Abstract
Emergence and worldwide spreading of resistant bacteria to antibiotic have raised the importance for finding therapeutic alternative to compensate antibiotic drawbacks. Quorum sensing (QS) is a cell-to-cell communication involved in the development of various common bacterial behaviors including virulence factors expression, and targeting QS seems to be relevant to the struggle against bacterial infection. In this report, relevant literature on intrication of QS system and antimicrobial sensitivity mechanisms in P. aeruginosa PAO1 are reviewed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the struggle against bacterial infectious diseases, antibiotic represent the most effective class of medication at our disposal. However, the recurrent development of bacterial antibiotic resistance limits considerably the effectiveness of these agents as well as our therapeutic options. Moreover, the increase of multidrug-resistant (MDR) strains is now a worldwide concern and represents a serious public health issue, recognized by the World Health Organization [39]. Among the most problematic MDR strains are Gram-negative bacteria which produce extended-spectrum β-lactamases (ESBLs) such as P. aeruginosa and Escherichia coli [23].
Depending on bacteria species, resistance to antibiotic can be inherent (constitutive or induced), developed through mutation, and/or received through encoding genetic material from different strains [6]. Thus, bacteria have the ability to adapt and develop mechanism to escape any intrusive product that could undermine the sustainability of the species, and the overuse of antibiotics presumably generates high level of selective pressure which facilitates growth of resistant bacteria [6]. However, bacterial adaption to aggressive compounds suggests a very complex and reactive arsenal of protection. Recent advances in the comprehension of bacterial behaviors demonstrated the presence of cell-to-cell communication mechanism termed quorum sensing (QS) which regulated social bacterial behaviors [19]. Indeed, bacteria are able to detect their population density by producing, releasing, and perceiving small diffusible molecules called autoinducers and allowing them to coordinate a common action implicated in infection success which rely on virulence expression and invasion abilities [7].
Nowadays, evidences have been reported on the implication of QS mechanism in the ability of bacteria to escape antibiotic aggression. For instance, in E. coli, overexpression of SdiA, a LuxR homologue protein that regulates cell division in a cell density-dependent manner [36], confers multidrug resistance and increases levels of AcrAB protein, a component of the antibiotic efflux pump AcrAB-TolC [34]. Moreover, a decreased level of AcrB protein is observed in sdiA null mutants exhibiting consequently a hypersensitivity to fluoroquinolones [34].
P. aeruginosa, an opportunistic Gram-negative pathogen with broad resistance to antibiotics [23] that causes severe infections in immune-compromised hosts [7], shares several mechanisms with E. coli such as antibiotic resistance including efflux systems and β-lactamase production [28] as well as the LuxR/I type QS mechanisms [9]. However, few studies have addressed the correlation between QS mechanisms and bacterial susceptibility to conventional antibiotics in this bacterial model [16, 35]. The aim of the present report is to review relevant literature related to the potential relationship between QS mechanisms and bacterial response to antibiotics in P. aeruginosa.
Overview of QS and Antibiotic Resistance Mechanisms in P. aeruginosa
QS Mechanisms in P. aeruginosa
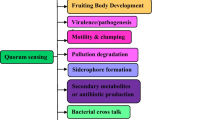
During the last three decades, P. aeruginosa QS mechanisms and their implication in pathogenicity have been largely studied [2, 21]. Briefly, P. aeruginosa possesses two main QS systems (las and rhl) which drive the production (by the synthetases LasI and RhlI) and the detection (by the transcription factors LasR and RhlR) of the autoinducer signaling molecules N-(3-oxododecanoyl)- l-homoserine lactone (3-oxo-C12-HSL) and N-butanoyl-l-homoserine lactone (C4-HSL), respectively [3]. In las system, the diffusible molecule 3-oxo-C12-HSL activates and interacts with the transcription factors LasR when they reach a putative threshold concentration in the cell environment. This interaction drives to the increase of lasI expression and triggers the production of virulence factors including LasB elastase, LasA protease, Apr alkaline protease, and exotoxin A (Fig. 1). Similarly, the interaction of C4-HSL with transcription factors RhlR increases rhlI expression and enhances the production of rhamnolipids, pyocyanin, LasB elastase, hydrogen cyanide, and cytotoxic lectins. The rhl system is also regulated, at the transcriptional and posttranscriptional levels, by the las system in a hierarchical manner [13]. A third QS system, based on the release of a 2-heptyl-hydroxy-4-quinolone [an intercellular signal designated the Pseudomonas quinolone signal (PQS)], interacts with the acyl-homoserine lactones (AHLs) systems in an intricate way [40]. This secondary metabolite of P. aeruginosa is incorporated into the QS hierarchy in times of cell stress and acts as a link between the las and rhl QS systems [8] (Fig. 1). Recently, a fourth intercellular communication signal termed “Integrated QS system” (IQS) has been discovered [20]. This QS system uses new class of quorum-sensing signal molecules (2-(2-hydroxyphenyl)-thiazole-4-carbaldehyde), which have been demonstrated to be able to partially take over the functions of the central las system under phosphate depletion stress conditions. Moreover, it positively regulates the production of PQS and C4-HSL signals, as well as the virulence factors such as pyocyanin, rhamnolipids, and elastase [21].
Mechanisms of Resistance to Antibiotic
Antibacterial drugs present mainly four key mechanisms of action which generate either bactericidal (i.e., killing the bacteria directly) or bacteriostatic effects (i.e., slowing down the reproduction of bacteria) [11]. These mechanisms are mainly based on the inhibition of bacterial cell wall synthesis (e.g., cefotaxime, class of cephalosporin), bacterial deoxyribonucleic acid synthesis (e.g., ofloxacin, class of fluoroquinolone), bacterial protein synthesis (e.g., tobramycin, class of aminoglycosides), or essential metabolites (such as folate) synthesis (e.g., trimethoprim, class of sulfonamides) [12]. The biochemical mechanisms of acquiring resistance to most classes of antibiotics are not completely understood with some exceptions [1]. It is suggested that when first exposed to a new antibiotic, bacteria tend to be largely susceptible and only a few may survive from this antibiotic exposure. Those that survive usually have some genetic characteristic which arise from random mutations, and can be spread among bacteria and transmitted by reproduction to the offspring which become less sensitive to antibiotic by carrying the genetic characteristics of the parent microbe [5]. Drug resistance may also be carried by plasmids or small segments of DNA called transposons, and some plasmids can be horizontally transferred between bacterial cells in a population and between different, but closely related bacterial populations [4].
Antibiotic resistance in P. aeruginosa is mediated via several distinct mechanisms including the production of enzyme that inactivate antibiotics (e.g., production of β-lactamase that degrade β-lactamin antibiotics), the production of efflux pumps (e.g., MexAB-OrpM efflux pumps extruding β-lactamin antibiotics), and the modification and/or alteration of target site or outer membrane (e.g., mutation in gyrA gene encoding the target enzyme of fluoroquinolone antibiotics, a DNA gyrase) [18] (Fig. 2). Beyond the resistance of pathogenic microorganisms to individual antibiotics, P. aeruginosa can develop resistance to multiple antibiotics which is the result association of different mechanism in a single bacteria or the action of single potent mechanism that generate a “cross- or co-resistance” [23]. In this case, Pseudomonas resistance mainly relies on β-lactamase production and overexpression of efflux pumps [37].
Bacterial antibiotic resistance mechanisms [4, 18]. (i) Active drug efflux systems that remove toxic compounds from cells (i.e., resistance to tobramycin and cefotaxime); (ii) mutations resulting in altered cell permeability and decreased penetration of antibiotic (i.e., resistance to ofloxacin); (iii) enzymatic degradation of antimicrobials by the synthesis of specific enzymes for each antimicrobial classes (i.e., resistance to tobramycin and cefotaxime); (iv) alteration/modification of the target site through mutation of key binding elements (i.e., resistance to ofloxacin and trimethoprim)
QS and Efflux Pump Systems Correlation in P. aeruginosa
Few studies have clearly addressed the linkage between QS systems and antimicrobial susceptibility mechanisms. However, QS was found to be involved in antimicrobial resistance regulation via efflux pump genes [25].
Among different types of antibiotic efflux system in bacteria, the resistance-nodulation-division (RND)-type efflux pumps that use proton motive force as sources of energy are commonly found in Gram-negative bacteria [33]. P. aeruginosa encodes dozen possible RND-type efflux systems [38] among which MexAB-OprM, MexCD-OprJ, MexEF-OprN, and MexXY-OprM have been characterized [30, 31]. Efflux transporters are organized as tripartite systems where the pump located in the inner membrane (e.g., MexB, MexD, MexF, and MexY) works in conjunction with a periplasmic protein (e.g., MexA, MexC, MexE, and MexX) and an outer membrane protein (e.g., OprM, OprJ, and OprN). Functioning of each efflux pumps lead to extrusion of broad substrates including antibiotic and chemotherapeutic agent as well as AHLs with acyl chain lengths of C8–14 including 3-oxo-C12-HSL as summarized in Table 1. Indeed, in contrast to C4-HSL and 3-oxo-C6-HSL which are known to diffuse freely in and out the bacteria [15, 29], 3-oxo-Cn-HSLs with long acyl chain lengths of C8–14 requires active transport through the MexAB-OprM and MexEF-OprN efflux systems [15, 27]. Secretion of AHLs through MexXY-OprM and MexCD-OprJ has not been yet documented.
In regard to the relation between efflux and QS systems, Evans et al. [10] demonstrated that P. aeruginosa mutant strains overexpressing the mexAB-oprM exhibit reduced expression in lasI and produce less 3-oxo-C12-HSL and virulence factors (including pyocyanin, elastase, and casein protease) compared to wild-type strains. Likewise, Köhler et al. [31] reported that mutants overexpressing the mexEF-oprN efflux system exhibit reduced expression in rhlI and produce lower levels of C4-HSL and extracellular virulence factors (pyocyanin, elastase, and rhamnolipids) as compared to wild type. Interestingly, Maseda et al. [25] demonstrated that exogenous addition of the C4-HSL enhanced the expression of mexAB-oprM operon in laboratory-derived PAO4290 strains, whereas 3-oxo-C12-HSL had only a slight effect. This study also revealed that MexAB mutants accumulate 3-oxo-C12-HSL intracellularly which suppose a QS-efflux pumps correlation. The correlation between AHLs level production and efflux pumps expression (mexAB-oprM and mexEF-oprN) in P. aeruginosa are summarized in Fig. 3.
Interaction between QS and efflux pump systems in P. aeruginosa. Fact 1 overexpression of mexAB-orpM leads to increase of antibiotic extrusion but also to an important extrusion of 3-oxo-C12-HSLs and consequently decreases the formation of 3-oxo-C-12-HSL-LasR complex and virulence factors expression. Similarly, overexpression of mexEF-orpN leads to a decrease in rhlI expression and C4-HSL concentration [17]; consequently, high levels of antimicrobial resistance with reduction in virulence factor expression are recorded. Fact 2 Exogenous C4-HSL supply enhances expression of mexAB-orpM (Maseda et al.) [25] that presumably extrudes specific antimicrobials but could also extrude noncognate 3-oxo-Cn-HSLs [27] and consequently optimizes formation of 3-oxo-C-12-HSL-LasR complex leading to high QS-regulated and QS-related genes expression [13]. Fact 3 Lack of MexAB-OrpM pumps leads to an intracellular accumulation of 3-oxo-C-12-HSL that could limit cell-to-cell communication in P. aeruginosa
Recently, Pourmand et al. [32] reported a positive correlation between mexXY-oprM expression and las system among P. aeruginosa clinical strains isolated from wound infection where strains with increased lasI and lasR gene expression exhibit higher mexY gene expression and isolates with low lasI and lasR expression shown decrease mexY gene expression.
QS Regulation and Bacterial Sensitivity to Antibiotics in P. aeruginosa
In view of studies reported above, we are tempted to presume that the QS mechanism could influence bacterial susceptibility to antibiotics. Intriguingly, Karatuna and Yagci [16] reported that P. aeruginosa clinical isolates obtained from lower airways clinical samples that were deficient in QS genes (detected through PCR amplification by using oligonucleotide primers designed for lasI, lasR, rhlI, and rhlR genes) were generally less susceptible to antibiotics (including piperacillin, ceftazidime, tobramycin, and ciprofloxacin assessed by disk diffusion method) compared to P. aeruginosa PAO1. However, these clinical strains may harbor inherent or acquired antibiotic resistance mechanisms that have not been evidenced during experiments. Intriguingly, Rampioni et al. [35] demonstrated the importance of RsaL, negative regulator of lasI expression, in P. aeruginosa resistance to antibiotics through efflux pump systems modulation. Indeed, antibiotic susceptibility to trimethoprim, chloramphenicol, norfloxacin, and nalidixic acid is enhanced in rsaL mutant with respect to the wild type, and the microarray analysis showed that rsaL mutation leads to downregulation of mexEF-oprN multidrug-efflux system operons. However, authors also demonstrated that RsaL works as global regulator per se, independently of its repressive effect on lasI expression. Indeed, RsaL control at least 341 genes, including genes involved in biofilm formation and antibiotic resistance which could contribute to the increased sensitivity of rsaL mutant strain to antibiotics. Thus, implication of AHLs-based QS system and bacterial response to antibiotics are not clearly established. In our opinion, to better understand the link between QS mechanism regulation and bacterial response to different antibiotics, several experiments should be conducted. For instance, sensitivity to antibiotics should be evaluated in two different conditions where QS mechanism in PAO1 is altered. Such condition could be achieved by adding exogenous AHLs to PAO1 culture medium and by using PAO1 altered by mutations, specifically in LasI/R and RhlI/R systems. Moreover, to bring light on the hierarchical importance of QS systems and to precise the implications of QS in efflux pumps systems regulation, expression of efflux pump genes (e.g., mexA, mexB), and their corresponding regulator genes in Las and Rhl mutant strains as well as influence of both AHLs on efflux pump systems (e.g., mexAB-orpM) should also be assessed. These investigations are necessary to determine the precise role of QS actors (AHLs, regulator proteins, and QS genes) in antibiotic susceptibility and theirs interaction with conventional antibiotics.
Concluding Remarks
It is well-admitted that bacteria have the ability to adapt and develop mechanism in response to any intrusive compounds that could impair sustainability of its species [6]. In the case of antibiotic, its overuse presumably precipitate increase rate of resistant bacteria [9]. On one hand, an important potential strategy to resolve this issue is the development of new active agents capable to suppress bacterial resistance mechanisms [24]. On the other hand, research for anti-QS has been largely explored since last decades, in order to propose new alternative to struggle against bacterial infection with a limited selective pressure [14]. The present paper highlights that QS probably contribute to optimize some of the arsenals developed by bacteria to escape antibiotics aggression by upregulation of efflux pump genes. However, we have to admit that this interconnection remains nebulous and the hierarchical importance of QS systems in this resistance process needs to be investigated. While it is recognized that efflux pump systems represent an important “pivot” for antimicrobial resistance, AHLs management and diffusion, further investigation should be carried out to bring strong arguments for the importance of QS in the bacterial adaptation to antimicrobial aggression and thus, put QS as efficient anti-infective target. Furthermore, impact of RsaL on the modulation of P. aeruginosa antibiotic susceptibility eggs on investigating the impact of other negatives (e.g., global posttranscriptional regulator, RsmA and quorum-sensing control repressor, and QscR) and positive (e.g., GacS/GacA two component system and CRP-homologous regulator, and VfR) QS global regulator. Finally, studying interconnection between QS system and efflux system connection in antibiotic response should be also extended to the non-AHL-based QS system (PQS system and the recently known IQS) as they could represent the missing link in the hierarchical quorum-sensing system circuitry in P. aeruginosa.
References
Acar J, Davies J (2009) Antibiotic resistance: an ecological perspective on an old problem. A report from the American Academy of Microbiology, p 32
Bassler BL, Miller MB (2013) Quorum sensing. In: The prokaryotes, Springer, Berlin, pp 495–509
Bjarnsholt T, Givskov M (2007) The role of quorum sensing in the pathogenicity of the cunning aggressor Pseudomonas aeruginosa. Anal Bioanal Chem 387(2):409–414
Blair JM, Webber MA, Baylay AJ, Ogbolu DO, Piddock LJ (2015) Molecular mechanisms of antibiotic resistance. Nat Rev Microbiol 13(1):42–51
Cantas L, Shah SQ, Cavaco LM, Manaia C, Walsh F, Popowska M et al (2013) A brief multi-disciplinary review on antimicrobial resistance in medicine and its linkage to the global environmental microbiota. Front Microbiol 4:96
Davies J, Davies D (2010) Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev 74(3):417–433
Deep A, Chaudhary U, Gupta V (2011) Quorum sensing and bacterial pathogenicity: from molecules to disease. J Lab Physicians 3(1):4–11
Diggle SP, Winzer K, Chhabra SR, Worrall KE, Cámara M, Williams P (2003) The Pseudomonas aeruginosa quinolone signal molecule overcomes the cell density-dependency of the quorum sensing hierarchy, regulates rhl-dependent genes at the onset of stationary phase and can be produced in the absence of LasR. Mol Microbiol 50(1):29–43
English BK, Gaur AH (2010) The use and abuse of antibiotics and the development of antibiotic resistance. In: Hot Topics in infection and immunity in children VI. Springer, New York, pp 73–82
Evans K, Passador L, Srikumar R, Tsang E, Nezezon J, Poole K (1998) Influence of the MexAB-OprM multidrug efflux system on quorum sensing in Pseudomonas aeruginosa. J Bacteriol 180(20):5443–5447
Frost KJ, Fernandes T (2007) An overview of antibiotic therapy. Nurs Stand 22(9):51–57
Hills T (2010) Antibacterial chemotherapy. In: Lymn J, Bowskill D, Bath-Hextall F, Knaggs R (eds) The new prescriber: an integrated approach to medical and non-medical prescribing. Wiley, Chichester, pp 444–460
Jimenez PN, Koch G, Thompson JA, Xavier KB, Cool RH, Quax WJ (2012) The multiple signaling systems regulating virulence in Pseudomonas aeruginosa. Microbiol Mol Biol Rev 76(1):46–65
Kalia VC, Purohit HJ (2011) Quenching the quorum sensing system: potential antibacterial drug targets. Crit Rev Microbiol 37(2):121–140
Kaplan HB, Greenberg E (1985) Diffusion of autoinducer is involved in regulation of the Vibrio fischeri luminescence system. J Bacteriol 163(3):1210–1214
Karatuna O, Yagci A (2010) Analysis of quorum sensing-dependent virulence factor production and its relationship with antimicrobial susceptibility in Pseudomonas aeruginosa respiratory isolates. Clin Microbiol Infect 16(12):1770–1775
Köhler T, van Delden C, Curty LK, Hamzehpour MM, Pechere J-C (2001) Overexpression of the MexEF-OprN multidrug efflux system affects cell-to-cell signaling in Pseudomonas aeruginosa. J Bacteriol 183(18):5213–5222
Lambert P (2002) Mechanisms of antibiotic resistance in Pseudomonas aeruginosa. J R Soc Med 95(Suppl 41):22–26
LaSarre B, Federle MJ (2013) Exploiting quorum sensing to confuse bacterial pathogens. Microbiol Mol Biol Rev 77(1):73–111
Lee J, Wu J, Deng Y, Wang J, Wang C, Wang J et al (2013) A cell-cell communication signal integrates quorum sensing and stress response. Nat Chem Biol 9(5):339–443
Lee J, Zhang L (2015) The hierarchy quorum sensing network in Pseudomonas aeruginosa. Protein Cell 6(1):26–41
Lister PD, Wolter DJ, Hanson ND (2009) Antibacterial-resistant Pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin Microbiol Rev 22(4):582–610
Magiorakos AP, Srinivasan A, Carey R, Carmeli Y, Falagas M, Giske C et al (2012) Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18(3):268–281
Malléa M, Mahamoud A, Chevalier J, Alibert-Franco S, Brouant P, Barbe J (2003) Alkylaminoquinolines inhibit the bacterial antibiotic efflux pump in multidrug-resistant clinical isolates. Biochem J 376:801–805
Maseda H, Sawada I, Saito K, Uchiyama H, Nakae T, Nomura N (2004) Enhancement of the mexAB-oprM efflux pump expression by a quorum-sensing autoinducer and its cancellation by a regulator, MexT, of the mexEF-oprN efflux pump operon in Pseudomonas aeruginosa. Antimicrob Agents Chemother 48(4):1320–1328
Masuda N, Sakagawa E, Ohya S, Gotoh N, Tsujimoto H, Nishino T (2000) Substrate specificities of MexAB-OprM, MexCD-OprJ, and MexXY-OprM efflux pumps in Pseudomonas aeruginosa. Antimicrob Agents Chemother 44(12):3322–3327
Minagawa S, Inami H, Kato T, Sawada S, Yasuki T, Miyairi S et al (2012) RND type efflux pump system MexAB-OprM of Pseudomonas aeruginosa selects bacterial languages, 3-oxo-acyl-homoserine lactones, for cell-to-cell communication. BMC Microbiol 12(1):70
Murakami S, Nakashima R, Yamashita E, Yamaguchi A (2002) Crystal structure of bacterial multidrug efflux transporter AcrB. Nature 419(6907):587–593
Pearson JP, Van Delden C, Iglewski BH (1999) Active efflux and diffusion are involved in transport of Pseudomonas aeruginosa cell-to-cell signals. J Bacteriol 181(4):1203–1210
Poole K, Srikumar R (2001) Multidrug efflux in Pseudomonas aeruginosa components, mechanisms and clinical significance. Curr Top Med Chem 1(1):59–71
Poonsuk K, Chuanchuen R (2014) Detection of the mex efflux pumps in Pseudomonas aeruginosa by using a combined resistance-phenotypic markers and multiplex RT-PCR. Open J Med Microbiol 4(03):153–160
Pourmand MR, Sadighian H, Naderi M (2013) Relation between Expression of the las quorum-sensing system in clinical isolates of Pseudomonas aeruginosa and expression of efflux pump and ampC. J Med Bacteriol 2(3, 4):32–40
Putman M, van Veen HW, Konings WN (2000) Molecular properties of bacterial multidrug transporters. Microbiol Mol Biol Rev 64(4):672–693
Rahmati S, Yang S, Davidson AL, Zechiedrich EL (2002) Control of the AcrAB multidrug efflux pump by quorum-sensing regulator SdiA. Mol Microbiol 43(3):677–685
Rampioni G, Schuster M, Greenberg EP, Zennaro E, Leoni L (2009) Contribution of the RsaL global regulator to Pseudomonas aeruginosa virulence and biofilm formation. FEMS Microbiol Lett 301(2):210–217
Sitnikov DM, Schineller JB, Baldwin TO (1996) Control of cell division in Escherichia coli: regulation of transcription of ftsQA involves both rpoS and SdiA-mediated autoinduction. Proc Natl Acad Sci USA 93(1):336–341
Tam VH, Chang K-T, Abdelraouf K, Brioso CG, Ameka M, McCaskey LA et al (2010) Prevalence, resistance mechanisms, and susceptibility of multidrug-resistant bloodstream isolates of Pseudomonas aeruginosa. Antimicrob Agents Chemother 54(3):1160–1164
Venter H, Mowla R, Ohene-Agyei T, Ma S (2015) RND-type drug efflux pumps from gram-negative bacteria: molecular mechanism and inhibition. Front Microbiol 6:377. doi:10.3389/fmicb.2015.00377
WHO (2014) Antimicrobial resistance: global report on surveillance. WHO Press, Geneva Switzerland, 257 pages (ISBN: 978 92 4 156474 8), http://www.who.int/drugresistance/documents/surveillancereport/en
Wilder CN, Diggle SP, Schuster M (2011) Cooperation and cheating in Pseudomonas aeruginosa: the roles of the las, rhl and pqs quorum-sensing systems. ISME J 5(8):1332–1343
Acknowledgments
This research was supported by the Project PIC-Madagascar 2009 and the postdoctoral fellowship programs “ELAN 2015” and “ELAN 2016” of the ARES-CCD (Académie de Recherche et d’Enseignement Supérieur-Commission Coopération au Développement, Belgium).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Rasamiravaka, T., El Jaziri, M. Quorum-Sensing Mechanisms and Bacterial Response to Antibiotics in P. aeruginosa . Curr Microbiol 73, 747–753 (2016). https://doi.org/10.1007/s00284-016-1101-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-016-1101-1