Abstract
This work aimed to rely expression of the fengycin promoter to fengycin production under different culture conditions. To this end, Bacillus subtilis BBG208, derived from BBG21, which is a fengycin overproducing strain carrying the green fluorescent protein (GFP) under the control of fengycin promoter, was used to assess the effects of different carbon and nitrogen sources on surfactin and fengycin production and the fengycin promoter expression. The data showed that some carbon sources oriented synthesis of one family of lipopeptides, while most of the nitrogen sources allowed high co-production of fengycin and surfactin. High expressions of promoter Pfen and fengycin synthesis were obtained with urea or urea + ammonium mixture as nitrogen source and mannitol as carbon source. Moreover, temperature, pH and oxygenation influenced their biosynthesis based on the nutrition conditions. Optimization of the production medium increased the fengycin production to 768 mg L−1, which is the highest level reported for this strain. This study defines the suitable nutrient conditions allowing as well the highest expression of the fengycin promoter and portrays the conditions relying on the fengycin and surfactin production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bacteria belonging to the Bacillus genus produce more than 20 different antagonistic secondary metabolites (Sinchaikul et al. 2002; Chen et al. 2007). The non-ribosomally synthesized lipopeptides are the most important secondary metabolites, which are structurally composed of cyclic peptides linked to a fatty acid chain. The lipopeptide group includes surfactins/lichenysins/pumilacidins, fengycins/plipastatins, iturins (bacillomycins/iturins A/mycosubtilins), kurstakins and locillomycins families (Hathout et al. 2000; Ongena and Jacques 2008; Chen et al. 2009; Luo et al. 2015). Fengycins are cyclic lipodecapeptides containing a β-hydroxy fatty acid chain of 14–19 carbon atoms. This family includes fengycins A and B, also named plipastatins (Jacques et al. 1999; Ramarathnam et al. 2007; Raaijmakers et al. 2010; Wei et al. 2010). Non-Ribosomal Peptide Synthetases (NRPS) responsible for fengycins/plipastatins biosynthesis are coded by five genes named fenA to fenE or ppsA to ppsE (Tosato et al. 1997; Steller et al. 1999; Wu et al. 2007). The antibacterial, antiviral and antifungal properties of fengycins as well as their capabilities to inhibit the growth of human lung cancer cells have been already reported (Tosato et al. 1997; Huang et al. 2006; Fracchia et al. 2012; Cawoy et al. 2015; Yin et al. 2013; Ditmer 2014). On the other hand, the environmental conditions determining the type of lipopeptide produced and its yield have been also analysed (Volpon et al. 2000; Islam et al. 2012). Fengycin production is dependent on the source of nutriments, oxygen transfer rate, temperature and pH (Pryor et al. 2007, Jacques 2011; Fahim et al. 2012, Varadavenkatesan and Murty 2013, Zhu et al. 2014). Fengycin synthesis is tightly correlated to nitrogen metabolism, as the nitrogen source appeared to influence the biosurfactant synthesis (Makkar and Cameotra 1997; Steller et al. 1999; Jacques 2011). To date, only few studies treating the effect of the aforementioned factors on the amount of fengycin produced have been reported. In our opinion, there are no data available on the fengycin promoter activity and the fengycin production under different biotic or abiotic conditions.
It is noteworthy that Bacillus strains producing fengycin most often produce surfactin (Roongsawang et al. 2002; Kim et al. 2010). The DNA operon coding for surfactin contains four large ORFs designated srfAA, srfAB, srfAC and srfAD (Galli et al. 1994; Lee et al. 2007). Mutations within the surfactin operon alter the production of fengycin, mycosubtilin and bacilysin (Karatas et al. 2003; Zeriouh et al. 2014).
Recently, the gene coding for the green fluorescent protein (GFP) was cloned under the control of the fengycin promoter in BBG21, leading to BBG208 (Yaseen et al. 2016). According to its fluorescence stability and absence of adverse effect, the GFP protein was used in BBG208 as a promoter biomarker. Here, we used the GFP expression to examine the effects of nutritional conditions and chemo-physical factors on the fengycin biosynthesis. The promoter expression and fengycin production were investigated under different experimental conditions.
Materials and methods
Media and bacterial strain
The Landy medium (Landy et al. 1948) containing 100 mM 3-(N-morpholino) propanesulfonic acid (MOPS) was used as the basic medium for lipopeptide production. All carbon and nitrogen sources in this medium were 0.2 µm filter-sterilized. BBG208 strain producing surfactin and fengycin was used for these experiments. Besides, BBG208 was genetically modified and, therefore, contains the gfp ORF under the control of the fengycin promoter (Yaseen et al. 2016).
Assessment of lipopeptide production and promoter activity
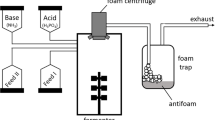
The promoter expression was tested using the high-throughput BioLector system of fermentation (Mp2-labs GmbH, Baesweiler, Germany). Pre-cultures were performed at 30 °C in 500 mL Erlenmeyer flasks containing 50 mL of Landy medium (10% working volume). Cultures were carried out in 48-well flower plate designed for the BioLector, containing pH, dissolved oxygen, fluorescence and biomass optodes in 1 mL of Landy medium at 30 °C, 1100 rpm with initial pH of 7.0 for 72 h. For all conditions tested, all these experiments were performed in triplicate wells and repeated at least twice. The standard deviation was <1% for all these means. Eleven carbon sources, at 20 g L−1, including galactose, lactose, maltose, mannitol, sucrose, starch, fructose, arabinose, glycerol, mannose and sorbitol, were used to replace glucose in Landy medium, Similarly, different nitrogen sources, at 5 g L−1, such as urea, beef extract, peptone, casein hydrolysate, tryptone, different amino acids such as alanine, proline, threonine, ornithine, valine, glycine, isoleucine and several inorganic nitrogen sources such as NH4NO3, NH4HCO3, NaNO3, KNO3 and NH4HCO3 mixed with urea were used to replace glutamic acid in Landy medium. Vegetable and hydrocarbon oils (including sunflower oil, olive oil, paraffin oil and silicon oil), benzene and diesel were tested individually by adding 1% (v/v) of any of this product into Landy medium. Biomass and optical density measurements for BioLector calibration were carried out as recently described (Yaseen et al. 2016).
Influence of the physico-chemical parameters on fengycin production
BBG208 grown overnight in Landy MOPS medium was inoculated in Landy glucose (20 g L−1) + urea (5 g L−1) medium or Landy glutamic acid (5 g L−1) + mannitol (20 g L−1) medium with an initial OD600 of 0.05. Cultures were performed at 30 °C, under agitation at 160 rpm with 10% working volume until synthesis of fengycin was observed. Afterward, the growth cultures were stopped. The time required to reach this point was previously determined from experimental data relying on the growth and production conditions on a single curve (data not shown). After 16 h of growth in Landy urea medium, the cultures were centrifuged (2300g, 20 °C, 10 min), and the supernatants were discarded. Cells were inoculated in seven different media (Table 1) to quantify the lipopeptide production. Similarly, after 15 h of growth in Landy mannitol medium, cultures were centrifuged as mentioned above, and the cells were inoculated in seven different media (Table 1). For all these cultures, pH, OD600 and fengycin concentration were recorded at 24 and 48 h.
Influence of nitrogen and carbon sources on fengycin production
BBG208 was grown for 16 h in Landy MOPS glucose + urea to test for the nitrogen source or for 15 h in Landy MOPS glutamic acid + mannitol to test for the carbon source. In both cases, the media were inoculated with an initial OD600 of 0.05. Upon these periods of incubation, each culture was centrifuged (2300g, 20 °C, 10 min) and the resulting supernatants were eliminated. The cells were placed in fresh Landy MOPS media complemented with glutamic acid and one carbon source (glucose, maltose or sucrose) and grown at 30 °C, under agitation 160 rpm with 10% working volume. Otherwise, they were grown under similar conditions in fresh Landy MOPS media complemented with glucose and one nitrogen source (glutamic acid or ammonium nitrate).
Influence of nitrogen or carbon source concentration on lipopeptide production
The influence of nitrogen concentration was tested in Landy medium complemented with glucose (20 g L−1) and 4, 5, 6, 8 or 10 g L−1 of urea. The influence of carbon concentration was tested in Landy medium complemented with glutamic acid (5 g L−1) and 15, 20, 25, 35 or 45 g L−1 of mannitol.
Lipopeptide purification and quantification
The supernatant (1 mL) was extracted using C18 cartridges (Extract-clean SPE 500 mg, Grace Davison-Alltech, Deerfield, IL, USA). Lipopeptide productions were quantified by HPLC (Waters Corporation, Milford, MA, USA) using a C18 column (5 μm, 250 × 4.6 mm, VYDAC 218 TP, Hesperia, CA, USA). Analyses of fengycin and surfactin were performed as previously described (Coutte et al. 2010).
Results
High-throughput screening of the media components on the lipopeptide production
To establish the effect of several substrates on the surfactin and the fengycin productions as well as the fengycin promoter expression, we tested 18 nitrogen sources and 18 carbon sources including 6 complex carbon sources, among which were hydrocarbon and vegetable oil sources. These components were incorporated in Landy MOPS medium. The seven amino acids tested as nitrogen sources appeared as precursors of the fengycin peptide chain. Otherwise, most of the other substrates were previously studied and reported as positively acting on the lipopeptide production (Fahim et al. 2012; Ghribi and Ellouze-Chaabouni 2011; Huang et al. 2015).
Surfactin production
BBG208 produces surfactin and fengycin. The specific production of the surfactin was measured under the 36 conditions, after 72 h growth, at 30 °C (Fig. 1). No significant difference in the growth rate was registered under these different experimental conditions (data not shown). With respect to the effect of nitrogen source, the specific production of surfactin ranged from 20.34 to 285 mg L−1 OD −1600 . The conditions showing the highest specific productions were urea, urea + ammonium carbonate + alanine, i.e., 257.8 mg L−1 OD −1600 (2.1 g L−1), 285 mg L−1 OD −1600 (2.43 g L−1) and 276 mg L−1 OD −1600 (2 g L−1), respectively. A second set of interesting nitrogen sources included glutamic acid (213 mg L−1 OD −1600 ), which is usually recognized as a central point in the nitrogen metabolism and present in Landy medium and ammonium carbonate (192 mg L−1 OD −1600 ). Peptone, casein and glycine gave intermediary results, while the nitrate salts, the other amino acid residues, the beef extract and the tryptone led to low specific production of surfactin (Fig. 1a). Most of the carbon sources permitted specific production ranging from 100 to 224 mg L−1 OD −1600 . However, when galactose or arabinose was used, the production decreased to 30 mg L−1 OD −1600 . Remarkably, when lactose was used, the levels obtained were similar to those of glucose and galactose. Interestingly, maltose was less efficient than glucose and starch (Fig. 1b). In the media supplemented with 1% vegetable oil and hydrocarbon sources, surfactin production did not increase comparatively to the standard Landy medium (Fig. 1c).
Surfactin production by B. subtilis BBG208 after 48 h of growth in the presence of different substrates. a Nitrogen sources: Landy medium (control) was modified by replacing the glutamic acid with 5 g of 17 different nitrogen sources: KNO3, NaNO3, NH4HCO3, NH4NO3, beef extract, casein, peptone, tryptone, urea, urea (2.5 g) + NH4HCO3 (2.5 g), alanine, glycine, isoleucine, ornithine, proline, threonine, and valine. b Carbon sources: Landy medium (control) was modified by replacing the glucose with 20 g of 11 different carbon sources: arabinose, fructose, galactose, glycerol, maltose, mannitol mannose, lactose, sorbitol, starch, and sucrose. c Addition of hydrocarbon sources: Landy medium (control) was modified by supplying the medium with 1% of six different hydrocarbon sources: benzene, diesel, olive oil, paraffin, silicon oil, and sunflower oil
Fengycin production
The fengycin-specific production ranged from 7.0 to 61.4 mg L−1 OD −1600 . The highest productions were obtained with urea, urea + NH4CO3, NH4CO3, alanine and glutamic acid at 52, 61.46, 48.39, 56.38 and 43.3 mg L−1 OD −1600 , respectively. In contrast to surfactin, NH4NO3 led to highly elevated production of fengycin (43.8 mg L1 OD −1600 ). Low productions were observed with casein, tryptone, beef extract and peptone. As depicted on the Fig. 2a, we registered less than <28 mg L−1 OD −1600 when amino acids other than alanine and glutamic acid are used. Among 12 different carbon sources tested, mannitol has significantly enhanced the production of fengycin to 77.7 mg L−1 OD −1600 . This level is 17-fold up than that observed for mannose, which is about 5.7 mg L−1 OD −1600 . The production was 47.7 mg L−1 OD −1600 with starch as carbon source. The production with maltose, sucrose and lactose was comprised between 37 and 44 mg L−1 OD −1600 . With arabinose or sorbitol, production was less than 20 mg L−1 OD −1600 (Fig. 2b). When composite carbon sources (diesel, benzene, sunflower oil, silicon oil, olive oil and paraffin) were added (1%) to the medium, a slight increase was observed with sunflower oil (53.6 mg L−1 OD −1600 ) comparatively to the control. Equivalent production to the control was obtained with diesel and olive oil. As shown on the Fig. 2c, the media containing paraffin oil, silicon oil, benzene or olive oil led to productions <30 mg L−1 OD −1600 .
Fengycin production by B. subtilis BBG208 after 48 h of growth in the presence of different substrates. a Nitrogen sources: Landy medium (control) was modified by replacing the glutamic acid with 5 g of 17 different nitrogen sources: KNO3, NaNO3, NH4HCO3, NH4NO3, beef extract, casein, peptone, tryptone, urea, urea (2.5 g) + NH4HCO3 (2.5 g), alanine, glycine, isoleucine, ornithine, proline, threonine, and valine. b Carbon sources: Landy medium (control) was modified by replacing the glucose with 20 g of 11 different carbon sources: arabinose, fructose, galactose, glycerol, lactose, maltose, mannitol mannose, sorbitol, starch and sucrose. c Addition of hydrocarbon sources: Landy medium (control) was modified by supplying the medium with 1% of six different hydrocarbon sources: benzene, diesel, olive oil, paraffin, silicon oil, and sunflower oil
Expression of fengycin operon promoter
The production of fengycin was assessed through expression of the promoter under the above-cited conditions using BBG208, in which the Pfen::gfp cassette was integrated at the amyE gene. The use of the BioLector device allowed following the fluorescence of the GFP and, therefore, the expression of the fengycin promoter in different nitrogen, carbon or hydrocarbon and vegetable sources. Therefore, GFP high expression level does not systematically fit with high fengycin production. Some conditions induced the GFP promoter, but the production was not significantly important. In contrast, a good production was correlated with a moderate gene expression.
Nitrogen sources
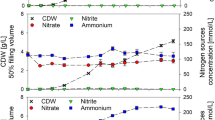
Figure 3 shows the promoter expression under different nitrogen sources. The highest fluorescence intensity was registered for urea, urea + NH4HCO3 and alanine (1.9, 1.48 and 1.24 au OD −1600 , respectively). Under these conditions, the promoter expression was correlated to high levels of fengycin. In contrast, low expression (0.3–0.66 au OD −1600 ) was observed for glutamic acid, NH4NO3 and NH4HCO3, and the levels reached under the same conditions were 43–48 mg L−1 OD −1600 . Nevertheless, no significant expression and synthesis were observed under other conditions (Fig. 3a). Assessment of the fengycin promoter activity placed the expression between 10 h and 18–20 h of cultivation in most of the conditions tested here. The pH decreased to the least value after 30 h and started to increase again. The highest pH value (8.1) was recorded for urea after 36 h, and the lowest pH value (5.8) was also obtained similarly after 36 h using NH4HCO3. Otherwise, the pH level ranged from 6.2 to 7.2 under the other conditions (data not shown).
Fengycin promoter expression by B. subtilis BBG208 after 48 h of growth in the presence of different substrates (All experiments were performed in triplicate wells for each condition and the values of standard deviation were less than 1%). a Nitrogen sources: Landy medium (control) was modified by replacing the glutamic acid with 5 g of 17 different nitrogen sources: KNO 3 , NaNO 3 , NH 4 HCO 3 , NH 4 NO 3 , beef extract, casein, peptone, tryptone, urea, urea (2.5 g) + NH4HCO3 (2.5 g), alanine, glycine, isoleucine, ornithine, proline, threonine, and valine. b Carbon sources: Landy medium (control) was modified by replacing the glucose with 20 g of 11 different carbon sources: arabinose, fructose, galactose, glycerol, lactose, maltose, mannitol, mannose, sorbitol, starch, and sucrose. c Addition of hydrocarbon sources: Landy medium (control) was modified by supplying the medium with 1% of six different hydrocarbon sources: benzene, diesel, olive oil, paraffin, silicon oil, and sunflower oil
Carbon sources
Starch permitted highly elevated promoter expression (1.2 au OD −1600 ), but with a lesser level of fengycin produced compared to that obtained with mannitol. Clear differences in fengycin genes expression and fengycin production using mannitol, lactose, glucose or sucrose were observed. The expression of fengycin promoter was estimated at 0.63 OD −1600 with mannitol, and 0.8 OD −1600 with lactose. Using lactose, the fengycin production was estimated at 37 mg L−1 OD −1600 . Expression with sucrose was reduced by about 50% comparatively to glucose, albeit the productions remained identical at 40 mg L−1 OD −1600 (Fig. 3b).
For most of the conditions tested here, the expression was stopped after 18 h. The highest promoter activity was recorded after 22 h in Landy starch, and after 16–18 h in Landy mannitol. During these experiments, pH changes were not important. Indeed, the pH remained between 6.7 and 7.2 under all the conditions.
Influence of the addition of different hydrocarbon sources
Figure 3c shows the effects of the addition of different hydrocarbon sources (1% (w/v) on the promoter expression. The promoter expression in Landy complemented with sunflower oil was 0.78 OD −1600 ). This value was twice that obtained with Landy medium used as control. The specific fengycin production was 1.28-fold up to the control medium. Notably, no significant differences in the expression level were observed under the other conditions tested. The pH remained between 6.7 and 7.2 along these experiments (data not shown).
Optimization of fengycin production
The effects of temperature, pH and aeration rate
In the presence of urea, the highest fengycin production was obtained after 48 h at 30 °C (550 mg L−1), with a working volume of 10% at pH 7.0 (C2) (Table 2). With the shift of temperature to 25 °C or 37 °C, the production was altered. Similarly, when the oxygen rate was reduced and the initial pH of the medium also was modified, the production was not changed. For mannitol, the maximum production was obtained after 48 h at 25 °C (M 1) with 588 mg L−1 (Table 3). At 30 °C, a high aeration rate and an initial pH of 7.0 led to better conditions. Notably, the influence of the initial pH was less important than in the presence of urea.
Influence of urea and mannitol concentrations on fengycin production
As depicted on Fig. 4, the best specific fengycin production was obtained by the addition of 20 or 25 g L−1 of mannitol (60.5 mg L−1 OD −1600 ), whereas 5 or 6 g L−1 of urea in the culture was enough to allow the highest fengycin concentration at 43.8 mg L−1 OD −1600 . Moreover, 78.5 mg L−1 OD −1600 (648 mg L−1) of fengycin was obtained using Landy medium with 6 g L−1 of urea and 25 g L−1 of glucose as optimal culture condition (data not shown).
Cultures with two successive different nitrogen or carbon sources
The sources allowing fengycin production but not fengycin operon expression were selected for this assessment. Thus, optimal temperature (30 °C), initial pH (7.0) and working volume (10%) were selected as described in “Materials and methods”. The successive use of urea and glutamic acid or ammonium nitrate, or mannitol and glucose or maltose did not significantly increase the specific fengycin production (Fig. 5). However, the successive use of mannitol and sucrose increased the specific fengycin production by 20.7% (85.3 mg L−1 OD −1600 ) compared to mannitol alone (70.7 mg L−1 OD −1600 ). At these conditions, the fengycin production was also the highest (768 mg L−1).
Effect of successive cultivations in the presence of two different nitrogen or carbon sources on specific fengycin production by B. subtilis BBG208: a Nitrogen sources and b carbon sources. B. subtilis BBG208 was grown for 16 h in Landy glucose + urea to test nitrogen sources (a) or during 15 h in Landy glutamic acid + mannitol to test carbon sources (b). Cultures were centrifuged (2300g, 10 min) and the supernatants were discarded. According to the preculture (influence or carbon or nitrogen sources), the cells were then replaced in fresh Landy medium (control) or in a Landy medium in which glucose was replaced by maltose or sucrose and incubated at 160 rpm, with 10% filling volume at 30 °C or in fresh Landy medium or in a Landy medium in which glutamic acid was replaced by ammonium nitrate
Discussion
In this study, we used the GFP reporter genes and BioLector device to screen the influence of a high set of 36 substrates on both promoter expression and lipopeptide production. The use of different experimental conditions was based on previously published reports, in which the influence of these substrates on the production of different lipopeptides was the main topic. To gain insights, we looked at the feeding of fengycin precursors, such as the amino acid residues of the peptide chain. This strategy should help distinguish between parameters affecting the first step of the synthesis from other ones, especially the supply of precursors to the synthetases. As strain BBG208 produces surfactin and fengycin, we assessed and compared the production of these lipopeptides using a high-throughput system for 36 experimental conditions. The ratio of specific production of surfactin/fengycin varied from 1 to 36 depending on the substrate tested. A remarkable difference was observed with mannose which favors surfactin production and with valine which led to equivalent amounts of these lipopeptides, but at low concentrations. Similarly, Singh et al. (2014) showed that environmental conditions can indeed further promote the production of a specific lipopeptide. On the other hand, Davis et al. (1999) underpinned the role of nitrogen sources on the surfactin regulation in B. subtilis ATCC 21332. Abushady et al. (2005) established the key role of ammonium salts and urea for surfactin production. In this study, the positive influence of urea and ammonium carbonate was confirmed for both lipopeptides. The low cost of these nitrogen sources versus glutamic acid which is currently used for lipopeptide production offers another advantage at the economical scale.
Cooper et al. (1981) showed that ammonium nitrate was more suitable for fengycin production. Fahim et al. (2012) using BBG21 established that the oxygen transfer rate can modulate surfactin/fengycin ratio, and a moderate oxygen transfer rate further promotes fengycin synthesis. In this study, we underline that mannose, sorbitol, silicon oil and paraffin oil are more favorable for surfactin production. Recently, Zheng et al. (2013) highlighted the role of starch as a carbon source for surfactin production in B. subtilis NEL-01. Abdel-Mawgoud et al. (2008) showed that galactose and lactose increased the surfactin production, arguing therefore that lipopeptide production is strain dependent with regard to environmental conditions and factor sources.
To study the influence of these environmental factors on fengycin operon expression, we placed the GFP gene expression under the control of the fengycin promoter. Fengycin was preferably and highly produced using nitrogen sources including urea, urea + ammonium carbonate, alanine and ammonium carbonate. The main significant expression was obtained with urea, urea with ammonium carbonate or alanine as the sole nitrogen source in the medium. Indeed, a 20-fold higher expression was obtained with Landy urea than Landy beef extract, while no significant difference in biomass was observed between these two conditions. NH4 was found to be the main nitrogen source for B. subtilis ATCC 21332 and therefore use of NH4 increased B. subtilis cell growth (Makkar and Cameotra 1997; Huang et al. 2015). This ammonia release could slightly increase the intracellular pH, and the alkaline pH positively influences surfactin expression according to Cosby et al. (1998). The use of NH4 as nitrogen source allowed high biosurfactant production in B. subtilis DM-03 and B. subtilis ATCC 21332 (Mukherjee and Das 2005; Huang et al. 2015). The use of urea as the sole nitrogen source may lead to the activation of a switching signal to the secondary metabolism that promotes the synthesis of fengycin.
Among the 12 carbon sources tested in the Landy medium, starch, lactose, mannitol and glucose improved the promoter expression. The highest fengycin production was obtained with mannitol (14-fold more than the lowest one and 1.6-fold more than with glucose). Mannitol was studied in several studies to be a preferable carbon source for fengycin production (Besson et al. 1987; Wei et al. 2010; Islam et al. 2012). The level of fengycin operon expression for Bacillus in the presence of glucose which is defined as the reference carbon source occurred after 18 h, more rapidly than with other carbon sources. On the other hand, the use of starch as carbon source is inconclusive. Zheng et al. ( 2013) reported that starch was the best source for lipopeptide production, but this compound was difficult to metabolize in B. subtilis ATCC 21332, albeit the amylase gene was present (Thompson et al. 2001). Moreover, we established that the addition of sunflower oil in Landy medium enhanced both promoter expression and fengycin production. Related to that, the use of sunflower oil was also reported to improve biosurfactant production in Bacillus sp. (Ghribi and Ellouze-Chaabouni 2011; Rajendran et al. 2014). In B. subtilis, the complex metabolism of the carbon sources can lead to myriad hypotheses to explain the effect of each carbon source on fengycin production.
To gain insights into fengycin production, we investigated the effects of temperature, pH and aeration in two different nutrient conditions, which allowed good promoter expression level. These conditions required the use of urea as nitrogen source and mannitol as carbon source. The temperature required for the optimal production of fengycin can be substrate dependent. Indeed, the temperature for the maximum specific fengycin production was 30 °C in the presence of urea, and 25 °C in the presence of mannitol. The role of pH, as investigated here, indicated that the initial and final pH were key elements for fengycin production. Related to this, a least production was recorded with a final pH 8.9 (urea with initial pH 7.5), and a significant decrease in production was observed with mannitol with a final pH of 6.1 (mannitol with an initial pH of 6.5). A recent study of Varadavenkatesan and Murty (2013) indicated that the optimum pH for fengycin production ranged from 6.5 to 7.5. In the case of our study, a pH ranging from 6.3 to 8.1 still allowed elevated production of lipopeptide.
Reducing the oxygen supplied to the culture was accompanied by a decrease in fengycin production under both conditions. In contrast, a maximal fengycin production (about 0.3 g L−1) was recently obtained at moderate oxygen supply with a kLa of 0.01 s−1 (Chtioui et al. 2014).
Furthermore, we found here that the successive use of mannitol and sucrose enhanced the specific fengycin production of 97% compared to the control, 93% compared to the production obtained in Landy sucrose and 20% compared to the production obtained in Landy mannitol.
To sum up, the use of different nitrogen and carbon sources strongly influences the ratio of specific production surfactin/fengycin from 1 to 36 and the fengycin promoter expression, which can either or not reflect directly on the fengycin production. Activation of the promoter expression by the addition of mannitol followed by sucrose permitted the highest specific productions. This strategy enabled us to increase the fengycin production to 768 mg L−1, which is the highest level ever reported for this strain. The maximum surfactin production (2.33 g L−1) was observed with a mix of urea and ammonium carbonate as nitrogen source, which is also a favorable source for fengycin production. Taking all these data together, it can be hypothesized that fengycin and surfactin produced in this strain probably share several direct or indirect regulation factors.
References
Abdel-Mawgoud M, Aboulwafa M, Hassouna H (2008) Optimization of surfactin production by Bacillus subtilis isolate BS5. Appl Biochem Biotechnol 150:305–325. doi:10.1007/s12010-008-8155-x
Abushady M, Bashandy S, Aziz H, Ibrahim M (2005) Molecular characterization of Bacillus subtilis surfactin producing strain and the factors affecting its production. Int J Agric Biol 3:337–344
Besson F, Chevanet C, Michel G (1987) Influence of the culture medium on the production of iturin A by Bacillus subtilis. J Gen Microbiol 3:767–772
Cawoy H, Debois D, Franzil L, De Pauw E, Thonart P, Ongena M (2015) Lipopeptides as main ingredients for inhibition of fungal phytopathogens by Bacillus subtilis/amyloliquefaciens. Microb Biotechnol 8:281–295. doi:10.1111/1751-7915.12238
Chen XH, Koumoutsi A, Scholz R, Eisenreich A, Schneider K, Heinemeyer I et al (2007) Comparative analysis of the complete genome sequence of the plant growth–promoting bacterium Bacillus amyloliquefaciens FZB42. Nat Biotechnol 25:1007–1014. doi:10.1038/nbt1325
Chen XH, Koumoutsi A, Scholz R, Schneider K, Vater J, Süssmuth R (2009) Genome analysis of Bacillus amyloliquefaciens FZB42 reveals its potential for biocontrol of plant pathogens. J Biotechnol 140:27–37. doi:10.1016/j.jbiotec.2008.10.011
Chtioui O, Dimitrov K, Gancel F, Dhulster P, Nikov I (2014) Selective fengycin production in a modified rotating discs bioreactor. Bioprocess Biosyst Eng 37:107–114. doi:10.1007/s00449-013-0964-9
Cooper DG, Macdonald CR, Duff SJ, Kosaric N (1981) Enhanced production of surfactin from Bacillus subtilis by continuous product removal and metal cation additions. Appl Environ Microbiol 42:408–412
Cosby M, Vollenbroich D, Lee H, Zuber P (1998) Altered srf expression in Bacillus subtilis resulting from changes in culture pH is dependent on the Spo0 K oligopeptide permease and the ComQX system of extracellular control. J Bacteriol 180:1438–1445
Coutte F, Lecouturier D, Yahia SA, Leclère V, Béchet M, Jacques P (2010) Production of surfactin and fengycin by Bacillus subtilis in a bubbleless membrane bioreactor. Appl Microbiol Biotechnol 87:499–507. doi:10.1007/s00253-010-2504-8
Davis A, Lynch C, Varley J (1999) The production of surfactin in batch culture by Bacillus subtilis ATCC 21332 is strongly influenced by the conditions of nitrogen metabolism. Enzyme Microb Technol 25:322–329. doi:10.1016/S0141-0229(99)00048-4
Ditmer EM (2014) Evaluation of the anti-proliferative effect of the lipopeptides iturin A and fengycin of Bacillus spp. and the viral fusion protein PTD4-VP3 on human cancer and normal cells. Masters Dissertation, Federal University of Rio Grande do Sul, Brazil, p 115
Fahim S, Dimitrov K, Gancel F, Vauchel P, Jacques P, Nikov I (2012) Impact of energy supply and oxygen transfer on selective lipopeptide production by Bacillus subtilis BBG21. Bioresour Technol 126:1–6. doi:10.1016/j.biortech.2012.09.019
Fracchia L, Cavallo M, Giovanna M, Banat IM (2012) Biosurfactants and bioemulsifiers biomedical and related applications, present status and future potentials. In: Ghista DN (ed.) Biomedical Science, Engineering and Technology, pp 325–370
Galli G, Rodriguez F, Cosmina P, Pratesi C, Nogarotto R, de Ferra F, Grandi G (1994) Characterization of the surfactin synthetase multi-enzyme complex. Biochim Biophys Acta Protein Struct Mol Enzymol 1205:19–28. doi:10.1016/0167-4838(94)90087-6
Ghribi D, Ellouze-Chaabouni S (2011) Enhancement of Bacillus subtilis lipopeptide biosurfactants production through optimization of medium composition and adequate control of aeration. Biotechnol Res Int. doi:10.4061/2011/653654 (Epub 2011)
Hathout YA, Ho YP, Ryzhov V, Demirev P, Fenselau C (2000) Kurstakins: a new class of lipopeptides isolated from Bacillus thuringiensis. J Nat Prod 63:1492–1496. doi:10.1021/np000169q
Huang QX, Lu ZX, Zhao HZ, Bie XM, Lu FX, Yang SJ (2006) Antiviral activity of antimicrobial lipopeptide from Bacillus subtilis fmbj against pseudorabies virus, porcine parvovirus, Newcastle disease virus and infectious bursal disease virus in vitro. Int J Pept Res Ther 12:373–377. doi:10.1007/s10989-006-9041-4
Huang X, Liu JN, Wang Y, Liu J, Lu L (2015) The positive effects of Mn2 + on nitrogen use and surfactin production by Bacillus subtilis ATCC 21332. Biotechnol Biotechnol Equip 29:381–389. doi:10.1080/13102818.2015.1006905
Islam M, Jeong T, Lee S, Song H (2012) Isolation and identification of antifungal compounds from Bacillus subtilis C9 inhibiting the growth of plant pathogenic fungi. Mycobiology 40:59–66. doi:10.5941/MYCO.2012.40.1.059
Jacques P (2011) Surfactin and other lipopeptides from Bacillus spp., In: Soberon-Chavez G (ed.) Biosurfactants, microbiology monographs 20, pp 57–91. doi: 10.1007/97836421449053
Jacques P, Hbid C, Destain J, Razafindralambo H, Paquot M, De Pauw E (1999) Optimization of biosurfactant lipopeptide production from Bacillus subtilis S499 by Plackett–Burman design. Appl Biochem Biotechnol 77–79:223–233
Karatas Y, Çetin S, Özcengiz G (2003) The effects of insertional mutations in comQ, comP, srfA, spo0H, spo0A and abrB genes on bacilysin biosynthesis in Bacillus subtilis. Biochim Biophys Acta Gene Struct Expr 1626:51–56. doi:10.1016/S0167-4781(03)00037-X
Kim I, Ryu J, Kim H, Chi T (2010) Production of biosurfactant lipopeptides Iturin A, fengycin and surfactin A from Bacillus subtilis CMB32 for control of Colletotrichum gloeosporioides. J Microbiol Biotechnol 20:138–145. doi:10.4014/jmb.0905.05007
Landy M, Warren GH, Rosenman SB, Colio LG (1948) Bacillomycin, an antibiotic from Bacillus subtilis active against pathogenic fungi. Proc Soc Exp Biol Med 67:530–541
Lee K, Yoon D, Yoon H, Lee G, Song J, Kim G, Kim S (2007) Cloning of srfA operon from Bacillus subtilis C9 and its expression in E. coli. Appl Microbiol Biotechnol 75:567–572. doi:10.1007/s00253-007-0845-8
Luo C, Liu X, Zhou X, Guo J, Truong J, Wang X, Zhou H, Li X, Chen Z (2015) Unusual biosynthesis and structure of locillomycins from Bacillus subtilis 916. Appl Environ Microbiol 81:6601–6609. doi:10.1128/AEM.01639-15
Makkar S, Cameotra S (1997) Biosurfactant production by a thermophilic Bacillus subtilis strain. J Ind Microbiol Biotechnol 18:37–42
Mukherjee AK, Das K (2005) Correlation between diverse cyclic lipopeptides production and regulation of growth and substrate utilization by Bacillus subtilis strains in a particular habitat. FEMS Microbiol Ecol 54:479–489. doi:10.1016/j.femsec.2005.06.003
Ongena M, Jacques P (2008) Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol 16:115–125. doi:10.1016/j.tim.2007.12.009
Pryor SW, Siebert KJ, Gibson DM, Gossett JM, Walker LP (2007) Modeling production of antifungal compounds and their role in biocontrol product inhibitory activity. J Agric Food Chem 55:9530–9536. doi:10.1021/jf0719252
Raaijmakers M, De Bruijn I, Nybroe O, Ongena M (2010) Natural functions of lipopeptides from Bacillus and Pseudomonas: more than surfactants and antibiotics. FEMS Microbiol Rev 34:1037–1062. doi:10.1111/j.1574-6976.2010.00221.x
Rajendran D, Venkatachalam P, Ramakrishnan J (2014) Response surface methodology: optimisation of antifungal bioemulsifier from novel Bacillus thuringiensis. Sci World J. doi:10.1155/2014/423289 (Epub 2014 Oct 14)
Ramarathnam R, Bo S, Chen Y, Fernando WGD, Xuewen G, de Kievit T (2007) Molecular and biochemical detection of fengycin- and bacillomycin D-producing Bacillus spp., antagonistic to fungal pathogens of canola and wheat. Can J Microbiol 53:901–911. doi:10.1139/W07-04
Roongsawang N, Thaniyavarn J, Thaniyavarn S, Kameyama T, Haruki M, Imanaka T, Kanaya S (2002) Isolation and characterization of a halotolerant Bacillus subtilis BBK-1 which produces three kinds of lipopeptides: bacillomycin L, plipastatin, and surfactin. Extremophiles 6:499–506. doi:10.1007/s00792-002-0287-2
Sinchaikul S, Sookkheo B, Topanuruk S, Juan HF, Phutrakul S, Chen ST (2002) Bioinformatics, functional genomics, and proteomics study of Bacillus sp. J Chromatog B 771:261–287
Singh K, Rautela R, Cameotra S (2014) Substrate dependent in vitro antifungal activity of Bacillus sp. strain AR2. Microb Cell Fact 13:67–77. doi:10.1186/1475-2859-13-67
Steller S, Vollenbroich D, Leenders F, Stein T, Conrad B, Hofemeister J, Jacques P, Thonart P, Vater J (1999) Structural and functional organization of the fengycin synthetase multienzyme system from Bacillus subtilis b213 and A1/3. Chem Biol 6:31–41. doi:10.1016/S1074-5521(99)80018-0
Thompson N, Fox L, Bala A (2001) The effect of pretreatments on surfactin production from potato process effluent by Bacillus subtilis. In: Davison BH, McMillan JD, Finkelstein M (eds) Twenty-second symposium on biotechnology for fuels and chemicals. Humana Press, Springer, New York, pp 487–501
Tosato V, Albertini AM, Zotti M, Sonda S, Bruschi CV (1997) Sequence completion, identification and definition of the fengycin operon in Bacillus subtilis 168. Microbiology 143:3443–3450
Varadavenkatesan T, Murty R (2013) Production of a lipopeptide biosurfactant by a novel Bacillus sp and its applicability to enhanced oil recovery. ISRN Microbiol. doi:10.1155/2013/621519
Volpon L, Besson F, Lancelina JM (2000) NMR structure of antibiotics plipastatins A and B from Bacillus subtilis inhibitors of phospholipase A2. FEBS Lett 485:76–80. doi:10.1016/S0014-5793(00)02182-7
Wei YH, Wang LC, Chen WC, Chen SY (2010) Production and characterization of fengycin by indigenous Bacillus subtilis F29-3 originating from a potato farm. Int J Mol Sci 11:4526–4538. doi:10.3390/ijms11114526
Wu CY, Chen CL, Lee YH, Cheng YC, Wu YC, Shu HY (2007) Nonribosomal synthesis of fengycin on an enzyme complex formed by fengycin synthetases. J Biol Chem 282:5608–5616. doi:10.1074/jbc.M609726200
Yaseen Y, Gancel F, Drider D, Béchet M, Jacques P (2016) Influence of promoters on the production of fengycin in Bacillus spp. Res Microbiol 167:272–281. doi:10.1016/j.resmic.2016.01.008
Yin H, Guo C, Wang Y, Liu D, Lv Y, Lv F, Lu Z (2013) Fengycin inhibits the growth of the human lung cancer cell line 95D through reactive oxygen species production and mitochondria-dependent apoptosis. Anticancer Drugs 24:587–598. doi:10.1097/CAD.0b013e3283611395
Zeriouh H, Vicente A, Pérez-García A, Romero D (2014) Surfactin triggers biofilm formation of Bacillus subtilis in melon phylloplane and contributes to the biocontrol activity. Environ Microbiol 16:2196–2211. doi:10.1111/1462-2920.12271
Zheng M, Wang P, Wu Q (2013) Optimization of culture conditions for biosynthesis of lipopeptide by Bacillus subtilis with starch. Appl Mechanics Mat 291:225–229. doi:10.4028/www.scientific.net/AMM.291-294.225
Zhu Z, Sun L, Huang X, Ran W, Shen Q (2014) Comparison of the kinetics of lipopeptide production by Bacillus amyloliquefaciens XZ-173 in solid-state fermentation under isothermal and non-isothermal conditions. World J Microbiol Biotechnol 30:1615–1623. doi:10.1007/s11274-013-1587-7
Acknowledgements
Yazen Yaseen received a PhD grant from the Iraqi and French governments through Campus France. We thank our colleagues from the REALCAT platform for their collaboration. This platform benefited from a government subvention administered by the French National Research Agency (ANR) with the contractual reference ANR-11-EQPX-0037.The authors also thank the CPER/FEDER Alibiotech obtained from la region des Hauts-de-France.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Erko Stackebrandt.
Rights and permissions
About this article
Cite this article
Yaseen, Y., Gancel, F., Béchet, M. et al. Study of the correlation between fengycin promoter expression and its production by Bacillus subtilis under different culture conditions and the impact on surfactin production. Arch Microbiol 199, 1371–1382 (2017). https://doi.org/10.1007/s00203-017-1406-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-017-1406-x