Abstract
Predation by Bdellovibrio-like organisms (BLOs) results in bacterial community succession in aquatic ecosystems. The effects of nutrient loading on the distribution and phylogeny of BLOs remain largely unknown. To this end, we present our findings on BLO diversity from four north-Indian lakes that are variable in their trophic status; Nainital is eutrophic, both, Bhimtal and Naukuchiatal are mesotrophic and Sattal remains oligotrophic, respectively. Initially, total heterotrophic bacteria and BLOs were quantified by most probable number (MPN) analyses using Pseudomonas putida and Escherichia coli as prey bacteria. Total bacterial numbers were at least two-logs higher in the eutrophic lake samples compared with oligotrophic lake. Similarly, BLO numbers were approximately 39-fold higher using Pseudomonas sp., which is likely the preferred prey within these lakes. Conversely, significant differences were not observed between mesotrophic and oligotrophic BLO numbers when E. coli was used as the prey. PCR-RFLP of small subunit rDNA (SSU rDNA) of BLOs, followed by cloning, sequencing, and taxonomic categorization revealed distinct differences such that, eutrophic lake consisted of higher BLO diversity compared with mesotrophic and oligotrophic lake, most likely due to both, higher numbers and availability of a diverse population of prey bacteria resulting from nutrient loading in this ecosystem.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bacterial community succession in aquatic systems is largely believed to be a function of both, bottom up substrate supply (i.e. dissolved and particulate organic material as well as inorganic nutrients) [18] and top down factors through a variety of processes including protistan grazing and viral lysis [5, 15]. Therefore, a combination of factors including biotic (predation, viral lysis) and abiotic (salinity, water resident time, temperature) likely drive bacterial community succession in aquatic ecosystems [33]. While studying marine systems, Mou et al. [22] proposed that rather than the transient fluxes of DOC, marine bacteria are significantly controlled by viral lysis, protistan grazing, or even physico-chemical conditions. Since, both, protistan grazing and viral lyses are selective, a variety of factors including non-susceptibility, morphology, size, and motility offer protection to certain bacterial groups [24].
Further, one of the least studied processes within the aquatic microbial loop is the relatively non-specific and obligate bacterial mortality caused by the predacious Bdellovibrio-like organisms [38]. We recently reported the short-term effect of predation and proposed that BLOs exert a significant ‘sideways’ control over estuarine bacterial communities, causing shifts within the susceptible microbiota [9]. This sideways control of aquatic bacteria via BLOs is similar to other known mechanisms that include both, positive (syntrophy) and/or negative (allelopathy) effects [13]. In another study, we surveyed the taxonomic diversity of BLOs from marine, salt lake, and estuarine environments from 30 locations around the world, which clearly indicated this group to be taxonomically more diverse than previously thought [26]. For the most part, halotolerant BLOs are grouped under clusters III, IV, V, IX, X, XI, XII, XIII, and three outlier isolates (GSL371, NZ7, and IP1), based on ≥96.5% of 16S rDNA sequence similarity. All the freshwater or terrestrial isolates are included in the other five clusters (I, II, VI, VII, VIII) [26]; clusters I and VII have recently been included within Peredibacteraceae family [27].
Previous reports have shown a direct correlation between the trophic status of the environment to bacterial numbers and even activities [8, 14, 20, 29, 40]. In another study, total bacterial biomass was found to increase 2-fold in hypertrophic vs. oligotrophic streams [3]. This leads to the question—whether nutrient status of an ecosystem has any influence on the diversity and abundance of predacious Bdellovibrio-like organisms? BLOs are obligately dependent on native prey bacteria for their propagation, such that the extremely high metabolic activity of free-living BLOs can lead to rapid starvation if prey cells are not encountered. Therefore, in all likelihood, BLO environmental niche preferences are not merely a function of the physico-chemical conditions but more importantly, likely upon the availability of a suite of susceptible prey bacteria [7, 26]. Since nutrient loading generally results in enrichment of bacterial species, abundance and even diversity of total assemblages [14, 20, 29, 40], we hypothesized that nutrient-rich systems should possess an increased diversity of obligate bacterial predators, triggered by the availability of both, higher numbers and diversity of native prey microbiota. Results presented here from lakes with variable trophic status validate our hypothesis.
Methods
Sampling Site Details
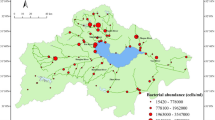
Samples were collected in June 2006 along piers from Nainital, Bhimtal, Naukuchiatal, and Sattal. These lakes are situated in Northern India at an altitude of 1220—1937 m, at latitude 29oN, within a short distance of each other (Fig. 1). These lakes are small with the exception of Bhimtal, which is the largest covering approximately 72 ha. Naukuchiatal has been found to be the deepest at approximately 40.8 m. Water samples from each lake were obtained within an area of approximately 25 m2 by submerging a sterile container to an approximate depth of 0.5 m, placed in a cooler and shipped to the laboratory at Florida A&M University, Tallahassee, USA. Replicate samples were separately and gently passed through 0.8 μm filters to exclude debris and grazers [6, 7], and stored at 4°C. Experiments were set up within a week after sampling.
Samples for this study were collected from the water column at Nainital (Eutrophic), Bhimtal, Naukuchiatal (mesotrophic), and Sattal (oligotrophic) lakes of North India, as shown in the map. Adapted from http://www.fao.org/docrep/003/x2614e/x2614e05.htm#P5_0
Physicochemical Parameters
Selected sample parameters such as temperature, conductivity, pH, and dissolved oxygen were measured by a Portable Water Checker U-10 probe (Horiba, Kyoto, Japan).
Heterotrophic Bacteria and BLO MPNs
BLOs were quantified by the 3-tube dilution-most probable numbers (MPNs) assay as previously described [7]. Each dilution series was amended with a dense suspension of overnight grown culture of either Pseudomonas putida or E. coli strains (final OD600 of 0.5 nm). Tubes that showed a reduction in absorbance compared to control tubes that contained only prey cells were considered positive. The presence of BLO predators in tubes showing positive response was further confirmed by acridine orange staining and epifluorescence microscopy. Similarly, 3-tube MPN dilutions were set up in nutrient broth for estimating total bacteria in the lake samples; dilution series were scored positive based on growth of bacteria at 600 nm.
Nucleic Acid Extraction and PCR Amplification
DNA from environmental samples and terminal MPN dilution series were extracted using the UltraClean water nucleic acid extraction kit (MoBio, Solana Beach, CA), with the exception that the DNA was eluted in sterile PCR-grade water for downstream processing. Quality of the DNA was evaluated by electrophoresis through a 0.7% agarose gel with Tris-acetate-EDTA (TAE) buffer, and quantity was estimated by the NanoDrop technique (Thermo Fisher Scientific, DE). Primers for PCR amplification of total bacterial 16S rDNA genes were 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-GGCTACCTTGTTACGACTT-3′) [19]. BLO diversity was assessed by specific primers that target either the freshwater or marine BLOs; Bd529F (5′-GGTAAGACGAGGGATCCT-3′)-Bd1007R (5′-TCTTCCAGTACATGTCAAG-3′) or Bac676F (5′-ATTTCGCATGTAGGGGTA-3′)-Bac1442R (5′-GCCACGGCTTCAGGTAAG-3′) [11]. For samples that failed to amplify with the BLO-specific primers, a semi-nested approach was followed by first amplifying the sample with BLO-specific forward primer and ‘universal’ bacterial reverse primer—1492R. PCR product from this first amplification was then used as template and amplified with BLO-specific primers. All amplifications were performed in a myCycler (Bio-Rad, Hercules, CA) using HotStartTaq Master Mix (Qiagen, Valencia, CA). PCR programs were those earlier reported [6–9]. PCR products (5 μl) were analyzed by electrophoresis through a 1% agarose gel in TAE buffer to check for estimated size and purity.
Cloning of 16S rDNA and RFLP Analyses
16S ribosomal gene was cloned using fresh PCR products ligated into pCRII-TOPO cloning vector and transformed into E. coli TOP10F′ cells according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA). Clones were screened on indicator plates made in Luria–Bertani agar supplemented with 50 μg/ml of kanamycin, X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside), and IPTG (isopropyl-β-d-thiogalactopyranoside). Colony PCR was performed on the positive clones by using T7 (5′-GTAATACGACTCACTATAGGGC-3′) and SP6 (5′-ATTTAGGTGACACTATAG-3′) primers homologous to the RNA polymerase binding sites that flank the insertion site in the vector. Restriction fragment length polymorphism (RFLP) analyses were conducted by separately digesting 10 μl of PCR products using restriction enzymes HhaI and HaeIII with subsequent analyses in a 2.5% agarose gel. Combinations of these enzymes were able to clearly distinguish the BLO community. This was also confirmed by in-silico analyses using CloneMap v2.11 software (CGC Scientific Inc., MO) and previously published enzyme selections for BLO species [11]. Clone libraries were subjected to rarefaction using aRarefactWin (version 1.3; S. Holland, Stratigraphy Lab, University of Georgia, Athens (http://www.uga.edu/~strata/software/) to confirm that sufficient numbers of RFLP groups were sequenced to represent BLO diversity originating from different lake samples.
DNA Sequencing and Phylogenetic Analysis
Restriction banding pattern of the clone libraries were compared by RFLP and grouped in operational taxonomic units (OTUs). Selected clones were then sequenced at the DNA Sequencing Laboratory at Florida State University with Bd529F/Bac676F/27F primer. Chimera evaluation was performed online via Belerophon, available at the RDP-II website [10]. Sequences generated from this study were compared with sequences contained in the National Center for Biotechnology Information database using the Basic Local Alignment Search Tool [2]. Sequences were aligned with ClustalX v. 1.8 [36], and evolutionary relationships among taxa were inferred using the maximum parsimony method using MEGA4 software [35]. The bootstrap consensus tree that was inferred from 100 replicates is shown in Fig. 2 to represent the evolutionary relationships of taxa identified in this study. Branches that resulted in bootstrap values of less than 50% have been shown as collapsed. The positions that contained gaps or data that was missing were eliminated. This analysis resulted in a total of 559 positions, 110 of which were found to be parsimony informative.
Phylogenetic tree represents partial BLO 16S rDNA gene sequences from eutrophic (Nainital), mesotrophic (Bhimtal and Naukuchiatal), and oligotrophic (Sattal) Kumaon lakes of North India. Phylotree represents evolutionary relationships among taxa inferred using the maximum parsimony method using MEGA4 software. Numbers at nodes represent bootstrap values (100 times resampling analysis); only values >50% are presented. Bacteriovorax sp. ETC. (NCBI Accession number AY294217) was used as outgroup
Statistical Analyses
BLO sequences generated from the four lakes were subjected to statistical analyses using UniFrac [21] as described in our recent reports [7, 9]. Comparative analyses were run to test which environments significantly differed using P-test, UniFrac metric test, and Principal Coordinate Analyses (PCA) with the scatter plot option. Further analyses were also performed by a lineage-specific analysis, applying the G-test of significance to each BLO lineage separately to determine whether sequences had different distributions over the lakes with variable trophic status.
Nucleotide sequence accession numbers
The partial 16S rRNA gene sequences obtained in this study are available in GenBank under accession numbers of FJ405259-FJ405272 (BLO sequences) and FJ405273-FJ405278 (Bacterial sequences).
Results and Discussion
Biogeochemical Parameters and Historical Perspective of Kumaon Lakes
The values for selected environmental parameters in the four lake samples were within a close range; temperature (25–27°C), pH (8.5–9.0), and dissolved oxygen (6–14 mg/l). Generally, these lakes are stratified in spring but the water column is well mixed during winter. These lakes are variable in their trophic states; Nainital is eutrophic, with carbonate values in the range of 288 mg l−1, nitrogen 512 g l−1, and phosphorus 22.5 g l−1; Bhimtal and Naukuchiatal have intermediate levels of nutrients in the range of being mesotrophic and Sattal is poor in nutrients [32]. Conductivity in these lakes are in the range of 350–500 μmhos (Nainital), 350–500 μmhos (Bhimtal), 120–200 μmhos (Naukuchiatal), and 110–210 μmhos (Sattal), respectively [23, 32]. Of major concern is the rising concentration of nitrogen in Lake Nainital [23]. These studies indicate that a combined action of a net increase in biological production and significantly higher organic matter production and deposition within the sediments have resulted in anoxic conditions in the bottom water layers. Resultant eutrophication has caused considerable fish kills from Nainital and Naukuchiatal, which are more severe during winter months. To compound problems, these lakes increasingly witness blooms of aquatic macrophytes such as Polygonum, Hydrilla, Potamogeton, Myriophyllum, and Vallisneria. Additionally, lake waters and sediments also receive large amounts of detritus from human recreational and fishing activities on a year round basis.
Bacterial and BLO MPNs
Given that the trophic status of lake ecosystems have significant influences on total bacterial communities [14, 20, 29, 40], we measured predator–prey numbers in lakes with variable trophic status. Estimates of the numbers of total bacteria and BLOs in water samples collected from the four lakes were determined by the 3-tube MPN method. These results are shown in Table 1. Total bacterial numbers were approximately two-log higher in eutrophic (Nainital) site as compared to the oligotrophic (Sattal) site. Intermediate numbers and variability were observed between the two mesotrophic sites; Bhimtal consisted of higher numbers than Naukuchiatal.
BLO numbers corresponded very well to the numbers of the allochthanous bacteria, such that samples with higher bacterial numbers also had higher numbers of BLOs. An approximately, 39-fold higher BLO numbers were observed from eutrophic lake compared with oligotrophic lake, when Pseudomonas putida was used as prey (Table 1). Interestingly, these numbers were significantly lower when E. coli was the prey bacterium, indicating that some bacteria, such as Pseudomonas in this study, are preferred by BLOs over others as shown previously [30, 31, 34]. Recently, we utilized a stable isotope probing approach using 13C-labeled prey bacteria and found Vibrio spp., as one of the most preferred prey for estuarine BLOs [9]. Mechanisms behind such prey preferences are not very clear; it is likely that susceptible prey may contain motifs or receptor sites in their cell wall surfaces that are recognized by BLOs during the predatorial response.
Direct relationships between bacterial abundances and viral predators have been reported previously [1]. We observed a similar relationship between BLOs and their prey bacterial community, such that samples from eutrophic lakes consisted of both, higher bacterial and predator numbers (Table 1). This data is consistent with our recent tidal study, in which BLO numbers and diversity responded rapidly to an increase in the numbers of available prey species in an estuarine system [9]. Given that BLOs are highly motile and chemotactic towards autochthanous environmental bacteria [6], which is a function of prey cell densities [37], in all likelihood, increased numbers of potential prey bacteria at nutrient enriched sites elicited the stronger response and diversity from the BLO community (Table 1). A similar response of BLOs has been observed earlier in which higher numbers were found in environments abounding with prey, such as biofilms [7, 39] and soils [16]. Biofilms harbor high densities of bacteria; upto 108–109 cells/ml has been reported, which are 100–10,000-fold higher than the water column [28]. Correspondingly, BLO guilds were found in higher numbers from aquatic biofilms or submerged surfaces but not in the surrounding waters [17, 40]. Although BLO numbers represent only those guilds that were able to attack and consume prey used in this study and MPNs, like other culture-based methods, underestimate bacterial abundances, nevertheless, the results indicate a quantitative and tightly coupled relationship between bacterial predator–prey interactions in a natural setting which appeared to be a function of eutrophication. Further, acridine orange staining confirmed that the drop in optical density of MPN tubes was due to the predation activity of BLOs; cleared tubes showed abundant, highly motile community of small sized BLOs that were distinguishable from other bacteria cells (data not shown).
Phylogenetic Analysis
BLO diversity from the lake samples was assessed by PCR-RFLP followed by 16S rDNA gene sequence analysis. Phylotypes were assigned to operational taxonomic unit (OTUs) based on RFLP banding patterns. Forty-eight clones were screened from each library; Nainital samples consisted of 5 OTUs, Bhimtal, Naukuchiatal, and Sattal consisted of 3 OTUs each. Rarefaction curves indicated that sufficient numbers of clones were sequenced from each sample to represent the BLO diversity (data not shown). Relative distribution of sequences within the individual lake clone libraries are presented in Table 2. At least two clones from each OTU were sequenced and taxonomically identified by BLAST.
Currently, marine/estuarine BLO species belong to clusters III, IV, V, IX, X, XI, XII, XIII and freshwater/terrestrial species are grouped under clusters I, II, VI, VII, VIII with three outlier isolates (GSL371, NZ7, and IP1) [26]. More recently, clusters I and VII were reclassified within Peredibacteraceae family [27]. Table 2 represents relative phylotype abundances of BLO clusters that were identified from Kumaon lakes with variable trophic status. Of major interest, were BLO clusters I and IV, which were identified from all sites regardless of trophic status (Fig. 2, Table 2). The relative distribution of cluster IV was more than 55% in mesotrophic lakes. Therefore, these clusters may represent ‘generalist’ BLO species such that they can survive along a wide gradient of nutrient concentrations. Additionally, Nainital (eutrophic) samples consisted of clusters VII and XI which were not found in any other sites; these likely represent those BLO communities that are ‘specialists’ in utilizing particular prey species that may not be present in other lakes. BLO sequences common between eutrophic and mesotrophic sites (Bhimtal and Naukuchiatal) aligned with cluster II.
In addition, the oligotrophic site, Sattal, consisted of BLO sequences that aligned with cluster V, which were not identified from other lake samples. Our previous studies have shown that cluster V represents a unique clade that has, thus far, been recovered only from low salinity regions of Chesapeake Bay, Pamilco Sound/Neuse River area, and Apalachicola Bay systems [7, 9, 26]. In all likelihood, cluster V, along with clusters IV and XI identified from these freshwater lakes, represent a broad category of ‘generalist’ BLO species that are well adapted to survive in both, brackish and freshwater habitats. Identification of BLO clusters previously identified only from halophilic environments have also been shown by Davidov et al. [11], while studying the diversity of Bdellovibrio and like organisms in soils. It is very likely that some BLO species, such as clusters IV, V, and XI identified in this study, represent those relatives of halo tolerant BLO species that are adapted to freshwater or brackish environments during evolutionary processes.
Additionally, 16S rDNA from the last two positive bacteria and BLO MPN dilution series were cloned to identify the dominant predator and prey from all four lakes. Regardless of trophic status, we identified BLO cluster IV (sequence identity, 98%–99%) and Gamma-proteobacteria—Stenotrophomonas sp. (89%–96%) from all terminal dilutions. Additionally, from eutrophic sub-terminal dilution series, Xanthomonas bacterium LA37 (82%) was identified, which also represents Gamma-proteobacteria. Other bacterial species identified from eutrophic and mesotrophic dilution series clustered with Beta-proteobacterium—Achromobacter xylosoxidans strain TF (79%) and Achromobacter xylosoxidans strain TFD47 (76%). It appears that Gamma-proteobacteria and specifically, Stenotrophomonas sp., may be the dominant cultivable bacteria from within these lake ecosystems. This appears to be somewhat contrary to the previous findings which propose Gamma-proteobacteria to be of minor importance in fresh water systems, which are thought to be mainly dominated by Beta-proteobacteria [4]. Stenotrophomonas spp., are affiliated to Xanthomonadaceae and have been identified from lake plankton [12] and high activity of Stenotrophomonas spp., has been found to coincide with a spike in the concentrations of ammonium [25]. We speculate that the dominance of Stenotrophomonas spp., in these lakes is likely due to anthropogenic influences. As documented in other studies, these lakes receive increased influx of organic carbon by terrestrial runoff and recreational activities that is likely enriching for potentially pathogenic bacteria, as has been shown previously in blooms of Stenotrophomonas spp., in other systems [25].
We conclude that niche preferences for obligatory predacious BLO species are based on both, physico-chemical conditions and also availability of a diverse bacterial community on which they proliferate. Since the eutrophic and mesotrophic sites in this study consisted of significantly higher prey communities, it resulted in higher numbers and diversity of BLOs. Conversely, we observed lower numbers and diversity in the oligotrophic site (Table 1, Fig. 2), potentially due to limiting nutrients in these sites that results in a lower diversity of prey. Ecological implications of this study are, however, limited since we were unable to examine spatio-temporal variations in the predator–prey community, due in part to being limited on further sampling of these lakes. If such variables were taken into account, the results would likely be more conclusive on the effects of nutrient loading on BLO guilds and resultant carbon cycling potential within the microbial loop in aquatic ecosystems.
Statistical Analyses
Statistical analyses was performed by UniFrac to determine which of the environments i.e., eutrophic, mesotrophic, or oligotrophic consist of similar BLO diversity. Prior to UniFrac analysis, the 16S rRNA sequences were grouped based on their origin i.e., Nainital (eutrophic), Bhimtal, Naukuchiatal (mesotrophic), and Sattal (oligotrophic). Principal coordinates axis are shown in Fig. 3; BLO species from eutrophic sites separated out from those that were identified from ologotrophic sites; mesotrophic BLO species segregated out on a third PCA axes. This suggested that significant differences exist between BLO species present along these trophic gradients. Further, based on the P-test values, eutrophic sequences were found to be significantly different (<0.001) than oligotrophic sequences. PCA axis 1 explained 63.52% of the variability, and PCA axis 2 explained 36.48%, with a cumulative percentage of 100%, which provided for a strong statistical correlation to other data.
Shown are the first two principal coordinates from a principal coordinate analysis (PCA) after sequences that originated from eutrophic (Nainital), mesotrophic (Bhimtal and Naukuchiatal), and oligotrophic (Sattal) lakes were subjected to UniFrac analyses. Percentages represented in the axis labels are percentages of variation that are explained by the principal coordinates. PCA analyses indicate that BLO diversity is a function of the nutritional status of the ecosystem
Eutrophication impacts are known to last for decades and include a variety of changes to the ecosystem such as increased bacterial numbers and activities [3, 8, 14, 20, 29, 40]. Taken together, these results are indicative that nutrient enrichments result in increased diversity of specific bacterial processes such as predation by BLOs, which potentially impact carbon cycling within the aquatic microbial food web processes.
References
Almeida MA, Cunha MA, Alcantara F (2001) Loss of estuarine bacteria by viral infection and predation in microcosm conditions. Microb Ecol 42:562–571
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Baldy V, Gobert V, Guerold F, Chauvet E, Lambrigot D, Charcosset JV (2007) Leaf litter breakdown budgets in streams of various trophic status: effects of dissolved inorganic nutrients on microorganisms and invertebrates. Freshwater Biol 52(7):1322–1335
Bouvier TC, del Giorgio PA (2002) Compositional changes in free-living bacterial communities along a salinity gradient in two temperate estuaries. Limnol Oceanogr 47:453–470
Bouvier T, del Giorgio PA (2007) Key role of selective viral-induced mortality in determining marine bacterial community composition. Environ Microbiol 9(2):287–297
Chauhan A, Williams HN (2006) Response of Bdellovibrio and like organisms (BALOs) to the migration of naturally occurring bacteria to chemo attractants. Curr Microbiol 53(6):516–522
Chauhan A, Williams HN (2007) Biostimulation of estuarine microbial community on substrate coated agar slides: a novel approach to study the phylogeny of Bdellovibrio-and like organisms. Microb Ecol 55:640–650
Chauhan A, Ogram A, Reddy KR (2004) Syntrophic-methanogenic associations along a nutrient gradient in the Florida Everglades. Appl Environ Microbiol 70:3475–3484
Chauhan A, Cherrier J, Williams HN (2009) Impact of sideways and bottom up control factors on bacterial community succession over a tidal cycle. Proc Natl Acad Sci. doi:10.1073/pnas.0809671106. http://www.pnas.org/cgi/doi/10.1073/pnas.0809671106
Cole JR, Chai B, Marsh TL, Farris RJ, Wang Q, Kulam SA, Chandra S, McGarrell DM, Schmidt TM, Garrity GM, Tiedje JM (2003) The ribosomal database project (RDP-II): previewing a new autoaligner that allows regular updates and the new prokaryotic taxonomy. Nucleic Acids Res 31:442–443
Davidov Y, Friedjung A, Jurkevitch E (2006) Structure analysis of a soil community of predatory bacteria using culture-dependent and culture-independent methods reveals a hitherto undetected diversity of Bdellovibrio-and-like organisms. Environ Microbiol 8(9):1667–1673
De Wever A, Muylaert K, Van der Gucht K, Pirlot S, Cocquyt C, Descy JP, Plisnier PD, Vyverman W (2005) Bacterial community composition in Lake Tanganyika: vertical and horizontal heterogeneity. Appl Environ Microbiol 71:5029–5037
Fuhrman JA, Hagstrom Ake (2008) Bacterial and archaeal community structure and its pattern. In: Kirchman DL (ed) Microbial ecology of the oceans, 2nd edn. John Wiley, New Jersey
Jones JG, Orlandi MJLG, Simon BM (1979) A microbiological study of sediments from the Cumbrian lakes. J Gen Microbiol 115:37–48
Jürgens K, Matz C (2002) Predation as a shaping force for the phenotypic and genotypic composition of planktonic bacteria. Antonie Van Leeuwenhoek 81(1–4):413–434
Jurkevitch E (2000) The genus Bdellovibrio. In: Dworkin M et al. (eds) The Prokaryotes: an evolving electronic resource for the microbiological community, 3rd edn, release 3.7, November 2, 2001, Springer-Verlag, New York. http://link.springer-ny.com/link/service/books/10125/
Kelley JI, Williams HN (1992) Bdellovibrios in Callinectus sapidus, the blue crab. Appl Environ Microbiol 58:1408–1410
Kirchman DL, Dittel AI, Findlay SEG, Fisher D (2004) Changes in bacterial activity and community structure in response to dissolved organic matter in the Hudson River, New York. Aquat Microb Ecol 35:243–257
Lane DJ (1991) 16S/23S rRNA sequencing. In: Goodfellow M, Stackebrandt E (eds) Nucleic acid techniques in bacterial systematics. Wiley, New York, pp 115–175
Lindstrom ES (2000) Bacterioplankton community composition in five lakes differing in trophic status and humic content. Microb Ecol 40:104–113
Lozupone C, Knight R (2005) UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol 71(12):8228–8235
Mou X, Sun S, Edwards RA, Hodson RE, Moran MA (2008) Bacterial carbon processing by generalist species in the coastal ocean. Nature 451(7179):708–711
Pant MC, Sharma PC, Sharma AP (1985) Physico-chemical limnology of Lake Nainital, Kumaon Himalaya (U.P.), India. Acta Hydrochim Hydrobiol 13(3):331–349
Pernthaler J (2005) Predation on prokaryotes in the water column and its ecological implications. Nat Rev Microbiol 3:537–546
Piccini C, Conde D, Alonso C, Sommaruga R, Pernthaler J (2006) Blooms of single bacterial species in a coastal lagoon of the Southwestern Atlantic Ocean. Appl Environ Microbiol 72:6560–6568
Pineiro SA, Stine OC, Chauhan A, Steyert SR, Smith R, Williams HN (2007) Global survey of diversity among environmental saltwater Bacteriovoracaceae. Environ Microbiol 9(10):2441–2450
Piñeiro SA, Williams HN, Stine OC (2008) Phylogenetic relationships amongst the 435 saltwater members of the genus Bacteriovorax using rpoB sequences and reclassification of 436 Bacteriovorax stolpii as Bacteriolyticum stolpii gen nov., comb.nov. Int J Syst Evol Microbiol 58:1203–1209
Ploug H, Grossart HP (2000) Bacterial growth and grazing on diatom aggregates: respiratory carbon turnover as a function of aggregate size and sinking velocity. Limnol Oceanogr 45:1467–1475
Porter J, Morris SA, Pickup RW (2004) Effect of trophic status on the culturability and activity of bacteria from a range of lakes in the English Lake District. Appl Environ Microbiol 70(4):2072–2078
Sanchez-Amat A, Torrella F (1990) Formation of stable Bdelloplasts as a starvation-survival strategy of marine Bdellovibrios. Appl Environ Microbiol 56(9):2717–2725
Schoeffield AJ, Williams HN (1990) Efficiencies of recovery of Bdellovibrios from brackish-water environments by using various bacterial species as prey. Appl Environ Microbiol 56:230–236
Sharma PC, Pant MC (1985) Species composition of zooplankton in two Kumaon lakes (U.P.). Arch Hydrobiol 102(3):387–402
Strom SL (2008) Microbial ecology of ocean biogeochemistry: a community perspective. Science 320(5879):1043–1045
Sutton DC, Besant PJ (1994) Ecology and characteristics of Bdellovibrios from three tropical marine habitats. Mar Biol 119:313–320
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 24:4876–4882
Varon M, Shilo M (1968) Interaction of Bdellovibrio bacteriovorus and host bacteria I. Kinetic studies of attachment and invasion of Escherichia coli B by Bdellovibrio bacteriovorus. J Bacteriol 95:744–753
Williams HN, Pineiro S (2006) Ecology of the predatory Bdellovibrio and like organisms. In: Jurkevitch E (ed) Predatory Prokaryotes. Springer-Verlag, Berlin, pp 214–244
Williams HN, Scheoffield AJ, Guether D, Kelley J, Shah D, Falker WA (1995) Recovery of Bdellovibrios from submerged surfaces and other aquatic habitats. Microb Ecol 29:39–48
Yannarell AC, Kent AD, Lauster GH, Kratz TK, Triplett EW (2003) Temporal patterns in bacterial communities in three temperate lakes of different trophic status. Microb Ecol 46(4):391–405
Acknowledgments
Funding for this study was provided by National Science Foundation’s Historically Black Colleges and Universities Research Infrastructure for Science and Engineering (HBCU-RISE) initiative grant (No. 0531523). Partial support was also obtained from Department of Education’s Title III program and Faculty Research Development Program awarded to AC through FAMU’s RCMI program sponsored by the National Institute of Health.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chauhan, A., Fortenberry, G.Z., Lewis, D.E. et al. Increased Diversity of Predacious Bdellovibrio-Like Organisms (BLOs) as a Function of Eutrophication in Kumaon Lakes of India. Curr Microbiol 59, 1–8 (2009). https://doi.org/10.1007/s00284-009-9385-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-009-9385-z