Abstract
Filamentous fungi play a dynamic role in health and the environment. In addition, their unique and complex hyphal structures are involved in their morphogenesis, integrity, synthesis, and degradation, according to environmental and physiological conditions and resource availability. However, in biotechnology, it has a great value in the production of enzymes, pharmaceuticals, and food ingredients. The beginning of nomenclature of overall fungi started in early 1990 after which the categorization, interior and exterior mechanism, function, molecular and genetics study took pace. This mini-review has emphasized some of the important aspects of filamentous fungi, their pattern of life cycle, history, and development of different strategic methods applied to exploit this unique organism. New trends and concepts that have been applied to overcome obstacle because of their basic structure related to genomics and systems biology has been presented. Furthermore, the future aspects and challenges that need to be deciphered to get a bigger and better picture of filamentous fungi have been discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There are varieties of fungal species involved in most common opportunistic mycotic infection, mainly Candida spp. and Cryptococcus neoformans [102], while some serious infections occur due to Aspergillus spp. and other filamentous fungi [52, 67, 102]. These are becoming a threat to the medical and research field regarding infectious morbidity and mortality worldwide [36, 51, 101, 102]. The filamentous fungus mainly attacks immunocompromised host which worsens the health condition more seriously. Therefore, many studies on pathogenic fungus have been performed in recent years [55, 63, 86, 102]. Until 2000 AD, Amphotericin B was the standard therapy for mycotic infections caused by a number of hyaline filamentous fungi such as Fusarium, Acremonium, Penicillium, and Scedosporium species, Zygomycetes, and Dematiaceous filamentous fungi such as Bipolaris, Alternaria, and Exophiala species; however, the effect is only suboptimal. Hence, more research on active and potential therapeutics is needed. Because of the unique behavior and distinctive cellular organization, they have been creating extraordinary challenges in describing their form and function, which has been an essential factor for the breakout diseases [74]. The rigid hyphal network and the cytoplasm that can be moved within the hyphal network are the two peculiar characteristics that make filamentous fungi a unique group over all organisms. Moreover, the heterokaryotic nature of organisms [54] undergoing cell fusion and the growth mode resulting into hyphae with multinuclear cellular compartments represent another wicked setback in antifungal clinical setting [35, 91, 95]. In spite of these special features that are an obstacle from a therapeutic aspect, filamentous fungi are yet another important class of eukaryotic organisms of significant commercial relevance, especially in the production of antibiotics, food additives, or recombinant proteins at the industrial scale [35, 72]. For example, the market value of cholesterol-lowering statins represents almost US$15 billion per year in the USA [72]. Fermentation of filamentous fungi for producing many enzymes, antibiotics, fermented food, pigments, medicine, and many useful compounds is most commonly used. Some filamentous fungi are potential producers of a wide range of lignocellulolytic enzymes, which has drawn great interest in many industries [43, 47]. Some of the fungi that are industrially important for the production of various enzymes are listed in Table 1. Based on previous publications, the study on filamentous fungi has been done in 1853 which advanced with the study of nomenclature in 1904. Until now, the number of studies on filamentous fungi has increased drastically with new advancement and technologies that have provided solutions to lots of health, environmental, industrial, and economical problems.
Life Cycle and Microscopic Observation of Filamentous Fungi
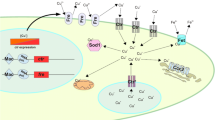
The complete life cycle of filamentous fungi lasts 2–3 laboratory weeks including both sexual reproduction (fusion of two haploid nuclei; karyogamy), followed by meiotic division of the diploid nucleus, and asexual reproduction (division of nuclei by mitosis) [25]. On the other hand, karyogamy may be followed immediately by the combination of two hyphal protoplasts (plasmogamy), or it may be separated in time [18, 25]. In contrast, identical haploid cells can be obtained by the asexual cycle, which can be used for mutagenesis and DNA-mediated transformation [25]. The schematic representation of the life cycle of filamentous fungi is presented in Fig. 1. Mainly, hyphal growth initiates by extension of hyphae at the tips followed by polarization [127]. The polarized growth can be partially identified by directional movement and vesicle accumulation carrying cell wall precursors and cell wall synthetases [25, 79, 127]. Ultrastructure study revealed that hyphal morphogenesis is a complicated organization of tip-growth-related organelles and cytoskeletal elements as well as chitin microfibrils at the apical dome of the hyphae [25, 63]. The chitosomes which control the activity of membrane-bound chitin synthetase may arise from Golgi-like bodies or by a process of self-assembly of subunits freely within the cytoplasm or within larger vesicular bodies. [25]. Many studies performed on microscopic observation of filamentous fungi including fluorescence microscopy, electron microscopy, or even confocal microscopy have successfully obtained the clear picture of the life cycle and reproduction process of filamentous fungi. Similarly, tagging of chimeric green fluorescent proteins (GFPs) to the target protein sequence of AfMp1, AfGel1, and AfEcm33, respectively, has also been proved to be an important method for identifying localizations of certain proteins based on fluorescence tag [97]. Phosphomannose isomerase (Pmi1) [41], GDP-mannose pyrophosphorylase [62], and O-mannosyltransferase 1 (AfPmt1) [129] in A. fumigatus are found to be crucial for cell wall integrity and conidium morphology, while GPI-anchor is essentially required for morphogenesis and virulence [78]. In addition, glucosidase I (AfCwh41) [127] and α-mannosidase (AfMsdC) [79] are required for cell wall synthesis, conidiation, septation, and polarity in A. fumigatus.
Sexual reproduction in fungi typically involves the fusion of two haploid nuclei (karyogamy), followed by meiotic division of the resulting diploid nucleus. In some cases, sexual spores are produced only by fusion of two nuclei of different mating types, which necessitates prior conjugation of different thalli. This condition of sexual reproduction is known as heterothallism, and the nuclear fusion is referred to as heterokaryosis [25, 29]. Normally, plasmogamy (union of two hyphal protoplasts which brings the nuclei close together in the same cell) is followed almost immediately by karyogamy [25]. In certain members of the Basidiomycotina, however, these two processes are separated in time and space, with plasmogamy resulting in a pair of nuclei (dikaryon) contained within a single cell. The development of a dikaryotic mycelium results from simultaneous division of the two closely associated nuclei and separation of the sister nuclei into two daughter cells [25]. An alternative mechanism of sexual reproduction in the fungi is homothallism, in which a nucleus within the same thallus can fuse with another nucleus of that thallus (i.e., homokaryosis) [25, 29]. An understanding of these nuclear cycles is fundamental to the investigation of fungal genetics. Despite the absence of meiosis during the life cycle of these imperfect fungi, recombination of hereditary properties and genetic variation still occur by a mechanism called parasexuality [25]. The major events of this process include the production of diploid nuclei in a heterokaryotic, haploid mycelium that results from plasmogamy and karyogamy, multiplication of the diploid along with haploid nuclei in the heterokaryotic mycelium, sorting out of a diploid homokaryon, segregation and recombination by mitotic crossing over, and haploidization of the diploid nuclei. Some fungi that reproduce sexually also exhibit parasexuality, which could also provide genetic remuneration of meiosis that is achieved through mitotic means [25, 103].
Genetic Engineering and Molecular Approach
According to DNA analysis, the history behind the successful study of this unique group of organisms started since fungi diverged from other life around 1500 million years ago [20]. The typical features of fungi corresponding to earliest fossils possessing date belong to the Proterozoic eon [some 1430 million years ago (Ma)] where the multicellular benthic organisms were found to posses filamentous structures with septa and were capable of anastomosis [21]. In earlier 1930s, the genetic study of any organism was mainly based on mutation. The first heterologous gene of filamentous fungus N. crassa pyr4 gene was isolated by Buxton and Radford in 1983 by complementation of E. coli pyr (F) mutant [22]. Earlier studies already revealed that in higher eukaryotes genetic studies through mutation can be accompanied by light microscopy for the determination of the karyotype. However, small chromosome size in fungi appeared to be problematic for microscopic studies of their karyotype. Thus, the mutation using different mutagens was the ultimate method for the genetic study. But the major problems with this method were the induction of chromosome aberrations with single gene mutations by mutagens [118] and the reduction of interchromosomal recombination and falsely indicated mitotic linkage [65]. Further, the genetic map could only give accurate genesis only if the species is genetically well understood which was lacking during that era. Teow et al. somehow solved those problems alternately using spontaneous mutations in the ascomycete A. nidulans as a model organism [119]. Previous studies have identified sequence and annotation of 18 different species of filamentous fungi such as A. clavatus [72, 124], A. flavus [93, 99], A. fumigatus [45, 93], A. nidulans [45], A. niger [100], A. terreus [124], F. verticillioides [19], N. crassa [17, 45], and P. chrysosporium [85]. Moreover, ten genome sequences of the most important industrial and medical Aspergilli are publically accessible, making this genus one of the best to be studied by comparative genome analysis [72]. The trends of genomics studies done on filamentous fungi in the last 20 years are depicted in Fig. 2a. Nevertheless, the great effort of researchers brought a fascinating period of new discoveries and breakthroughs in many new genetic tools and techniques in the past decades such as efficient genetic transformation systems, expression systems for high-level and controlled protein production, high-throughput gene targeting tools, and even live imaging techniques for cell structures [89].
PEG-mediated transformation system was first established in yeast (Saccharomyces cerevisiae) in 1978 [69], which was later followed by transformation of filamentous fungi in 1989 for the first time [42]. Although asexual spores such as conidia or sporangia are most favorable for filamentous fungi, sometimes the use of mycelia and multinuclear state of conidia make the transformation system in filamentous fungi less efficient than in yeast [10]. Later in late 90s, Chakraborti and Ruiz-Diez studied electroporation for the transformation using protoplast, conidia, or young germlings [33, 106]. A great progress in microbial metabolomics has been achieved in the last 37 years. However, it is clear that there appears to be no universal methodology in microbial metabolomics for instantaneous quenching of microbial metabolic activity, extraction of all low-molecular weight metabolites, and analysis of these metabolites of interest. The use of genomics, transcriptomics, proteomics, and metabolomics toward an improved and novel understanding of the biochemical processes has been proved to be important for understanding the mechanism and massive overproduction of secreted proteins [75].
A number of genetic and genomic tools have been developed to obtain modified and improved strains to enhance the production of specific enzymes from fungi. Zou et al. [130] showed the direct engineering of the cbh1 promoter of T. reesei by replacing the three binding sites of the carbon catabolite repressor CREI with binding sites of different transcription activators to improve the strength of promoter. The heterologous expression of thermostable endoglucanase E1 from A. cellulolyticus in T. reesei showed that the fusion proteins greatly improved the quality of carbohydrate metabolic enzyme with increased enzyme activity and better thermostability to release sugars from complex biomass. Similarly, molecular techniques such as cloning or denaturing gradient gel electrophoresis (DGGE) have provided better knowledge about microbial community structure [48]. A limited knowledge on microbial enzymes because of the narrow traditional microbiological and biochemical methods to characterize the enzymes of complex sugar degradation has been solved by much advanced genetic and molecular technology.
Yet another alternative strategy for gene targeting and in particular gene deletion has been in use in recent years. This strategy was also found to be more effective as compared with the tedious gene knockout strategies [116]. The RNA-based methods that silence gene expression post-transcriptionally are especially helpful either when gene targeting approach could not be attained, isogenes might compensate for the knockout, or when multiple copies of a gene of interest are present in the genome [88]. Many successful stories on gene silencing using artificial antisense constructs have been reported on filamentous fungi [15, 73, 92, 128]. Similarly, the effects of silencing of the transcriptional regulator toward the total expression of xylanases in A. niger was studied by measuring the relative expression levels of two highly transcribed target genes encoding d-xylose reductase and endo-β-1,4-xylanase B [82]. This method described homologous recombination between a general destination vector and a specific entry clone to generate the corresponding dsRNA expression [82]. Likewise, RT-PCR analysis revealed the inducible expression of an RNAi construct for efficient gene expression in A. fumigatus [71, 96]. In T. koningii YC01, a cellulase-hyperproducing strain with genetic similarity to T. reesei, the improved expression and production of cellulase and xylanase was studied by constructing ACEI through RNAi [121]. Similarly, CRISPR–Cas9 is another powerful and most recent approach for genome editing in a variety of organisms including filamentous fungi. CRISPR/Cas9 system has been studied in Trichoderma reesei by specific codon optimization and in vitro RNA transcription through inducible Cas9 expression. This system can generate site-specific mutations, even using short homology arms in target genes through efficient homologous recombination. This tool also provided an applicable and promising approach to target multiple genes simultaneously [77, 81, 94].
Proteomics Study on Filamentous Fungi
Several fungi possess exceptional capacity for protein production which provides one of the important aspects for identifying the protein function. The ideal targets for protein could be more easily exposed by proteomics techniques rather than conventional methods. The proteomic study has opened a method for screening the secreted protein through their peptide sequence. The published data reveal that the progress particularly in proteomics and its techniques has been high only after 2000 (Fig. 2b). In the last few years, the secreted lignocellulolytic enzymes of individual strains and their co-cultures were analyzed by high-throughput isobaric tag for relative and absolute quantification (iTRAQ) coupled with liquid chromatography tandem mass spectrometry (LC–MS/MS) [5, 112]. Using 2D gel electrophoresis, Sato et al. [107] identified 18 proteins with the expression patterns of cellulolytic proteins from P. chrysosporium in cellulose-grown cultures and wood-grown cultures. It was reported that there are several disadvantages such as reagent cross-reactivity and detection sensitivity with colorimetric estimation and identification of enzymes in a complex secretome. Gene expression technologies such as transcriptome profiling are somehow flawless and advantageous but also include limitations such as RNA stability, choice of primer set, and a high number of false-positive and false-negative findings. However, proteomic technology is much more advanced, sensitive, and suitable to identify various sets of proteins from intricate biological samples [83, 84]. In recent years, an iTRAQ-based quantitative proteomics is a method of choice for proteomics analysis in order to understand cytosolic and membrane proteins, cellular process mechanisms, their regulations and protein–protein interactions, and possible metabolic pathway for enhanced cellulose hydrolysis potential of bacterial and fungal secretomes [5]. The proteomics analysis of P. chrysosporium, T. fusca, A. nidulans, A. fumigatus, A. niger, and T. reesei and other bacterial and fungal enzymes has provided a useful means to improve the understanding of their unique enzyme system and evaluate their use in industries for lignocellulosic bioenergy [3,4,5, 59, 80, 83].
The genome analysis of T. fusca exposed 45 hydrolytic enzymes, 28 putative glycoside hydrolases, and other enzymes optimally active at 55 °C [5, 61, 76]. It is assumed that membrane proteins could exhibit a major function in many biological processes, secretory protein export, nutrient transport, and signal transduction to provide special cellular strategies to survive and secrete thermostable hemi/cellulolytic enzymes. Cellulase genes present in T. reesei and A. niger have been identified from secreted protein by 2DGE and LC–MS [37, 64]. The protein secretion profile of A. niger identified 102 unique proteins including many hydrolyzing enzymes, such as cellulases, hemicellulases, hydrolases, proteases, peroxidases, and protein-translocating transporter proteins [4]. Moreover, most of the hydrolases have potential application in lignocellulosic biomass hydrolysis for biofuel production. For example, some enzymes such as endoglucanase, glucan 1,4-α-glucosidase, β-mannosidase, glycosyl hydrolase, proteases, and other enzymes like cytochrome C oxidase and glucose oxidase were highly expressed in A. niger, which have a major role in cellulolysis. In addition, specific enzyme production can be stimulated by controlling pH of the culture medium [2, 4, 9, 26]. However, the role of PTMs is not very clear although Adav et al. [59] showed deamidation in the secretome of A. fumigatus.
In LC-based platform, the proteins are first digested into peptides, followed by separation with strong cation exchange and then with reversed phase chromatography [125]. Numerous labeling methods depending on heavy isotopes such as 2H, 13C, 15N, and 18O have been developed and allow relative quantitation using MS. There are various quantitative methods that can be used in LC-based approach including stable isotope labeling by amino acids in cell culture (SILAC), cleavable isotope-coded affinity tag (cICAT) labeling, and isobaric tags for relative and absolute quantitation (iTRAQ). In iTRAQ approach, a corresponding balance group and an amine-reactive group are used to label primary amino group in peptides using either 4-plex or 8-plex isobaric tags consisting of a reporter mass of 114-117 (4-plex) or 113-121 (excluding 120, 8-plex). The labeled peptides are pooled, fractionated, and subjected to mass spectrometry whereby MS/MS fragmentation releases the reporter ions which would reflect the relative abundance of the proteins [56, 125]. In iTRAQ labeling for relative and absolute quantitation, the iTRAQ reagents react with primary amines of amino termini or lysine residues and label most peptides and proteins present in cells. iTRAQ reporter ions are released upon collision-induced dissociation (CID) and their relative intensities are used for protein quantitation. In contrast to ICAT and SILAC (where only two or three samples can be compared), iTRAQ permits labeling and quantitation of four or eight samples. Further, by mixing multiple samples in a single run, the instrument time for analyses can be reduced, and the results are not affected by variations between different LC/MS runs. Among these three approaches, iTRAQ has a higher sensitivity [46]; however, the three methods covered cell lysates’ protein profiles which have little overlaps, suggesting that these methods may complement each other [14, 125].
Recent Advancement and Future Prospect
It is clear that the formation of heterokaryons in filamentous fungi leads to the formation of hyphae with multinuclear cellular compartment and webs of mycelia that limit the research of their cell cycle pattern [54, 91, 95]. Moreover, because of this nature the genetic and molecular techniques usually involve more laborious, tedious, and/or time-consuming procedures with greater risk of failure [109]. However, in recent years Delgado-Ramos et al. studied flow cytometry procedure in yeast and filamentous fungi by microencapsulation of single spore in which growth was monitored by light or fluorescent microscopy and complex object parametric analyzer and sorter large-particle flow cytometry [35]. Similarly, Beneyton et al. developed a high-throughput screening method of filamentous fungi using nanoliter-range droplet-based microfluidics [13]. These represent a great achievement in the production of industrially important strains with low cost, space, and time which could bring enormous benefits for improving the genetic and molecular viability in filamentous fungi [35]. Furthermore, live cell imaging of hyphal fusion (anastomosis) process in growing colonies of N. crassa was also successfully observed by staining with the membrane-selective dyes FM1-43 and FM4-64 [13, 55]. In addition, the apoptosis occurring in these fungi was performed by fluorescent labeling of the nuclei and analyzed by SCAN©, a System for Counting and Analysis of Nuclei [113]. These researches provide a great value as nuclei are divided between compartments in filamentous fungi containing a number of nuclei which further migrate between the compartments [35, 113]. A versatile technique in which RNA-guided mutagenesis by transforming a target fungus with a single plasmid has also been successfully performed in yeast, Aspergillus sp. and Trichoderma sp. by using CRISPR-Cas9 vectors furnished with fungal markers permitting the selection in a broad range of fungi. This method definitely helps in improving the efficiency and stability of recombination and transformation and also improves the gene targeting or gene replacement in filamentous fungi [44, 77, 94]. However, many new and advanced methods have been applied in the study of filamentous fungi; still more studies are required for fully understanding the further mechanism of their cell cycle. The mechanism of their polarized growth and the relation of their growth with their metabolism such as carbohydrate metabolism or change in glycosylation still need lots of efforts to be fully understood. For this, better and efficient methods should be developed because the filamentous and multinucleated nature of filamentous fungi always creates a mystery and complexity in gaining better understanding.
Conclusion
Filamentous fungi can be categorized not only as a pathogenic microorganism but also as a microbial cell factory or the source of enzymes, chemicals, and pharmaceuticals. With the great effort of researchers and scientists, genetic engineering and molecular approaches have been devised to understand their unique features and functions. Although many techniques and strategies including DNA–RNA and genomics-based approach, protein and enzymes approach using various biotechnological aspects have been successfully used to exploit the basis of filamentous fungi, still more efficient, stable, valid, and productive tools are essentially required to solve the obstacles in having a better insight into many industrially and commercially important filamentous fungi so as to improve their productivities. This will in future unlock the door for further improving the strain to obtain the product of our interest. Furthermore, in future the fungal genomics, transcriptomics, and proteomics could even provide a network for profiling their importance in relation to other organisms including human and other prokaryotes and eukaryotes.
References
Abrahao Neto J, Rossini C, El-Gogary S, Henrique-Silva F, Crivellaro O, El-Dorry H (1995) Mitochondrial functions mediate cellulase gene expression in Trichoderma reesei. Biochemistry 34(33):10456–10462
Acharya PB, Acharya DK, Modi HA (2008) Optimization for cellulase production by Aspergillus niger using saw dust as substrate. Afr J Biotechnol 7(22):4147–4152
Adav SS, Chao LT, Sze SK (2012) Quantitative secretomic analysis of Trichoderma reesei strains reveals enzymatic composition for lignocellulosic biomass degradation. Mol Cell Proteomics 11(7):1–15
Adav SS, Li AA, Manavalan A, Punt P, Sze SK (2010) Quantitative iTRAQ secretome analysis of Aspergillus niger reveals novel hydrolytic enzymes. J Proteome Res 9(8):3932–3940. doi:10.1021/pr100148j
Adav SS, Ng CS, Sze SK (2011) iTRAQ-based quantitative proteomic analysis of Thermobifida fusca reveals metabolic pathways of cellulose utilization. J Proteomics 74(10):2112–2122. doi:10.1016/j.jprot.2011.05.038
Adav SS, Ravindran A, Cheow ES, Sze SK (2012) Quantitative proteomic analysis of secretome of microbial consortium during saw dust utilization. J Proteomics 75(18):5590–5603. doi:10.1016/j.jprot.2012.08.011
Adav SS, Ravindran A, Sze SK (2015) Data for iTRAQ secretomic analysis of Aspergillus fumigatus in response to different carbon sources. Data Br 3:175–179
Adav SS, Ravindran A, Sze SK (2015) Quantitative proteomic study of Aspergillus fumigatus secretome revealed deamidation of secretory enzymes. J Proteomics 119:154–168
Andersen MR, Salazar MP, Schaap PJ, van de Vondervoort PJ, Culley D, Thykaer J, Frisvad JC, Nielsen KF, Albang R, Albermann K (2011) Comparative genomics of citric-acid-producing Aspergillus niger ATCC 1015 versus enzyme-producing CBS 513.88. Genome Res 21(6):885–897
Anke T, Weber D (2009) Physiology and genetics: selected basic and applied aspects. Springer, Berlin
Baig M, Baig M, Baig M, Yasmeen M (2005) Saccharification of banana agro-waste by cellulolytic enzymes. Afr J Biotech 3(9):447–450
Benedict C, Kohel R, Jividen G (1994) Crystalline cellulose and cotton fiber strength. Crop Sci 34(1):147–151
Beneyton T, Wijaya IPM, Postros P, Najah M, Leblond P, Couvent A, Mayot E, Griffiths AD, Drevelle A (2016) High-throughput screening of filamentous fungi using nanoliter-range droplet-based microfluidics. Sci Rep 6:27223
Besson D, Pavageau AH, Valo I, Bourreau A, Belanger A, Eymerit-Morin C, Mouliere A, Chassevent A, Boisdron-Celle M, Morel A, Solassol J, Campone M, Gamelin E, Barre B, Coqueret O, Guette C (2011) A quantitative proteomic approach of the different stages of colorectal cancer establishes OLFM4 as a new nonmetastatic tumor marker. Mol Cell Proteomics. doi:10.1074/mcp.M111.009712
Blanco FA, Judelson HS (2005) A bZIP transcription factor from Phytophthora interacts with a protein kinase and is required for zoospore motility and plant infection. Mol Microbiol 56(3):638–648
Boisset C, Fraschini C, Schülein M, Henrissat B, Chanzy H (2000) Imaging the enzymatic digestion of bacterial cellulose ribbons reveals the endo character of the cellobiohydrolase Cel6A from Humicola insolens and its mode of synergy with cellobiohydrolase Cel7A. Appl Environ Microbiol 66(4):1444–1452
Borkovich KA, Alex LA, Yarden O, Freitag M, Turner GE, Read ND, Seiler S, Bell-Pedersen D, Paietta J, Plesofsky N (2004) Lessons from the genome sequence of Neurospora crassa: tracing the path from genomic blueprint to multicellular organism. Microbiol Mol Biol Rev 68(1):1–108
Boyd RF, Hoerl BG (1991) Basic medical microbiology, vol 442. Little, Brown, New York
Brown DW, Cheung F, Proctor RH, Butchko RA, Zheng L, Lee Y, Utterback T, Smith S, Feldblyum T, Glenn AE (2005) Comparative analysis of 87,000 expressed sequence tags from the fumonisin-producing fungus Fusarium verticillioides. Fungal Genet Biol 42(10):848–861
Brundrett MC (2002) Coevolution of roots and mycorrhizas of land plants. New Phytol 154(2):275–304
Butterfield NJ (2005) Probable proterozoic fungi. Paleobiology 31(01):165–182
Buxton F, Radford A (1983) Cloning of the structural gene for orotidine 5′-phosphate carboxylase of Neurospora crassa by expression in Escherichia coli. Mol Gen Genet MGG 190(3):403–405
Cagas SE, Jain MR, Li H, Perlin DS (2011) Profiling the Aspergillus fumigatus proteome in response to caspofungin. Antimicrob Agents Chemother 55(1):146–154. doi:10.1128/AAC.00884-10
Campbell WG (1932) The chemistry of the white rots of wood: the effect on wood substance of Ganoderma applanatum (Pers.) Pat., Fomes fomentarius (Linn.) Fr., Polyporus adustus (Willd.) Fr., Pleurotus ostreatus (Jacq.) Fr., Armillaria mellea (Vahl.) Fr., Trametes pini (Brot.) Fr., and Polystictus abietinus (Dicks.) Fr. Biochem J 26(6):1829
Casselton L, Zolan M (2002) The art and design of genetic screens: filamentous fungi. Nat Rev Genet 3(9):683–697
Charita Devi M, Sunil Kumar M (2012) Production, optimization and partial purification of cellulase by Aspergillus niger fermented with paper and timber sawmill industrial wastes. J Microbiol Biotech Res 2(1):120–128
Chen XA, Ishida N, Todaka N, Nakamura R, Maruyama J, Takahashi H, Kitamoto K (2010) Promotion of efficient saccharification of crystalline cellulose by Aspergillus fumigatus Swo1. Appl Environ Microbiol 76(8):2556–2561. doi:10.1128/AEM.02499-09
Chow C-M, Yagüe E, Raguz S, Wood DA, Thurston CF (1994) The cel3 gene of Agaricus bisporus codes for a modular cellulase and is transcriptionally regulated by the carbon source. Appl Environ Microbiol 60(8):2779–2785
Cole GT (1996) Basic biology of fungi. University of Texas Medical Branch, Galveston
Comtat J (1983) Isolation, properties, and postulated role of some of the xylanases from the basidiomycete Sporotrichum dimorphosphorum. Carbohyd Res 118:215–231
Coughlan MP (1985) The properties of fungal and bacterial cellulases with comment on their production and application. Biotechnol Genet Eng Rev 3(1):39–110
Coutts A, Smith R (1976) Factors influencing the production of cellulases by Sporotrichum thermophile. Appl Environ Microbiol 31(6):819–825
Cushman M, Nagarathnam D, Gopal D, Chakraborti AK, Lin CM, Hamel E (1991) Synthesis and evaluation of stilbene and dihydrostilbene derivatives as potential anticancer agents that inhibit tubulin polymerization. J Med Chem 34(8):2579–2588
de Souza WR, de Gouvea PF, Savoldi M, Malavazi I, de Souza Bernardes LA, Goldman MH, de Vries RP, de Castro Oliveira JV, Goldman GH (2011) Transcriptome analysis of Aspergillus niger grown on sugarcane bagasse. Biotechnol Biofuels 4:40. doi:10.1186/1754-6834-4-40
Delgado-Ramos L, Marcos AT, Ramos-Guelfo MS, Sánchez-Barrionuevo L, Smet F, Chávez S, Cánovas D (2014) Flow cytometry of microencapsulated colonies for genetics analysis of filamentous fungi. G3 4(11):2271–2278
Denning D, Marinus A, Cohen J, Spence D, Herbrecht R, Pagano L, Kibbler C, Kermery V, Offner F, Cordonnier C (1998) An EORTC multicentre prospective survey of invasive aspergillosis in haematological patients: diagnosis and therapeutic outcome. J Infect 37(2):173–180
Do Vale LH, Gomez-Mendoza DP, Kim MS, Pandey A, Ricart CA, Ximenes FFE, Sousa MV (2012) Secretome analysis of the fungus Trichoderma harzianum grown on cellulose. Proteomics 12(17):2716–2728. doi:10.1002/pmic.201200063
Durand H, Soucaille P, Tiraby G (1984) Comparative study of cellulases and hemicellulases from four fungi: mesophiles Trichoderma reesei and Penicillium sp. and thermophiles Thielavia terrestris and Sporotrichum cellulophilum. Enzyme Microb Technol 6(4):175–180
Dutta T, Sahoo R, Sengupta R, Ray SS, Bhattacharjee A, Ghosh S (2008) Novel cellulases from an extremophilic filamentous fungi Penicillium citrinum: production and characterization. J Ind Microbiol Biotechnol 35(4):275–282
Eriksson K, Pettersson B (1975) Extracellular enzyme system utilized by the fungus Sporotrichum pulverulentum Chrysosporium lignorum) for the breakdown of cellulose. Eur J Biochem 51(1):193–206
Fang W, Yu X, Wang B, Zhou H, Ouyang H, Ming J, Jin C (2009) Characterization of the Aspergillus fumigatus phosphomannose isomerase Pmi1 and its impact on cell wall synthesis and morphogenesis. Microbiology 155(10):3281–3293
Fincham J (1989) Transformation in fungi. Microbiol Rev 53(1):148–170
Frisvad JC, Andersen B, Thrane U (2008) The use of secondary metabolite profiling in chemotaxonomy of filamentous fungi. Mycol Res 112(2):231–240
Fuller KK, Chen S, Loros JJ, Dunlap JC (2015) Development of the CRISPR/Cas9 system for targeted gene disruption in Aspergillus fumigatus. Eukaryot Cell 14(11):1073–1080
Galagan JE, Calvo SE, Cuomo C, Ma L-J, Wortman JR, Batzoglou S, Lee S-I, Baştürkmen M, Spevak CC, Clutterbuck J (2005) Sequencing of Aspergillus nidulans and comparative analysis with A. fumigatus and A. oryzae. Nature 438(7071):1105–1115
Ge P, Hao P, Cao M, Guo G, Lv D, Subburaj S, Li X, Yan X, Xiao J, Ma W, Yan Y (2013) iTRAQ-based quantitative proteomic analysis reveals new metabolic pathways of wheat seedling growth under hydrogen peroxide stress. Proteomics 13(20):3046–3058. doi:10.1002/pmic.201300042
Gerngross TU (2004) Advances in the production of human therapeutic proteins in yeasts and filamentous fungi. Nat Biotechnol 22(11):1409–1414
Gong X, Gruninger RJ, Qi M, Paterson L, Forster RJ, Teather RM, McAllister TA (2012) Cloning and identification of novel hydrolase genes from a dairy cow rumen metagenomic library and characterization of a cellulase gene. BMC Res Notes 5(1):566
Grigorevski-Lima AL, Da Vinha FN, Souza DT, Bispo AS, Bon EP, Coelho RR, Nascimento RP (2009) Aspergillus fumigatus thermophilic and acidophilic endoglucanases. Appl Biochem Biotechnol 155(1–3):321–329. doi:10.1007/s12010-008-8482-y
Grigorevski-Lima AL, de Oliveira MM, do Nascimento RP, Bon EP, Coelho RR (2013) Production and partial characterization of cellulases and xylanases from Trichoderma atroviride 676 using lignocellulosic residual biomass. Appl Biochem Biotechnol 169(4):1373–1385. doi:10.1007/s12010-012-0053-6
Groll A, Walsh T (2001) Uncommon opportunistic fungi: new nosocomial threats. Clin Microbiol Infect 7:8–24
Hajjeh RA, Brandt ME, Pinner RW (1995) Emergence of cryptococcal disease: epidemiologic perspectives 100 years after its discovery. Epidemiol Rev 17(2):303–320
Halliwell G, Riaz M (1970) The formation of short fibres from native cellulose by components of Trichoderma koningii cellulase. Biochem J 116(1):35–42
Hartwell LH, Culotti J, Reid B (1970) Genetic control of the cell-division cycle in yeast I-Detection of mutants. Proc Natl Acad Sci USA 66(2):352–359
Hickey PC, Jacobson DJ, Read ND, Glass NL (2002) Live-cell imaging of vegetative hyphal fusion in Neurospora crassa. Fungal Genet Biol 37(1):109–119
Hou Q, Tan HT, Lim KH, Lim TK, Khoo A, Tan IB, Yeoh KG, Chung MC (2013) Identification and functional validation of caldesmon as a potential gastric cancer metastasis-associated protein. J Proteome Res 12(2):980–990. doi:10.1021/pr3010259
Hrmová M, Biely P, Vršanská M, Petráková E (1984) Induction of cellulose-and xylan-degrading enzyme complex in the yeast Trichosporon cutaneum. Arch Microbiol 138(4):371–376
Hrmová M, Petráková E, Biely P (1991) Induction of cellulose-and xylan-degrading enzyme systems in Aspergillus terreus by homo-and heterodisaccharides composed of glucose and xylose. Microbiology 137(3):541–547
Hsieh CW, Cannella D, Jorgensen H, Felby C, Thygesen LG (2015) Cellobiohydrolase and endoglucanase respond differently to surfactants during the hydrolysis of cellulose. Biotechnol Biofuels 8:52. doi:10.1186/s13068-015-0242-y
Ilmen M, Saloheimo A, Onnela M-L, Penttilä ME (1997) Regulation of cellulase gene expression in the filamentous fungus Trichoderma reesei. Appl Environ Microbiol 63(4):1298–1306
Jeoh T, Wilson DB, Walker LP (2002) Cooperative and competitive binding in synergistic mixtures of Thermobifida fusca Cellulases Cel5A, Cel6B, and Cel9A. Biotechnol Prog 18(4):760–769
Jiang H, Ouyang H, Zhou H, Jin C (2008) GDP-mannose pyrophosphorylase is essential for cell wall integrity, morphogenesis and viability of Aspergillus fumigatus. Microbiology 154(9):2730–2739
Jin C (2011) Protein glycosylation in Aspergillus fumigatus is essential for cell wall synthesis and serves as a promising model of multicellular eukaryotic development. Int J Microbiol. doi:10.1155/2012/654251
Jun H, Kieselbach T, Jonsson LJ (2011) Enzyme production by filamentous fungi: analysis of the secretome of Trichoderma reesei grown on unconventional carbon source. Microb Cell Fact 10:68. doi:10.1186/1475-2859-10-68
Käfer E (1965) Origins of translocations in Aspergillus nidulans. Genetics 52(1):217
Kanda T, Wakabayashi K, Nisizawa K (1976) Synergistic action of two different types of endo-cellulase components from Irpex lacteus (Polyporus tulipiferae) in the hydrolysis of some insoluble celluloses. J Biochem 79(5):997–1006
Kao AS, Brandt ME, Pruitt WR, Conn LA, Perkins BA, Stephens DS, Baughman WS, Reingold AL, Rothrock GA, Pfaller MA (1999) The epidemiology of candidemia in two United States cities: results of a population-based active surveillance. Clin Infect Dis 29(5):1164–1170
Kaur B, Oberoi HS, Chadha BS (2014) Enhanced cellulase producing mutants developed from heterokaryotic Aspergillus strain. Biores Technol 156:100–107. doi:10.1016/j.biortech.2014.01.016
Kawai S, Hashimoto W, Murata K (2010) Transformation of Saccharomyces cerevisiae and other fungi: methods and possible underlying mechanism. Bioeng Bugs 1(6):395–403
Kawasaki L, Wysong D, Diamond R, Aguirre J (1997) Two divergent catalase genes are differentially regulated during Aspergillus nidulans development and oxidative stress. J Bacteriol 179(10):3284–3292
Khalaj V, Eslami H, Azizi M, Rovira-Graells N, Bromley M (2007) Efficient downregulation of alb1 gene using an AMA1-based episomal expression of RNAi construct in Aspergillus fumigatus. FEMS Microbiol Lett 270(2):250–254
Kim Y, Nandakumar M, Marten MR (2007) Proteomics of filamentous fungi. Trends Biotechnol 25(9):395–400
Kitamoto N, Yoshino S, Ohmiya K, Tsukagoshi N (1999) Sequence analysis, overexpression, and antisense inhibition of a β-xylosidase gene, xylA, from Aspergillus oryzae KBN616. Appl Environ Microbiol 65(1):20–24
Klein D, Paschke M (2004) Filamentous fungi: the indeterminate lifestyle and microbial ecology. Microb Ecol 47(3):224–235
Kubicek CP (2013) Systems biological approaches towards understanding cellulase production by Trichoderma reesei. J Biotechnol 163(2):133–142
Larsson AM, Bergfors T, Dultz E, Irwin DC, Roos A, Driguez H, Wilson DB, Jones TA (2005) Crystal structure of Thermobifida fusca endoglucanase Cel6A in complex with substrate and inhibitor: the role of tyrosine Y73 in substrate ring distortion. Biochemistry 44(39):12915–12922
Lee NC, Larionov V, Kouprina N (2015) Highly efficient CRISPR/Cas9-mediated TAR cloning of genes and chromosomal loci from complex genomes in yeast. Nucleic Acids Res 43(8):e55–e55
Li H, Zhou H, Luo Y, Ouyang H, Hu H, Jin C (2007) Glycosylphosphatidylinositol (GPI) anchor is required in Aspergillus fumigatus for morphogenesis and virulence. Mol Microbiol 64(4):1014–1027
Li Y, Zhang L, Wang D, Zhou H, Ouyang H, Ming J, Jin C (2008) Deletion of the msdS/AfmsdC gene induces abnormal polarity and septation in Aspergillus fumigatus. Microbiology 154(7):1960–1972
Liu D, Li J, Zhao S, Zhang R, Wang M, Miao Y, Shen Y, Shen Q (2013) Secretome diversity and quantitative analysis of cellulolytic Aspergillus fumigatus Z5 in the presence of different carbon sources. Biotechnol Biofuels 6(1):149. doi:10.1186/1754-6834-6-149
Liu R, Chen L, Jiang Y, Zhou Z, Zou G (2015) Efficient genome editing in filamentous fungus Trichoderma reesei using the CRISPR/Cas9 system. Cell Discovery 1:15007
Mach-Aigner AR, Omony J, Jovanovic B, van Boxtel AJ, de Graaff LH (2012) d-Xylose concentration-dependent hydrolase expression profiles and the function of CreA and XlnR in Aspergillus niger. Appl Environ Microbiol 78(9):3145–3155
Manavalan A, Adav SS, Sze SK (2011) iTRAQ-based quantitative secretome analysis of Phanerochaete chrysosporium. J Proteomics 75(2):642–654. doi:10.1016/j.jprot.2011.09.001
Manavalan T, Manavalan A, Thangavelu KP, Heese K (2012) Secretome analysis of Ganoderma lucidum cultivated in sugarcane bagasse. J Proteomics 77:298–309. doi:10.1016/j.jprot.2012.09.004
Martinez D, Larrondo LF, Putnam N, Gelpke MDS, Huang K, Chapman J, Helfenbein KG, Ramaiya P, Detter JC, Larimer F (2004) Genome sequence of the lignocellulose degrading fungus Phanerochaete chrysosporium strain RP78. Nat Biotechnol 22(6):695–700
Mashego MR, Rumbold K, De Mey M, Vandamme E, Soetaert W, Heijnen JJ (2007) Microbial metabolomics: past, present and future methodologies. Biotech Lett 29(1):1–16
McHale A, Coughlan MP (1980) Synergistic hydrolysis of cellulose by components of the extracellular cellulase system of Talaromyces emersonii. FEBS Lett 117(1–2):319–322
Meyer V (2008) Genetic engineering of filamentous fungi—progress, obstacles and future trends. Biotechnol Adv 26(2):177–185
Meyer V, Wu B, Ram AF (2011) Aspergillus as a multi-purpose cell factory: current status and perspectives. Biotech Lett 33(3):469–476
Miao Y, Li J, Xiao Z, Shen Q, Zhang R (2015) Characterization and identification of the xylanolytic enzymes from Aspergillus fumigatus Z5. BMC Microbiol 15:126. doi:10.1186/s12866-015-0463-z
Morris NR (1975) Mitotic mutants of Aspergillus nidulans. Genet Res 26(03):237–254
Ngiam C, Jeenes DJ, Punt PJ, Van Den Hondel CA, Archer DB (2000) Characterization of a foldase, protein disulfide isomerase A, in the protein secretory pathway of Aspergillus niger. Appl Environ Microbiol 66(2):775–782
Nierman WC, Pain A, Anderson MJ, Wortman JR, Kim HS, Arroyo J, Berriman M, Abe K, Archer DB, Bermejo C (2005) Genomic sequence of the pathogenic and allergenic filamentous fungus Aspergillus fumigatus. Nature 438(7071):1151–1156
Nødvig CS, Nielsen JB, Kogle ME, Mortensen UH (2015) A CRISPR-Cas9 system for genetic engineering of filamentous fungi. PLoS ONE 10(7):e0133085
Nurse P (1975) Genetic control of cell size at cell division in yeast. Nature 256:547–551
Oliveira JM, van der Veen D, de Graaff LH, Qin L (2008) Efficient cloning system for construction of gene silencing vectors in Aspergillus niger. Appl Microbiol Biotechnol 80(5):917–924
Ouyang H, Chen X, Lü Y, Wilson IB, Tang G, Wang A, Jin C (2013) One single basic amino acid at the ω-1 or ω-2 site is a signal that retains glycosylphosphatidylinositol-anchored protein in the plasma membrane of Aspergillus fumigatus. Eukaryot Cell 12(6):889–899
Pal P, Ghosh B (1965) Isolation, purification, and properties of cellulases from Aspergillus terreus and Penicillium variabile. Can J Biochem 43(1):81–90
Payne G, Nierman W, Wortman J, Pritchard B, Brown D, Dean R, Bhatnagar D, Cleveland T, Machida M, Yu J (2006) Whole genome comparison of Aspergillus flavus and A. oryzae. Medical Mycology 44(Supplement 1):S9–S11
Pel HJ, de Winde JH, Archer DB, Dyer PS, Hofmann G, Schaap PJ, Turner G, de Vries RP, Albang R, Albermann K (2007) Genome sequencing and analysis of the versatile cell factory Aspergillus niger CBS 513.88. Nat Biotechnol 25(2):221–231
Perfect JR, Schell WA (1996) The new fungal opportunists are coming. Clin Infect Dis 22(Supplement 2):S112–S118
Pfaller M, Messer S, Hollis R, Jones R (2002) Antifungal activities of posaconazole, ravuconazole, and voriconazole compared to those of itraconazole and amphotericin B against 239 clinical isolates of Aspergillus sp. and other filamentous fungi: report from SENTRY Antimicrobial Surveillance Program, 2000. Antimicrob Agents Chemother 46(4):1032–1037
Pontecorvo G, Käfer E (1958) Genetic analysis based on mitotic recombination. Adv Genet 9:71–104
Rautela GS, Cowling EB (1966) Simple cultural test for relative cellulolytic activity of fungi. Appl Microbiol 14(6):892–898
Royer JC, Nakas J (1990) Interrelationship of xylanase induction and cellulase induction of Trichoderma longibrachiatum. Appl Environ Microbiol 56(8):2535–2539
Ruiz-Díez B (2002) Strategies for the transformation of filamentous fungi. J Appl Microbiol 92(2):189–195
Sato S, Liu F, Koc H, Tien M (2007) Expression analysis of extracellular proteins from Phanerochaete chrysosporium grown on different liquid and solid substrates. Microbiology 153(9):3023–3033
Saykhedkar S, Ray A, Ayoubi-Canaan P, Hartson SD, Prade R, Mort AJ (2012) A time course analysis of the extracellular proteome of Aspergillus nidulans growing on sorghum stover. Biotechnol Biofuels 5(1):52. doi:10.1186/1754-6834-5-52
Seiler S, Plamann M (2003) The genetic basis of cellular morphogenesis in the filamentous fungus Neurospora crassa. Mol Biol Cell 14(11):4352–4364
Selby K, Maitland C (1965) The fractionation of Myrothecium verrucaria cellulase by gel filtration. Biochem J 94(3):578
Semighini CP, Marins M, Goldman MHS, Goldman GH (2002) Quantitative analysis of the relative transcript levels of ABC transporter Atr genes in Aspergillus nidulans by real-time reverse transcription-PCR assay. Appl Environ Microbiol 68(3):1351–1357. doi:10.1128/aem.68.3.1351-1357.2002
Sharma Ghimire P, Ouyang H, Wang Q, Luo Y, Shi B, Yang J, Lü Y, Jin C (2016) Insight into enzymatic degradation of corn, wheat, and soybean cell wall cellulose using quantitative secretome analysis of Aspergillus fumigatus. J Proteome Res 15(12):4387–4402
Shlezinger N, Eizner E, Dubinchik S, Minz-Dub A, Tetroashvili R, Reider A, Sharon A (2014) Measurement of apoptosis by SCAN©, a system for counting and analysis of fluorescently labelled nuclei. Microb Cell 1(12):406–415
Singhania RR, Saini JK, Saini R, Adsul M, Mathur A, Gupta R, Tuli DK (2014) Bioethanol production from wheat straw via enzymatic route employing Penicillium janthinellum cellulases. Biores Technol 169:490–495
Sison BC, Schubert WJ, Nord F (1958) On the mechanism of enzyme action. LXV. A cellulolytic enzyme from the mold Poria vaillantii. Arch Biochem Biophys 75(1):260–272
Sukumaran RK, Singhania RR, Pandey A (2005) Microbial cellulases-production, applications and challenges. J Sci Ind Res 64(11):832
Taniguchi M, Tanaka M, Matsuno R, Kamikubo T (1980) Effect of culture conditions on cellulase production by Pellicularia filamentosa: studies on the re-utilization of cellulosic resources (IV). J Ferment Technol 58(2):143–148
Tector MA, Käfer E (1962) Radiation-induced chromosomal aberrations and lethals in Aspergillus nidulans. Science 136(3521):1056–1057
Teow S, Upshall A (1983) A spontaneous mutation approach to genetic study of filamentous fungi. Trans Br Mycol Soc 81(3):513–521
Wang H, Jones RW (1995) Cloning, characterization and functional expression of an endoglucanase-encoding gene from the phytopathogenic fungus Macrophomina phaseolina. Gene 158(1):125–128
Wen WS, Liu G, Wang J (2012) Improving cellulase production in Trichoderma koningii through RNA interference on ace1 gene expression. J Microbiol Biotechnol 22(8):1133–1140
Wood T (1969) The cellulase of Fusarium solani resolution of the enzyme complex. Biochem J 115(3):457–464
Wood TM, McCRAE SI, Macfarlane CC (1980) The isolation, purification and properties of the cellobiohydrolase component of Penicillium funiculosum cellulase. Biochem J 189(1):51–65
Wortman J, Fedorova N, Crabtree J, Joardar V, Maiti R, Haas B, Amedeo P, Lee E, Angiuoli S, Jiang B (2006) Whole genome comparison of the Aspergillus fumigatus family. Med Mycol 44(sup1):3–7
Wu WW, Wang G, Baek SJ, Shen R-F (2006) Comparative study of three proteomic quantitative methods, DIGE, cICAT, and iTRAQ, using 2D gel-or LC-MALDI TOF/TOF. J Proteome Res 5(3):651–658
Yan J, Du T, Zhao W, Hartmann T, Lu H, Lü Y, Ouyang H, Jiang X, Sun L, Jin C (2013) Transcriptome and biochemical analysis reveals that suppression of GPI-anchor synthesis leads to autophagy and possible necroptosis in Aspergillus fumigatus. PLoS ONE 8(3):e59013
Zhang L, Zhou H, Ouyang H, Li Y, Jin C (2008) Afcwh41 is required for cell wall synthesis, conidiation, and polarity in Aspergillus fumigatus. FEMS Microbiol Lett 289(2):155–165
Zheng X, Kobayashi Y, Takeuchi M (1998) Construction of a low-serine-type-carboxypeptidase-producing mutant of Aspergillus oryzae by the expression of antisense RNA and its use as a host for heterologous protein secretion. Appl Microbiol Biotechnol 49(1):39–44
Zhou H, Hu H, Zhang L, Li R, Ouyang H, Ming J, Jin C (2007) O-Mannosyltransferase 1 in Aspergillus fumigatus (AfPmt1p) is crucial for cell wall integrity and conidium morphology, especially at an elevated temperature. Eukaryot Cell 6(12):2260–2268
Zou G, Shi S, Jiang Y, van den Brink J, de Vries RP, Chen L, Zhang J, Ma L, Wang C, Zhou Z (2012) Construction of a cellulase hyper-expression system in Trichoderma reesei by promoter and enzyme engineering. Microb Cell Fact 11(1):1
Acknowledgement
This work was sponsored by the Natural Science Foundation of China (31320103901 and 31630016).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Sharma Ghimire, P., Jin, C. Genetics, Molecular, and Proteomics Advances in Filamentous Fungi. Curr Microbiol 74, 1226–1236 (2017). https://doi.org/10.1007/s00284-017-1308-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-017-1308-9