and 42 °C and soil temperature ranged between 15 and 30° [25, 26]. This variability indicated that temperature could play a role in biocontrol activity, which formed the original hypothesis of this research. Thus, we designed the present study to determine the influence of temperature on growth of Bs006, the biosynthesis of CLPs, and the antagonistic activity against Foph. Three different culture media were included to discern the response of those variables under different nutritional levels.
Since the production of antifungal compounds and siderophores are the main strategies of Bacillus spp. to suppress phytopathogens [27], it is suitable to decipher the biotic and abiotic factors that modulate the synthesis of these metabolites [28]. Overall, the temperature is a factor that significantly affects the expression of biocontrol mechanisms [29], and has particularly been reported earlier for strains of Bacillus spp. [14, 30,31,32,33,34,35], and is confirmed herein. It was also confirmed that temperature differentially affects the production of CLPs by B. velezensis. After a thorough review of the literature, a single publication was found, by Pereira et al. (2016) [34], which measured the influence of temperature in interaction with the growth media on lipopeptides production by B. amyloliquefaciens strain 629. Despite the studies being similar, Pereira's work studied the interaction of the strain 629 with bean plants, while the present work addressed the interaction of B. velezensis with the target pathogen F. oxysporum (Foph). Although the current results could be useful to describe the interactions between a given strain of B. velezensis, a particular forma specialis of the pathogen F. oxysporum and abiotic conditions, these complex relationships remain difficult to predict and manage and its utility should be verified under greenhouse and field experiments.
Considering the components of the media used in this study and its quantities, the nutritional level of LB, Landy, and PZN media could be classified as low, intermediate, and high, respectively. LB is widely used for the growth of Bacillus spp. and other bacteria in the laboratory, since it allows rapid growth and high biomass yield [36]. Landy and PZN media were previously used for growth of B. amyloliquefaciens and production of lipopeptides [22, 23, 37, 38]. In this regard, we found the status of low nutritional level (LB) combined with low (15 °C) and moderate (25 °C) temperatures allowed to B. velezensis Bs006 to synthesize surfactins and iturins. In contrast, the combination of high nutritional level (PZN) and low temperature (15 °C) conditions significantly affected the growth rate of Bs006 and the production of surfactins and iturins concurrently; fengycins production was negatively affected by lower temperatures, independently of nutritional level. No growth of Bacillus at 15 °C, even on media with high nutritional levels, has already been observed [33, 34] as it was in this study for Bs006 under Landy medium. This complicates elucidation of the behavior of beneficial Bacillus strains in the rhizosphere, which requires further research to provide deeper insight and new knowledge to understand those interactions.
Our results suggest that under conditions of low nutritional level, temperature is a determining factor of the efficacy of Bs006 against Foph, but under high nutritional level, the bacterium shows high antagonistic activity at low and high temperatures. On the other hand, considering CLPs concentration and Bs006 cells population in culture medium, we observed that CLPs productivity by Bs006 was higher in LB than in the other two media, meaning that same population of Bs006 cells synthesized more CLPs in LB medium than PZN and Landy. Thus, productivity of surfactins was higher in LB at 15 °C, while iturins and fengycins productivity were higher in LB at 25 °C (Supplementary material Fig. S3).
Nihorimbere et al. (2009) [39] and Pertot et al. (2013) [11] demonstrated that B. subtilis/amyloliquefaciens strains favored the production of surfactins under low temperatures, which was also observed by Pereira et al. (2016) [34]. By contrast, we found a rapid growth by Bs006 under 15 °C in LB broth and low concentrations of surfactins during the first 24 h (5 μg mL−1) compared to 25 and 30 °C (15 and 12 μg mL−1, respectively). Even on PZN and Landy media, in which the concentration of carbon sources is higher than LB medium, the production of surfactins was very low at 15 °C. However, different responses between Bacillus strains to environmental conditions can be expected, which is supported by previous works [12, 32, 40, 41].
Nutritional sources and carbon concentration influence the production of lipopeptides by Bacillus spp. Willenbacher et al. (2015) [42] determined an optimal concentration of glucose to obtain a maximum production of surfactins by B. subtilis DSM 10T grown on Cooper medium at 30 °C. Singh et al. (2014) [13] reported that B. amyloliquefaciens AR2 produces a mixture of surfactins, iturins, and fengycins incubated at 30 °C in minimal salts moderately supplemented with dextrose, sucrose, or glycerol. The strain produced only iturins in those supplemented with maltose, lactose, or sorbitol.
Thus, our results suggest that compounds in the root exudates could also affect the biosynthesis of CLPs of Bacillus spp. For instance, surfactins production by B. subtilis BGS3 was higher in the presence of organic acids (citric acid and succinic acid) and amino acids (aspartic acid and glutamic acid) compared to the presence of sugars (glucose, fructose, and maltose) as carbon sources in a minimal medium [39]. Sources of nitrogen in the culture media of the present study are composed primarily of a variety of oligopeptides and free amino acids (tryptone in LB; soy peptone in PZN; glutamic acid in Landy) and carbon sources are also different (yeast extract in LB; maltodextrin in PZN; glucose and yeast extract in Landy). In all three cases Bs006 produced the three families of lipopeptides. However, it is clear that PZN medium provides more availability of nitrogen and carbon due to the high amount of soy peptone and maltodextrin (40 g/L in both cases), which probably favored both the growth of the bacteria and the production of iturins and fengycins in comparison with the other two culture media, where the lower availability of carbon was probably limiting the synthesis of lipopeptides.
Although we recently reported the capacity of Bs006 to colonize the root surface of golden berry and synthesize all three types of CLPs there [24], it is still intriguing to know on the biosynthesis of CLPs in the rhizosphere, in which solid, liquid, and gaseous phases vary throughout the day. However, the determination of CLPs production by this bacterium in the rhizosphere of golden berry in the presence of Foph requires further investigation. Meanwhile, analysis of CLPs on the agar close to B. velezensis Bs006 colonies in Petri dishes free of Foph indicated that the fungus neither stimulated nor suppressed accumulated CLPs for 7 days, suggesting that expression of genes for the biosynthesis of these CLPs is constitutive. However, this trait has been stimulated by the presence of pathogens at early stages of interaction under in vitro conditions [15].
Considering that the rhizosphere is characterized by low nutrient content, Bs006 must compete with the native microflora for space and nutrients, and as such, CLPs production would be expected to be lower than the in vitro conditions. Low lipopeptide production in the rhizosphere might explain the low consistency of biocontrol activity of Bs006 against Foph in golden berry. Additionally, in view of the soil temperature ranges from 10 to 20 °C in the areas where golden berry is grown, our results suggest that the rhizosphere would have low concentration of fengycins, compared to the production of iturins and surfactins. Low levels of fengycins could enable F. oxysporum to colonize the rhizosphere effectively and make it difficult to prevent the infection, since we have observed that this CLP reduces the growth of Foph and damages the cellular integrity [24]. However, the present study will help to design strategies that favor the success of the biological control agent. In this particular case, the application of Bs006 to the soil along with nutrients that promote both its establishment in the rhizosphere and CLPs production could generate better competition against Foph. Alternately, the complementary application of CLPs produced in vitro could help to reduce the pathogenic activity of the initial inoculum of the fungus.
Differences in metabolic activity among Bacillus spp., and even among strains of the same Bacillus species, reported in the literature, suggest that results in this area cannot be extrapolated and each specific interaction has to be studied to yield accurate use directions and to reduce the variability of biological control. As such, it is urgent to standardize methodologies to reliably detect and quantify the CLPs produced by Bacillus isolates in the rhizosphere and to study the persistence and the efficacy of these molecules in the soil in order to design successful strategies for their practical application.
Conclusion
Our experimental approach showed that the interaction between temperature and medium significantly affected the growing phases, cyclic lipopeptides profile production, and the antagonistic activity of B. velezensis Bs006 against F. oxysporum f. sp. physali. The available nutrients on LB medium allowed Bs006 to get a high cell population, but the fast growth limited the production of cyclic lipopeptides, as compared to what happened on PZN and Landy media that allowed high cell and cyclic lipopeptides concentration. Overall, our data showed that a cold environment reduced the production of CLPs and the antagonistic activity of Bs006. Moreover, a combination of low temperature with limited content of nutritional sources accentuates this effect. Since the environment of the rhizosphere is characterized by low nutrient availability and low to moderate temperatures, the results of this work in part might explain the variability of biocontrol activity of Bs006 against golden berry wilt disease caused by Foph. Further studies about plant pathogen Bs006 are necessary to clearly explain the in vivo behavior of this rhizobacterium.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Basu A, Prasad P, Das SN, Kalam S, Sayyed RZ, Reddy MS, El Enshasy H (2021) Plant growth promoting rhizobacteria (PGPR) as green bioinoculants: recent developments, constraints, and prospects. Sustainability 13:1140. https://doi.org/10.3390/su13031140
Lazarovits G, Turnbull A, Johnston-Monje D (2014) Plant health management: biological control of plant pathogens. Encycl Agric Food Syst 4:388–399. https://doi.org/10.1016/b978-0-444-52512-3.00177-7
Emmert EAB, Handelsman J (1999) Biocontrol of plant disease: a gram-positive perspective. FEMS Microbiol Lett 171:1–9. https://doi.org/10.1111/j.1574-6968.1999.tb13405.x
Raaijmakers JM, de Bruijn I, Nybroe O, Ongena M (2010) Natural functions of lipopeptides from Bacillus and Pseudomonas: more than surfactants and antibiotics. FEMS Microbiol Rev 34:1037–1062. https://doi.org/10.1111/j.1574-6976.2010.00221.x
Kloepper JW, Ryu CM, Zhang S (2004) Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology 94:1259–1266. https://doi.org/10.1094/PHYTO.2004.94.11.1259
Cawoy H, Mariutto M, Henry G, Fisher C, Vasilyeva N, Thonart P, Dommes J, Ongena M (2014) Plant defense stimulation by natural isolates of Bacillus depends on efficient surfactin production. Mol Plant Microbe Interact 27:87–100. https://doi.org/10.1094/MPMI-09-13-0262-R
Dunlap CA, Bowman MJ, Schisler DA (2013) Genomic analysis and secondary metabolite production in Bacillus amyloliquefaciens AS 43.3: a biocontrol antagonist of Fusarium head blight. Biol Control 64:166–175. https://doi.org/10.1016/j.biocontrol.2012.11.002
Compant S, Duffy B, Nowak J, Clément C, Barka EA (2005) Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl Environ Microb 71:4951–4959. https://doi.org/10.1128/AEM.71.9.4951-4959.2005
Thomashow LS (1996) Biological control of plant root pathogens. Curr Opin Biotech 7:343–347. https://doi.org/10.1016/S0958-1669(96)80042-5
Akpa E, Jacques P, Wathelet B, Paquot M, Fuchs R, Budzikiewicz H, Thonart P (2001) Influence of culture conditions on lipopeptide production by Bacillus subtilis. Appl Biochem Biotech 91:551–561. https://doi.org/10.1385/ABAB:91-93:1-9:551
Das P, Mukherjee S, Sen R (2009) Substrate dependent production of extracellular biosurfactant by a marine bacterium. Bioresource Technol 100:1015–1019. https://doi.org/10.1016/j.biortech.2008.07.015
Chen M, Wang J, Liu B, Zhu Y, Xiao R, Yang W, Ge C, Chen Z (2020) Biocontrol of tomato bacterial wilt by the new strain Bacillus velezensis FJAT-46737 and its lipopeptides. BMC Microbiol 20:160. https://doi.org/10.1186/s12866-020-01851-2
Singh AK, Rautela R, Cameotra SS (2014) Substrate dependent in vitro antifungal activity of Bacillus sp. strain AR2. Microb Cell Fact 13:67–77. https://doi.org/10.1186/1475-2859-13-67
Yaseen Y, Gancel F, Béchet M, Drider D, Jacques P (2017) Study of the correlation between fengycin promoter expression and its production by Bacillus subtilis under different culture conditions and the impact on surfactin production. Arch Microbiol 199:1371–1382. https://doi.org/10.1007/s00203-017-1406-x
Peypoux F, Michel G (1992) Controlled biosynthesis of Val7-and Leu7-surfactins. Appl Microbiol Biotechnol 16:515–517. https://doi.org/10.1007/BF00170194
Coutte F, Lecouturier D, Dimitrov K, Guez J-S, Delvigne F, Dhulster P, Jacques P (2017) Microbial lipopeptide production and purification bioprocesses, current progress and future challenges. Biotechnol J 12:1600566. https://doi.org/10.1002/biot.201600566
Malviya D, Sahu PK, Singh UB, Paul S, Gupta A, Gupta AR, Singh S, Kumar M, Paul D, Rai JP, Singh HV, Brahmaprakash GP (2020) Lesson from ecotoxicity: revisiting the microbial lipopeptides for the management of emerging diseases for crop protection. Int J Environ Res Public Health 17:1434. https://doi.org/10.3390/ijerph17041434
Moreno-Velandia CA, Izquierdo-García LF, Ongena M, Kloepper JW, Cotes AM (2019) Soil sterilization, pathogen and antagonist concentration affect biological control of Fusarium wilt of cape gooseberry by Bacillus velezensis Bs006. Plant Soil 435:39–55. https://doi.org/10.1007/s11104-018-3866-4
Landy M, Warren GH, Rosenman SB, Colio LG (1948) Bacillomycin, an antibiotic from Bacillus subtilis active against pathogenic fungi. Proc Soc Exp Biol Med 67:539–541
Leclère V, Béchet M, Adam A, Guez J-S, Wathelet B, Ongena M, Thonart P, Gancel F, Chollet-Imbert M, Jacques P (2005) Mycosubtilin overproduction by Bacillus subtilis BBG100 enhances the organism’s antagonistic and biocontrol activities. Appl Environ Microb 71:4577–4584. https://doi.org/10.1128/AEM.71.8.4577-4584.2005
Leclère V, Marti R, Béchet M, Fickers P, Jacques P (2006) The lipopeptides mycosubtilin and surfactin enhance spreading of Bacillus subtilis strains by their surface-active properties. Arch Microbiol 186:475–483. https://doi.org/10.1007/s00203-006-0163-z
Scholz R, Molohon KJ, Nachtigall J, Vater J, Markley AL, Süssmuth RD, Mitchell DA, Borriss R (2011) Plantazolicin, a novel microcin B17/streptolysin S-like natural product from Bacillus amyloliquefaciens FZB42. J Bacteriol 193:215–224. https://doi.org/10.1128/JB.00784-10
Scholz R, Vater J, Budiharjo A, Wang Z, He Y, Dietel C, Schwecke T, Herfort S, Lasch P, Borriss R (2014) Amylocyclicin, a novel circular bacteriocin produced by Bacillus amyloliquefaciens FZB42. J Bacteriol 196:1842–1852. https://doi.org/10.1128/JB.01474-14
Moreno-Velandia CA, Ongena M, Cotes AM (2021) Effects of fengycins and iturins on Fusarium oxysporum f. sp. physali and root colonization by Bacillus velezensis Bs006 protect golden berry against vascular wilt. Phytopathology. https://doi.org/10.1094/PHYTO-01-21-0001-R
Guacaneme A (2010) Biocontrol de Fusarium oxysporum y promoción de crecimiento por rizobacterias en plantas de uchuva (Physalis peruviana) Trabajo de Pregrado. Microbiología. Universidad de los Andes, Bogotá, p 11
Torres EL (2013) Determinación del efecto de Pseudomonas migulae Pf014 y Bacillus amyloliquefaciens Bs006 aplicadas de forma individual y en mezclas sobre la promoción de crecimiento y el control de Fusarium oxysporum en uchuva (Physalis peruviana). Trabajo de Maestría en Biotecnología. Universidad de Córdoba, Montería
Wulff EG, Mguni CM, Mansfeld-Giese K, Fels J, Lübeck M, Hockenhull J (2002) Biochemical and molecular characterization of Bacillus amyloliquefaciens, B. subtilis, and B. pumilus isolates with distinct antagonistic potential against Xanthomonas campestris pv. campestris. Plant Pathol 51:574–584. https://doi.org/10.1046/j.1365-3059.2002.00753.x
Raaijmakers JM, Vlami M, de Souza JT (2002) Antibiotic production by bacterial biocontrol agents. Antonie van Leeuwenhoek 81:537–547. https://doi.org/10.1023/A:1020501420831
Burpee LL (1990) The influence of abiotic factors on biological control of soilborne plant pathogenic fungi. Can J Plant Pathol 12:308–317. https://doi.org/10.1080/07060669009501005
Abushady HM, Bashandy AS, Aziz NH, Ibrahim HMM (2005) Molecular characterization of Bacillus subtilis surfactin producing strain and the factors affecting its production. Int J Agric Biol 7:337–344
Fickers P, Leclère V, Guez J-S, Béchet M, Coucheney F, Joris B, Jacques P (2008) Temperature dependence of mycosubtilin homologue production in Bacillus subtilis ATCC6633. Res Microbiol 159:449–457. https://doi.org/10.1016/j.resmic.2008.05.004
Krebs B, Höding B, Kübart S, Workie MA, Junge H, Schmiedeknecht G, Grosch R, Bochow H, Hevesi M (1998) Use of Bacillus subtilis as biocontrol agent. I. Activities and characterization of Bacillus subtilis strains. J Plant Dis Protect 105:181–197
Landa BB, Navas-Cortés JA, Jiménez-Díaz RM (2004) Influence of temperature on plant–rhizobacteria interactions related to biocontrol potential for suppression of fusarium wilt of chickpea. Plant Pathol 53:341–352. https://doi.org/10.1111/j.0032-0862.2004.01008.x
Pereira F, Vasconcelos F, Ongena M, de Franzil L, Souza P, de Souza J (2016) Effect of temperature, pH and substrate composition on production of lipopeptides by Bacillus amyloliquefaciens 629. Afr J Microbiol Res 10:1506–1512. https://doi.org/10.5897/AJMR2016.8222
Pertot I, Puopolo G, Hosni T, Pedrotti L, Jourdan E, Ongena M (2013) Limited impact of abiotic stress on surfactin production in planta and on disease resistance induced by Bacillus amyloliquefaciens S499 in tomato and bean. FEMS Microbiol Ecol 86:505–519. https://doi.org/10.1111/1574-6941.12177
Sezonov G, Joseleau-Petit D, D’Ari R (2007) Escherichia coli physiology in Luria-Bertani Broth. J Bacteriol 189:8746–8749. https://doi.org/10.1128/JB.01368-07
Argüelles-Arias A, Ongena M, Halimi B, Lara Y, Brans A, Joris B, Fickers P (2009) Bacillus amyloliquefaciens GA1 as a source of potent antibiotics and other secondary metabolites for biocontrol of plant pathogens. Microb Cell Fact 8:63. https://doi.org/10.1186/1475-2859-8-63
Chen XH, Scholz R, Borris M, Junge H, Mögel G, Kunz S, Borriss R (2009) Difficidin and bacilysin produced by plant-associated Bacillus amyloliquefaciens are efficient in controlling fire blight disease. J Biotechnol 140:38–44. https://doi.org/10.1016/j.jbiotec.2008.10.015
Nihorimbere V, Fickers P, Thonart P, Ongena M (2009) Ecological fitness of Bacillus subtilis BGS3 regarding production of the surfactin lipopeptide in the rhizosphere. Environ Microbiol Rep 1:124–130. https://doi.org/10.1111/j.1758-2229.2009.00017.x
Yu GY, Sinclair JB, Hartman GL (2002) Bertagnolli BL: production of iturin A by Bacillus amyloliquefaciens suppressing Rhizoctonia solani. Soil Biol Biochem 34(955):963. https://doi.org/10.1016/S0038-0717(02)00027-5
Chen M, Wang J, Zhu Y, Liu B, Yang W, Ruan C (2019) Antibacterial activity against Ralstonia solanacearum of the lipopeptides secreted from the Bacillus amyloliquefaciens strain FJAT-2349. J Appl Microbiol 126:1519–1529. https://doi.org/10.1111/jam.14213
Willenbacher J, Yeremchuk W, Mohr T, Syldatk C, Hausmann R (2015) Enhancement of surfactin yield by improving the medium composition and fermentation process. AMB Express 5:57–65. https://doi.org/10.1186/s13568-015-0145-0
Acknowledgements
The strains B. velezensis Bs006 and F. oxysporum f. sp. physali Map5 from AGROSAVIA Microorganisms Working Collection were evaluated in the research that gave rise to this paper. The authors acknowledge Laurent Franzil by technical support in the chromatography laboratory, and Drs. Felipe Borrero Echeverry and Andrea Paola Zuluaga by critical review of the manuscript.
Funding
C.A. Moreno-Velandia’s thesis was supported by commission studies for doctorate program from the Corporación Colombiana de Investigación Agropecuaria (AGROSAVIA) and a grant from Programa Nacional de Formación de Investigadores, Becas Colciencias, Colombia, Doctorado Nacional 567. This research was funded by AGROSAVIA and Ministerio de Agricultura y Desarrollo Rural through TV15 and TV16 agreements for the development of project 687. Microbial Processes and Interactions Laboratory, at Gembloux Agro-Bio Tech, University of Liege also contributed for the development of this work.
Author information
Authors and Affiliations
Contributions
CAM-V: conceptualization, methodology, investigation, formal analysis, and writing—original draft. MO: conceptualization, methodology, resources, and writing—review & editing. JWK: writing—review & editing. AMC: conceptualization, writing—review & editing, supervision, project administration, and funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Moreno-Velandia, C.A., Ongena, M., Kloepper, J.W. et al. Biosynthesis of Cyclic Lipopeptides by Bacillus velezensis Bs006 and its Antagonistic Activity are Modulated by the Temperature and Culture Media Conditions. Curr Microbiol 78, 3505–3515 (2021). https://doi.org/10.1007/s00284-021-02612-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-021-02612-8