Abstract
Endophytic actinomycetes isolated from Datura stramonium L. was evaluated for its effects against in vitro α-glucosidase inhibition, antioxidant, and free radical scavenging activities. Based on microbial cultural characteristic and 16S rRNA sequencing, it was identified as Streptomyces sp. loyola UGC. The methanolic extract of endophytic actinomycetes (MeEA) shows remarkable inhibition of α-glucosidase (IC50 730.21 ± 1.33 μg/ml), scavenging activity on 2,2-diphenyl-picrylhydrazyl (DPPH) (IC50 435.31 ± 1.79 μg/ml), hydroxyl radical (IC50 350.21 ± 1.02 μg/ml), nitric oxide scavenging (IC50 800.12 ± 1.05 μg/ml), superoxide anion radical (IC50 220.31 ± 1.47 μg/ml), as well as a high and dose-dependent reducing power. The MeEA also showed a strong suppressive effect on rat liver lipid peroxidation. Antioxidants of β-carotene linoleate model system revels significantly lower than BHA. The total phenolic content of the extract was 176 mg of catechol equivalents/gram extract. Perusal of this study indicates MeEA can be used as natural resource of α-glucosidase inhibitor and antioxidants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) is a metabolic disorder characterized by hyperglycemia resulting from defects in insulin secretion, insulin action, or both. Along with hyperglycemia and abnormalities in serum lipids, diabetes is associated with micro- and macrovascular complications, which are the major causes of morbidity and death in diabetic subjects [15]. The currently available antidiabetic agents including sulfonylureas, biguanide, and thiazolidinedione inhibitors are widely used to control the hyperglycemia and hyperlipidemia, but these drugs fail to significantly alter the course of diabetic complications and have limited use because of undesirable side effects and high rates of secondary failure [24]. Thus, it is essential to look for more effective antidiabetic agents with lesser side effects. There are many articles related to antidiabetic compounds from plants and also microbial resources have been proven to be a rewarding source of antidiabetic compounds reported from Streptomyces hygroscopicus-limoneus [6, 19], Streptomyces calvus [17]. However, normalizing blood glucose level is a formidable challenge in clinical practice. The pharmacological agents with greatest effect on postprandial hyperglycemia include insulin, lispro, amylin analogs, and α-glucosidase (acarbose and voglibose) inhibitors [10]. It has been well acknowledged that plant-derived extracts, phytochemicals, and microbial sources are potential alternatives to synthetic inhibitors against α-glucosidase.
Datura stramonium L. is a wild-growing herb known as Jimson weed belongs to the family Solanaceae. The plant distributed throughout most parts of temperate regions of the world [1]. Whole plant is used as anti-inflammatory, central nervous system stimulant [28], for the treatment of respiratory decongestion [35] dental and skin infections, toothache, and alopecia [5]. It is a hallucinogenic plant that causes serious poisoning. Consumption of any part of the plant may result in a severe anticholinergic reaction that may lead to toxicity and occasionally cause diagnostic difficulties [7]. It is used recreationally for its anticholinergic effects, resulting in hallucinations. The entire plant has anticholinergic compounds, but the seeds contain the highest concentration. An extract made by boiling crushed seeds retains the anticholinergic activity, has a rapid onset of action [3], and thus may be potentially useful as an alternative to atropine for the treatment of the muscarinic symptoms of organophosphate toxicity and some of the central anticholinergic effects [29]. There were overexploitations of this plant by the pharmaceutical industries without making any alternative methods to conserve this medicinally important plant. Attempt is made to isolate the endophytic actinomycetes from D. stramonium L. to evaluate the in vitro α-glucosidase and antioxidant potential of MeEA (Methanolic extract of endophytic actinomycetes).
Materials and Methods
Plant Materials
Datura stramonium L. was collected from Irula Tribal Women’s Welfare Society (ITWWS), Chengalpattu, Kanchipuram district, Tamil Nadu, South India, in the month of January. The species was identified and authenticated by Dr. D. Narashiman, Department of Botany, Madras Christian College, Chennai, South India.
Isolation of Actinomycetes
The roots and transition zones of D. stramonium L. were surface sterilized by the methodology of Johannes et al., [14] with some modifications. Samples were thoroughly washed with running tap water and all the visibly damaged materials were excluded. Plant parts were rinsed in 0.1 % Tween 20 for 30 s and followed by bevastin for 2–3 min to inhibit the fungal growth, sequentially immersed in 0.1 % sodium hypochlorite (NaClO) for 30 s and in 75 % ethanol for 3–5 min. After each treatment, samples were rinsed three times in sterile distilled water. Finally the surface-sterilized samples were thoroughly dried in a laminar flow chamber. The samples was aseptically dissected to expose cortex region and plated onto actinomycetes isolation medium, incubated for 12–15 days at 28 °C. The isolation medium was supplemented with nalidixic acid and actidion both to a final concentration of 50 μg/ml to inhibit the growth of non actinomycetes organisms.
Identification of Actinomycetes
The isolated actinomycetes were dereplicated for cultural and morphological characteristics, including morphology and color of aerial mycelium: characteristics of colonies on the plate, mycelium and spore color, color of diffusible pigments and growth. Visual observation by light microscopy and Gram-staining were performed for further identification [31]. The total genomic DNA was extracted by CTAB-Method. The actinomycetes DNA fragments were amplified using Universal primers 16S rRNA and PCR reaction were standardized as initial denaturation at 94 °C for 3 min, followed by 35 cycle of 1 min at 94 °C, 54 °C for 1 min, 72 °C for 2 min and a final extension at 72 °C for 8–10 min, stop at 4 °C for 1 h. The PCR products were stored at 4 °C and visualized by electrophoresis. The gel was photographed in gel documentation. The amplified product was purified and sequenced with two fragments of the 27F (5` AGT TTG ATC CTG GCT CAG 3`) and 1492R (5` ACG GCT ACC TTG TTA CGA CTT 3`) region in both the directions and the sequences obtained were submitted to Genbank for homology search with Blast.
Culture Media and Extraction
The spore suspensions of the culture were inoculated on the Modified Nutrient Glucose Broth (MNGB) medium with pH 7.0 and incubated at 28 °C for 7 days in a shaker. Then, the mycelium filtered and the culture fluid was extracted with methanol (1:1). The organic phase was evaporated to dryness under reduced pressure at 35 °C using rotor vacuum evaporator.
Determination of In Vitro α-Glucosidase Inhibition and Antioxidant Assay
α-Glucosidase Inhibition Activity
In order to investigate the inhibition activity of MeEA, an in vitro α-glucosidase inhibition test was performed. α-glucosidase from yeast is used extensively as a screening material for α-glucosidase inhibitors, but the results do not always agree with those obtained in mammals. Therefore, we used the mouse small intestine homogenate as α-glucosidase solution because we speculated that it would better reflect the in vivo state. The inhibitory effect was measured by the method slightly modified method of Dahlqvist [4]. After fasting for 20 h, the small intestine between the part immediately below duodenum and the part immediately above the cecum was cut, rinsed with ice-cold saline, and homogenized with 12 ml of maleate buffer (100 mM, pH 6.0). The homogenate was used as the α-glucosidase solution. The assay mixture consisted of 100 mM maleate buffer (pH 6.0), 2 % (w/v) each sugar substrate solution (100 μl), and the sample extract (200–1,000 μg/mL). It was preincubated for 5 min at 37 °C and the reaction was initiated by adding the crude α-glucosidase solution (50 μl) to it, followed by incubation for 10 min at 37 °C. Acarbose was used as a standard. The percentage of inhibition was calculated by the formula.
Inhibition (%) = [(amount of glucose produced by the positive control) – (amount of glucose produced by the addition of sample)/(amount of glucose produced by the positive control)] × 100.
Total Phenolic Content (TPC)
Total phenolic content was assessed according to the Folin–Ciocalteau method [27] with some modifications. Briefly, 0.1 ml of sample (200–1,000 μg/ml), 1.9 ml distilled water, and 1 ml of Folin–Ciocalteau’s reagent were added in a tube and then 1 ml of 100 g/l Na2CO3 was added. The reaction mixture was incubated at 25 °C for 2 h and the absorbance of the mixture was read at 765 nm. The sample was tested in triplicate and a calibration curve with six data points for catechol was obtained. The results were compared to a catechol calibration curve and the total phenolic content of MeEA was expressed as mg of catechol equivalents per gram of extract.
Reducing Power Activity
The reducing power of MeEA was determined according to Yen and Duh [32]. Different concentrations of MeEA (200–1,000 μg/ml) were mixed with 2.5 ml of phosphate buffer (200 mM, pH 6.6) and 2.5 ml of 1 % potassium ferricyanide. The mixtures were incubated for 20 min at 50 °C. After incubation, 2.5 ml of 10 % trichloroacetic acid was added to each mixture followed by centrifugation at 3,000 rpm for 10 min. The upper layer (5 ml) was mixed with 5 ml of distilled water and 1 ml of 0.1 % ferric chloride. The absorbance of the resultant solution was measured at 700 nm and was compared with standard BHT absorbance.
DPPH Radical Scavenging Assay
DPPH quenching ability of MeEA was measured according to Hanato et al. [11]. A methanol DPPH solution (0.15 %) was mixed with serial dilutions (200–1,000 μg/ml) of the MeEA and after 10 min, the absorbance was read at 515 nm. The antiradical activity was expressed as IC50 (μg/ml), (the antiradical dose required to cause a 50 % inhibition). Vitamin C was used as standard. The ability to scavenge the DPPH radical was calculated by the following formula:
where A0 is the absorbance of the control at 30 min and A1 is the absorbance of the sample at 30 min. All samples were analyzed in triplicate.
Hydroxyl Radical Scavenging Activity
The hydroxyl scavenging assay was performed as described by the method of Elizabeth and Rao [8] with minor changes. All solutions were prepared freshly. One milliliter of the reaction mixture contained 100 μl of 28 mM 2-deoxy-2-ribose (dissolved in phosphate buffer, pH 7.4), 500 μl solution of various concentrations of MeEA (200–1,000 μg/ml), 200 μl of 200 μM FeCl3 and 1.04 mM EDTA (1:1 v/v), 100 μl H2O2 (1 mM), and 100 μl ascorbic acid (1 mM). After an incubation period of 1 h at 37 °C, the extent of deoxyribose degradation was measured by the TBA reaction. The absorbance was read at 532 nm against the blank solution. Vitamin C was used as a positive control. The scavenging activity was calculated by the formula (1).
Nitric Oxide Scavenging Activity
Sodium nitroprusside in aqueous solution at physiologic pH spontaneously generates nitric oxide; it interacts with oxygen to produce nitrite ions, which can be estimated by the use of Griess Illosvoy reaction [9]. In the present investigation, Griess Illosvoy reagent was modified using naphthyl ethylenediamine dihydrochloride (0.1 % w/v) instead of 1-naphthylamine (5 %). The reaction mixture (3 ml) containing sodium nitroprusside (10 mM, 2 ml), phosphate buffer saline (0.5 ml), and different concentrations of MeEA (200–1,000 μg/ml) or standard solution (0.5 ml) was incubated at 25 °C for 150 min. After incubation, 0.5 ml of the reaction mixture containing nitrite was pipetted and mixed with 1 ml of sulphanilic acid reagent (0.33 % in 20 % glacial acetic acid) and allowed to stand for 5 min for completing diazotization. Then, 1 ml of naphthyl ethylenediamine dihydrochloride (1 %) was added, mixed, and allowed to stand for 30 min. A pink colored chromophore was formed in diffused light. The absorbance of these solutions was measured at 540 nm against the corresponding blank. Ascorbic acid was used as standard. The scavenging activity was calculated by Eq. (1).
Superoxide Scavenging Activity
Superoxide scavenging activity of MeEA was determined by monitoring the competition of those with NBT for the superoxide anion generated by the PMS–NADH system [16]. Superoxide radicals were generated in 1 ml of 20 mM Tris–HCl buffer pH 8.0 containing 0.05 mM nitroblue tetrazolium (NBT), 0.01 mM phenazine methosulphate (PMS), and different concentrations (200–1,000 μg/ml) of MeEA were preincubated for 2 min. The reaction was initiated by the addition of 0.078 mM NADH. Blue chromogen, formed due to NBT reduction, was read at 560 nm. Results were expressed as percentage of inhibition of superoxide radicals. Vitamin C was used as positive control. The scavenging activity was calculated by the formula (1).
Lipid Peroxidation Assay
The inhibition effect of MeEA on lipid peroxidation was determined according to the thiobarbituric acid method. FeCl2–H2O2 was used to induce the liver homogenate peroxidation [33]. In this method, 0.2 ml of MeEA (200–1,000 μg/ml) was mixed with 1.0 ml of 1 % liver homogenate (each 100 ml homogenate solution contains 1.0 g rat liver), then 50 μl of FeCl2 (0.5 mM) and H2O2 (0.5 mM) was added. The mixture was incubated at 37 °C for 60 min, 1.0 ml of trichloroacetic acid (15 %), and thiobarbituric acid (0.67 %) was added and the mixture was heated up in boiled water for 15 min. The absorbance was recorded at 532 nm. Ascorbic acid was used as the positive control. The percentage of inhibition effect was calculated according to the formula (1).
Antioxidant Assay Using β-Carotene Linoleate Model System
The antioxidant activity of MeEA was evaluated by the β-carotene linoleate model system [21]. A solution of β-carotene was prepared by dissolving 2 mg of β-carotene in 10 ml of chloroform. Two milliliters of this solution was pipetted into a 100-ml round-bottom flask. After chloroform was removed under vacuum, 40 mg of purified linoleic acid, 400 mg of Tween 40 emulsifier, and 100 ml of aerated distilled water were added to the flask with vigorous shaking. Aliquot (4.8 ml) of this emulsion was transferred into different test tubes containing different concentrations of MeEA (200–1,000 μg/ml). BHA was used for comparative purposes. As soon as the emulsion was added to each tube, the zero time absorbance was measured at 470 nm. The tubes were then placed at 50 °C in a water bath. Measurement of absorbance was continued until the color of β-carotene disappeared; a blank, devoid of β-carotene, was prepared for background subtraction. Antioxidant activity (AA) was calculated by the following equation:
Statistical Analysis
The data for biochemical and physiologic parameters were analyzed and expressed as mean ± SD. The IC50 values were calculated from linear regression analysis. Results were processed by computer program, Microsoft Excel (2007).
Results
Morphological Identification of the Isolated Strain
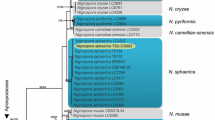
Seven different medium, AIA, ISP2, ISP3, ISP4, ISP5, ISP7, and MNGA were selected for the growth and morphological identification. The isolated strain was grown in each medium and it grew best in the Modified nutrient glucose agar (MNGA). A yellow pigment with raised colonies is observed (Table 1). The microscopic observation indicated that the isolated strain was aerobic with branched aerial hyphae and showed Gram-positive (Fig. 1). The 16S rRNA sequence data, whereby the closest match (99 % similarity) in the NCBI GenBank database was found to be with Streptomyces sp. loyola UGC (Fig. 2). The sequence of the strain has been deposited in the NCBI GenBank database (http://WWW.ncbi.nlm.nih.gov/nuccore/JN863117).
In Vitro α-Glucosidase Inhibition and Antioxidant Assays
α-Glucosidase Inhibition
The results for α-glucosidase inhibition assay are shown in (Table 2). The concentration for 50 % inhibition was found to be 730.21 ± 1.33 μg/ml of extract and all the concentration of standard exhibited above 50 % of inhibition.
Total Phenolic Content (TPC)
The total phenolic content of MeEA was 176 mg catechol equivalent/gram extract.
Reducing Power Activity
Figure 3a shows the reductive capability of MeEA compared to the standard butylated hydroxyl toluene. The reducing power of MeEA increased with increasing quantity of the sample.
a Reductive ability of different concentrations (200–1,000 μg/ml) of MeEA and BHT. Each value represents the mean ± SEM of triplicate experiments. b DPPH scavenging effect of different concentrations (200–1,000 μg/ml) of MeEA and vitamin C. Each value represents the mean ± SEM of triplicate experiments. c Hydroxyl radical scavenging effect of different concentrations (200–1,000 μg/ml) of MeEA and vitamin C. Each value represents the mean ± SEM of triplicate experiments. d Nitric oxide scavenging effect of different concentrations (200–1,000 μg/ml) of MeEA and vitamin C. Each value represents the mean ± SEM of triplicate experiments. e Superoxide scavenging effect of different concentrations (200–1,000 μg/ml) of MeEA and vitamin C. Each value represents the mean ± SEM of triplicate experiments. f Antilipid peroxidation effect of different concentrations (200–1,000 μg/ml) of MeEA and vitamin C. Each value represents the mean ± SEM of triplicate experiments. g Antioxidant activity of different concentrations (200–1,000 μg/ml) of MeEA in the β carotene bleaching assay and butylated hydroxyl anisole (BHA). Each value represents the mean ± SEM of triplicate experiments
DPPH Radical Scavenging Assay
The MeEA exhibited a significant dose dependent inhibition of DPPH activity with a 50 % inhibition (IC50) at a concentration of 435.31 ± 1.79 μg/ml. The results are presented in (Fig. 3b). The IC50 value of vitamin C was 490.21 ± 1.82 μg/ml.
Hydroxyl Radical Scavenging Activity
The results for hydroxyl scavenging assay is shown in (Fig. 3c). The concentrations for 50 % inhibition were found to be 350.21 ± 1.02 and 210.12 ± 0.62 μg/ml for the MeEA and vitamin C, respectively.
Nitric Oxide Scavenging Activity
The scavenging of nitric oxide by MeEA was increased in a dose-dependent manner as illustrated in (Fig. 3d). At concentration of 800.12 ± 1.05 μg/ml of extract, 50 % of nitric oxide generated by incubation was scavenged. The IC50 value of vitamin C was 510.20 ± 1.02 μg/ml.
Superoxide Scavenging Activity
Superoxide anion scavenging activity of MeEA is given in (Fig. 3e). The 50 % of superoxide anion radical generation was scavenged at the concentration of 220.31 ± 1.47 μg/ml. The IC50 value of vitamin C was 240.32 ± 0.69 μg/ml.
Lipid Peroxidation Assay
Activity of extract on lipid peroxidation is shown in (Fig. 3f). The extract showed inhibition of peroxidation at all concentration; it showed 50 % inhibition at 840.31 ± 2.18 μg/ml. The IC50 value of vitamin C was 610.30 ± 2.23 μg/ml.
Antioxidant Activity Using a β-Carotene Linoleate Model System
In the β-carotene linoleate system, β-carotene undergoes rapid discoloration in the absence of antioxidants. The addition of extracts to this system prevents the bleaching of β-carotene at different degrees. The MeEA hindered the extent of β-carotene bleaching in a dose-dependent manner. Based on 120-min reaction time (Fig. 3g), the extract showed 50 % inhibition at 420.12 ± 1.50 μg/ml and the value for BHA was 220.30 ± 1.48 μg/ml.
Discussion
In the present study, the strain Streptomyces sp. loyola UGC was isolated from the root/transition zone of D. stramonium L. Morphological and biochemical characteristics are the two important aspects for the classification in streptomycetaceae family [30]. There are many reports that support the use of antioxidant supplementation in reducing the level of oxidative stress and in slowing or preventing the development of complications associated with diseases [25]. In this study, the methanol extract of endophytic actinomycetes strain isolated from the D. stramonium. L was tested for different in vitro α-glucosidase inhibition and antioxidant properties. Agents with α-glucosidase inhibitory activity have been useful as oral anti hypoglycemic agents for the control of hyperglycemia in patients with diabetes. There are many natural sources with α-glucosidase inhibitory activity. These studies suggest that preventing an excessive postprandial rise of blood glucose level by α-glucosidase inhibition from natural resources is effective in real life as well. MeEA effectively reduced glucose level. In addition, some flavonoids and polyphenols as well as sugar derivatives were found to be effective on the inhibitory activities of α-glucosidase [34]. It appears that this effect is associated with Polyphenols present in MeEA. In the DPPH test, MeEA was able to reduce the stable radical DPPH to the yellow-colored diphenylpicrylhydrazine. The method is based on the reduction of alcoholic DPPH solution in the presence of a hydrogen-donating antioxidant due to the formation of the non-radical form DPPH-H by the reaction [2]. Superoxide anions derived from dissolved oxygen by the riboflavin/methionine/illuminate system will reduce NBT in this system. In this method, superoxide anion reduces the yellow dye (NBT2+) to produce the blue formazan. Antioxidants are able to inhibit the blue NBT formation [23]. The decrease in absorbance indicates the consumption of superoxide anion in the reaction mixture. In our study, the inhibition of superoxide radical by MeEA was lower than the vitamin C. Hydroxyl radical scavenging capacity of a compound is directly related to its antioxidant activity [26]. MeEA inhibited the free radical-mediated deoxyribose damage. Nitric oxides radical inhibition study showed that the extract was a potent scavenger of nitric oxide. The extract inhibited nitrite formation by competing with oxygen to react with nitric oxide directly and also to inhibit its synthesis. Scavengers of nitric oxide competed with oxygen leading to reduced production of nitric oxide [18]. MeEA inhibited free radical-mediated deoxyribose damage. Lipid peroxidation is an oxidative alteration of polyunsaturated fatty acids in the cell membranes that generates a number of degradation products. Malondialdehyde (MDA), one of the products of lipid peroxidation, has been studied widely as an index of lipid peroxidation and as a marker of oxidative stress [12]. MeEA showed strong inhibition of lipid peroxidation. β-Carotene in this model system undergoes rapid discoloration in the absence of an antioxidant. This is because of the coupled oxidation of β-carotene and linoleic acid, which generates free radicals. As a result, β-carotene will be oxidized and broken down in part; subsequently, the system looses its chromophore and characteristic orange color, which can be monitored spectrophotometrically [13]. In our study, the inhibition of β-carotene bleaching by MeEA was lower than the standard BHT. For measurement of reductive ability, we investigated the Fe3+ to Fe2+ transformation in the presence of methanol extract. The reducing power increased with increasing concentration of the extract. The reducing capacity of a compound may serve as a significant indicator of its potential antioxidant activity [20]. Polyphenols are the major plant compounds with antioxidant activity. This activity is believed to be mainly due to their redox properties [22] which play an important role in adsorbing and neutralizing free radicals, quenching singlet and triplet oxygen, or decomposing peroxides. Our study revealed the antioxidant property of MeEA by showing significant various scavenging activities. We believe that this was due to the presence of good amount of phenolics as estimated by Folin–Ciocalteau method. Even though this method has some limitations (possibility of the reagent reacting with other non-phenolic reducing compounds leading to the overevaluation of the phenolic content, possible interference of organic acids, sugars, and amino acids, and possible underestimation of some phenolic compounds due to low absorption) it has been used by many workers [22].
Conclusion
This study suggested that MeEA possessed antioxidant activity which might be helpful in preventing or slowing the progress of various oxidative stress-related diseases. Further investigation on the isolation and identification of antioxidant component(s) in the MeEA may lead to chemical entities for clinical use.
References
Berkov S, Zayed R, Doncheva T (2006) Alkaloids patterns in some varieties of Datura stramonium. Fitoterapia 77:179–182
Brand-Williams W, Cuvelier M, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. Lebensm-Wiss Technology 28:25–30
Chang SS, Wu ML, Deng JF, Lee CC, Chin TF, Liao SJ (1999) Poisoning by Datura leaves used as edible wild vegetables. Vet Hum Toxicol 41:242–245
Dahlqvist A (1964) Method for assay of intestinal disaccharidases. Anal Biochem 7:18–25
De Foe V, Senatore F (1993) Medicinal plants and phytotherapy in the Amal Fitan Cost, Salerno province Campania, Southern Italy. J Ethnopharmacol 39:39–51
De Melo EB, Gomes ADS, Carvalho I (2006) α- and β-Glucosidase inhibitors: chemical structure and biological activity. Tetrahedron 62(44):10277–10302. doi:10.1016/j.tet.08.055
Diker D, Markovitz D, Rothman M, Sendovski U (2007) Coma as a presenting sign of Datura stramonium seed tea poisoning. Eur J Intern Med 18(4):336–338
Elizabeth K, Rao MNA (1990) Oxygen scavenging activity of curcumin. Int J Pharm 58:237–240
Garratt DC (1964) The Quantitative Analysis of Drugs, vol 3. Chapman and Hall, Tokyo, pp 456–458
Goda T, Yamada K, Hosoya N, Moriuchi Y (1981) Effect of alpha glucosidase inhibitor BAY g 5421 on rat intestinal disaccharidases. EiyoToShokuryo (in Japanese) 34:139–143
Hanato T, Kagawa H, Yasuhara T, Okuda T (1988) Two new flavonoids and other constituents in licorice root: their relative astringency and radical scavenging effects. Chem Pharm Bull 36:2090–2097
Janero DR (1990) Malondialdehyde and thiobarbituric acid reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radical Biol Med 9:515–540
Jayaprakasha GK, Singh RP, Sakariah KK (2001) Antioxidant activity of grape seed (Vitis vinifera) extracts on peroxidation models invitro. Food Chem 73:285–290
Johannes H, Gabriele B, Barbara S (2006) Isolation procedures for endophytic microorganisms. Springer, Berlin, p 299
Kumar G, Murugesan AG (2008) Hypolipidaemic activity of Helicteres isora L. bark extracts in streptozotocin induced diabetic rats. J Ethnopharmacol 116:161–166
Liu F, Ooi VEC, Chang ST (1997) Free radical scavenging activities of mushroom polysaccharide extracts. Life Sci 60(10):763–771
Mahmud T (2003) The C7N aminocyclitol family of natural products. Natural Product Reports 20(1):137–166. doi:10.1039/b205561a
Marcocci L, Packer L, Droy-Lefai MT, Sekaki A, Gardes-Albert M (1994) Antioxidant action of Ginkgo biloba extracts EGb 761. Methods Enzymol 234:462–475
Matsui T, Ueda T, Oki T, Sugita K, Terahara N (2001) α-Glucosidase inhibitory action of natural acylated anthocyanins. Journal Agricultural Food Chemistry 49:1948–1951
Meir S, Kanner J, Akiri B, Hadas SP (1995) Determination and involvement of aqueous reducing compounds in oxidative defense systems of various senescing leaves. Journal of Agricultural Food Chemistry 43:1813–1817
Miller HE (1971) A simplified method for the evaluation of antioxidant. J Am Oil Chem Soc 18:439–452
Nitin KU, Yogendra Kumar MS, Asheesh G (2010) Antioxidant, cytoprotective and antibacterial effects of Sea buckthorn (Hippophae rhamnoides L.) leaves. Food Chem Toxicol 48:3443–3448
Parejo I, Viladomat F, Bastida J (2002) Comparison between the radical scavenging activity and antioxidant activity of six distilled and non distilled Mediterranean herbs and aromatic plants. Journal of Agricultural Food Chemistry 50:6882–6890
Ramesh Babu K, Maddirala Dilip R, Vinay Kumar K, Shaik Sameena F, Tiruvenkata Kumar EG, Swapna S, Ramesh B, Rao CA (2010) Antihyperglycemic and antihyperlipidemic activities of methanol:water (4:1) fraction isolated from aqueous extract of Syzygium alternifolium seeds in streptozotocin induced diabetic rats. Food Chem Toxicol 48:1078–1084
Rose WM, Creighton MO, Stewart DHPJ, Sanwal M, Trevithick GR (1972) In vivo effects of vitamin E on cataractogenesis in diabetic rats. Can J Ophthalmol 17:61–66
Shukla S, Mehta A, Bajpai VK, Shukla S (2009) In vitro antioxidant activity and total phenolic content of ethanolic leaf extract of Stevia rebaudiana Bert. Food Chem Toxicol 47:2338–2343
Slinkard K, Singleton VL (1977) Total phenol analyses: automation and comparison with manual methods. American Journal of Enology and Viticulture 8:4955
Spring MA (1989) Ethnopharmacologic analysis of medicinal plants used by Laotian Hmong refugees in Minnesota. J Ethnopharmacol 26:65–91
Theodore C, Bania MS, Chu Jasan, Bailes Dallas, O’Neill Melanie (2004) Jimson Weed Extract as a Protective Agent in Severe Organophosphate Toxicity. Acad Emerg Med 11(4):335–338
Waksman SA, Henrici AT (1943) The nomenclature and classification of the actinomycetes. J Bacteriol 46:337–341
Yan XC (1992) Isolation and identification of actinomycete. Science, Beijing, pp 45–68
Yen GC, Duh PD (1993) Antioxidative properties of methanolic extracts from Peanut Hulls. J Am Oil Chem Soc 70:383–386
Yen GC, Hsieh CL (1998) Antioxidant activity of extracts from Du-zhong (Eucommia urmoides) towards various peroxidation models in vitro. Journal of Agricultural and Food Chemistry 46:3952–3957
Yoshikawa M, Shimada H, Norihisa N, Li Y, Toguchida I, Yamahara J, Matsuda H (1998) Antidiabetic principles of natural medicines. II. Aldose reductase and a-glucosidase inhibitors from Brazilian natural medicine, the leaves of Myrcia multiflora DC. (Myrtaceae): structures of Myrciacitrins I and II and Myrciaphenones A and B. Chem Pharm Bull 46:113–119
Zagari A (1992) Medicinal plants, vol 3, 5th edn. Tehran University Publication, Tehran, p 889
Acknowledgments
Authors are thankful to the University Grant Commission, Government of India—UGC Major Research Project—under F-39-266/2010 (SR) for financial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nimal Christhudas, I.V.S., Praveen Kumar, P. & Agastian, P. In Vitro α-Glucosidase Inhibition and Antioxidative Potential of an Endophyte Species (Streptomyces sp. Loyola UGC) Isolated from Datura stramonium L. Curr Microbiol 67, 69–76 (2013). https://doi.org/10.1007/s00284-013-0329-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-013-0329-2