Abstract
Purpose
To evaluate the outcomes of intraperitoneal chemotherapy (IP) compared with those of intravenous chemotherapy (IV) in patients with advanced ovarian cancer after neoadjuvant chemotherapy (NACT) and interval debulking surgery (IDS) or primary debulking surgery (PDS).
Methods
Patients with advanced epithelial ovarian carcinoma treated with PDS or NACT and IDS from 2006 to 2015 were identified. Comparative statistics were used to evaluate covariates, and survival rates were calculated using the Kaplan–Meier method and compared with log-rank tests.
Results
Sixty-six patients received NACT followed by IDS with residual disease of ≤ 1 cm; 42 of these patients (63.6%) received IP therapy; and 24 patients (36.3%) had IV therapy only after IDS. The median progression-free survival (PFS) was 16.0 months in the IP group and 13.5 months in the IV group (p = 0.13). The estimated median overall survival (OS) was 64.0 months with IP and 50.0 months with IV (p = 0.44). During the same study period, 149 patients underwent optimal PDS after which 93 patients (62.4%) received IP and 56 patients (37.6%) were given IV chemotherapy. Patients after IP demonstrated improved survival outcomes when compared to patients after IV therapy. The median PFS was 28.0 months after IP and 16.5 months after IV (p = 0.0006), and the median OS was not reached for IP and 50.0 months after IV (p < 0.0001).
Conclusions
Although IP chemotherapy after PDS is associated with improved survival, IP therapy after NACT and IDS, despite high rates of completion, may not have the same degree of survival advantage over IV therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Standard treatment of ovarian cancer involves primary debulking surgery (PDS) followed by platinum–taxane-based chemotherapy. At a minimum, the aim of PDS is optimal debulking defined by disease less than 1 cm in maximum diameter at the completion of surgery, with the preferred goal to resect all visible disease. However, optimal debulking may not be feasible in cases with extensive tumor burden preventing complete resection or coexisting medical comorbidities that deem patients unfit for surgical intervention.
Suboptimal debulking defined as residual disease greater than 1 cm in maximum diameter at PDS confers a significantly worse survival than PDS with optimal debulking [1, 2]. For patients who are unlikely to achieve optimal PDS, an alternative treatment strategy of neoadjuvant chemotherapy (NACT) followed by interval debulking surgery (IDS) was adopted in an effort to reduce the rates of suboptimal PDS and, in turn, theoretically improve survival. Two large randomized-controlled phase III trials have shown that this regimen was not inferior to PDS for patients with advanced stage epithelial ovarian cancer [3, 4]. Though controversial, these results support NACT with IDS as an acceptable treatment option for patients who are unlikely to achieve optimal PDS. Nationally, there has been a gradual increase in the uptake of NACT for patients with both stage III and IV diseases [5].
Platinum–taxane-based intraperitoneal (IP) chemotherapy has been shown to improve survival in optimally debulked advanced stage ovarian cancer patients in several randomized--controlled trials [6, 7]. The Gynecologic Oncology Group (GOG) 172 trial found a 16-month improvement in overall survival in patients treated with IP chemotherapy after optimal PDS compared to patients treated with the conventional intravenous (IV) therapy [8]. This led the National Cancer Institute (NCI) to issue a clinical statement endorsing the use of IP chemotherapy in this patient population [9].
A standard chemotherapy regimen has not been established in patients who undergo NACT with optimal IDS. At our two institutions, we have been recommending IP chemotherapy after NACT and optimal IDS based on the prior phase III studies after optimal PDS and the NCI clinical announcement, hypothesizing that this strategy would improve survival outcomes relative to additional IV chemotherapy after IDS. The purpose of this study is to evaluate the progression-free survival (PFS) and overall survival (OS) of IP chemotherapy compared with that of IV chemotherapy in our patients with epithelial ovarian cancer treated with NACT followed by IDS as well as to compare the survival rates of IP and IV chemotherapy following PDS.
Materials and methods
After Institutional Review Board approval was obtained, all patients diagnosed with FIGO stage IIIC or IV epithelial ovarian, fallopian tube or primary peritoneal carcinoma at our two academic tertiary care centers between June 2006 and May 2015 were identified. Patients who received nonplatinum-based chemotherapy regimens and patients who did not receive their chemotherapy after PDS or IDS at our institutions were excluded. Patient demographics, clinical data, chemotherapy details, and survival outcomes were abstracted from medical records.
The decision to proceed with PDS or NACT was based on multiple factors including the extent of disease assessed on exam and imaging and the patient’s performance status, age, and medical comorbidities. Prior to NACT initiation, the diagnosis of Müllerian carcinoma was confirmed by biopsy or cytology along with CA-125/CEA ratio greater than 30. The utility and timing of IDS for eligible patients were decided upon review after at our divisional treatment planning conference.
Debulking status was determined by operative findings reporting the largest tumor size at the completion of cytoreductive surgery. Optimal debulking was defined as maximal tumor diameter less than or equal to 1 cm, while suboptimal debulking was assigned if the largest tumor diameter was greater than 1 cm. Patients who received at least one IP chemotherapy cycle following optimal IDS were categorized as the IP group, while patients who only received IV chemotherapy following optimal IDS were designated as the IV group. Disease recurrences were identified by measurable disease on imaging, which was obtained as clinically indicated, or pathology. PFS was measured from the date of diagnosis to the date of first recurrence, while OS was measured from the date of diagnosis to the date of death or last contact.
Median values and standard deviations were used to describe continuous data, and categorical variables were displayed as totals and frequencies. Categorical covariates were compared using Chi-square analyses, and continuous covariates were compared using two-tailed independent samples t tests, and Mann–Whitney tests. Survival rates were calculated using the Kaplan–Meier method. Log-rank tests and Cox regression analyses were used to determine associations with patient characteristics, stage, residual disease, BRCA mutation status, and treatment routes for univariate and multivariate analyses, respectively. All statistical analyses were performed using R Studio. The two-sided significance level was set at p < 0.05.
Results
Breakdown of all patients
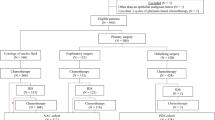
Two hundred and sixty-five subjects were diagnosed with FIGO stage IIIC or IV epithelial ovarian, tubal, or primary peritoneal carcinoma during this time period (Fig. 1), and of these, 178 underwent PDS (67.2%) and 87 received NACT (32.8%). Among the patients who had PDS, 149 were optimally cytoreduced (83.7%).
Characteristics of patients who underwent NACT followed by IDS
Among the 87 receiving NACT, 67 underwent IDS (77.0%) and all but three were optimally cytoreduced. The remaining 17 patients (19.5%) either died of disease or had disease progression during NACT and subsequently started the second-line chemotherapy without undergoing IDS.
After receiving NACT followed by optimal IDS, 42 of 66 patients received IP chemotherapy (63.6%), while the remaining 24 patients received IV chemotherapy (36.4%). Genetic testing in 35 of 66 patients (53.0%) identified BRCA mutations in 11 patients (11 of 35, 31.4%). Patients who underwent BRCA testing were more likely to receive IP chemotherapy following NACT. Among NACT patients, there were no significant differences in age at diagnosis, body mass index, race, origin of disease, histologic subtype, stage, tumor grade, residual disease at the conclusion of IDS, cancer antigen 125 (CA-125) levels, and platelet levels between the two groups (Table 1).
Of the 66 patients who underwent NACT followed by optimal IDS included in the analysis, 35 (53.0%) had stage IIIC disease and 31 (47.0%) had stage IV disease (Tables 1, 2). At the conclusion of IDS, 35 patients (53.0%) had no gross residual disease, 13 (19.7%) had residual tumor diameter of 1–5 mm, 16 (24.2%) had residual tumor diameter of 6–10 mm, and 2 (3.0%) were reported as optimal debulking without residual tumor measurements.
The median number of NACT cycles was three (range 3–8). Twenty-seven patients (40.9%) received a platinum agent (cisplatin or carboplatin) and a taxane (paclitaxel or docetaxel) every 3 weeks, and 39 patients (59.1%) received a platinum agent with divided-dose paclitaxel either every 7 or 10 days in the neoadjuvant setting. One patient received bevacizumab with her NACT regimen and was excluded from the analysis.
IP ports were placed at the time of IDS in 44 patients (44 of 66, 66.7%) and after IDS in one patient (1 of 66, 1.5%). Twenty-one patients (21 of 66, 31.8%) did not have IP ports placed. Of the 45 patients with IP ports, 42 received at least one cycle of IP chemotherapy (42 of 45, 93.3%). Six of the forty-two patients (14.3%) who received IP chemotherapy were unable to complete the prescribed dose of three or four cycles and subsequently were switched to IV chemotherapy. Of the six patients who discontinued IP chemotherapy, five were due to adverse effects and one was due to IP port extrusion. The remaining 24 patients received IV chemotherapy exclusively (24 of 66, 36%).
For all patients after NACT and IDS, the median number of postoperative chemotherapy cycles was three (range 2–6). The median number of postoperative cycles for the IP group was four (range 2–6) and that for the IV group was three (range 1–6). The majority of the IP group patients (27 of 42 patients, 64.3%) were treated with either the GOG 172 regimen or a modified GOG 172 regimen consisting of IV paclitaxel, IP cisplatin, and IP paclitaxel. Ten patients (23.8%) received IP cisplatin alone, two (4.8%) received IP cisplatin with IV docetaxel due to a prior reaction to paclitaxel, and the remaining three (7.1%) received IP carboplatin doublets with other chemotherapy agents. Among the IV group, 13 patients (52.0%) were administered chemotherapy every 3 weeks and 12 (48.0%) received chemotherapy every 7–10 days.
Characteristics of patients who underwent optimal PDS
There were 149 patients who underwent optimal PDS; and of these, 93 received IP therapy (62.4%) and 56 were treated with IV therapy (37.6%). Their demographics and characteristics are listed in Table 2. Patients who received IP chemotherapy were younger than those who underwent IV chemotherapy (median age 54 years versus 61 years, p = 0.004). Ninety-nine patients (66.4%) underwent genetic testing and 25 patients (11 of 99, 25.3%) were identified as BRCA mutation carriers. Genetic testing in 35 of 66 patients (53.0%) identified BRCA mutations in 11 patients (11 of 35, 31.4%). Patients who received IP chemotherapy had higher levels of CA-125 compared to patients who received IV therapy (median 458.7 versus 217.8 U/mL, p = 0.03). There were no significant differences in body mass index, race, origin of disease, histologic subtype, stage, tumor grade, BRCA mutation status, residual disease at the conclusion of PDS, and platelet levels between the two groups.
IP ports were placed in 101 patients (101 of 149, 69.8%); and of these, 78 were placed at the time of PDS (78 of 101, 77.2%) and 23 were placed postoperatively (23 of 101, 22.8%). Of the 101 patients with IP ports, 93 patients received at least one cycle of IP chemotherapy (92.1%). Twenty-seven patients (27 of 93, 29.0%) were unable to complete the prescribed dose of six cycles and were switched to IV chemotherapy. Of the 27 patients who discontinued IP chemotherapy, 16 (59.3%) were due to adverse effects, 4 (14.8%) were due to patient preference for IV chemotherapy, 3 (11.1%) were due to IP port malfunction, 2 (7.4%) were due to port extrusion, and 2 (7.4%) were discontinued due to unknown reasons.
The median number of chemotherapy cycles was six (range 2–8) for the entire cohort and also six for both the IP group and the IV group. Among the IP group, 71 (71 of 93, 76.3%) were treated with either the GOG 172 or a modified GOG 172 regimen including IV paclitaxel, IP cisplatin, and IP paclitaxel. Nine patients (9 of 93, 9.7%) were administered IP cisplatin alone and six patients (6 of 93, 6.5%) were given IP cisplatin with IV paclitaxel. For seven patients, their chemotherapy regimens were unknown. Among the patients who exclusively received IV chemotherapy, 30 patients (30 of 56, 53.6%) received chemotherapy every 3 weeks, 24 patients (24 of 56, 42.9%) were given chemotherapy every 7–10 days, and for 2 patients, the chemotherapy regimens were unknown.
Survival outcomes for patients who underwent NACT and IDS and patients after PDS
Of the 66 patients who received chemotherapy after IDS, six are without evidence of disease (9.1%), 39 recurred and are currently alive (59.1%), and 21 recurred and died of disease (31.8%), over a median follow-up period of 52.0 months (range 4.0–123.0 months). Specifically, the median follow-up time for the 6 patients without a recurrence was 36.0 months (range 18.0–71.0 months). In the IP group, PFS was 16.0 months (Fig. 2a) and OS was 64.0 months (Fig. 2b) compared to a median PFS of 13.5 months and OS of 50.0 months in the IV group. The median PFS after IDS was 11.5 months in the IP group and 10.0 months in the IV group. These differences in survival are not statistically significant. When evaluating the 35 patients who underwent IDS to no gross residual, there remained no difference in PFS and OS between the IP and IV groups.
Of the 149 patients who underwent PDS, 44 are without evidence of disease (29.5%), 51 recurred and currently alive with disease (34.2%) and 54 died of disease (36.2%). The patients who underwent optimal PDS and received IP therapy demonstrated a survival benefit when compared to the patients who underwent optimal PDS followed by IV therapy. The median PFS in the IP group was 28.0 months (Fig. 2c) and median OS was not yet reached (Fig. 2d), while the median PFS in the IV group was 16.5 months (p = 0.0005) and median OS was 50.0 months (p < 0.0001). After controlling for age, stage, BRCA mutation, and residual tumor volume in a Cox proportional hazards model, IP therapy after PDS had improved PFS (HR 0.54, 95% CI 0.36–0.83, p = 0.004) and OS (HR 0.39, 95% CI 0.22–0.69, p = 0.001).
Discussion
Intraperitoneal therapy has been shown to have a survival advantage in patients with stage III epithelial ovarian cancer who have undergone optimal primary cytoreductive surgery in three randomized phase III GOG trials [6,7,8], leading to the landmark 2006 NCI clinical announcement recommending the use of IP therapy in optimally debulked patients [9]. However, the recently presented preliminary results of the phase III randomized GOG 252 trial did not conclusively show a survival difference between patients who received IV dose-dense chemotherapy and patients who received IP chemotherapy after optimal PDS [10], raising questions regarding the benefit of IP chemotherapy compared to dose-dense IV chemotherapy. The interpretation of these results remains controversial due to the addition of bevacizumab to all study arms, which is largely considered to be a confounding factor.
We studied patients who underwent PDS and patients who received NACT followed by IDS during the same time period. Our rates of IP chemotherapy administration are higher than what had been recently reported in a prospective cohort study evaluating the use of IP chemotherapy after optimal PDS at six National Comprehensive Cancer Network institutions: 62.8% of patients after PDS and 65.6% of patients after NACT and IDS received IP chemotherapy in this study compared to 41% in the prior published study [11].
Our results show a significant benefit in both PFS and OS with IP chemotherapy following PDS, but this advantage was not seen in the NACT cohort following IDS. PFS after IDS are nearly the same for the two groups (11.5 months in IP group versus 10.0 months in IV group), and the difference in OS, while favoring IP, was not statistically different, although our OS data are still immature. In addition, the difference seen may be due to an effect of the second- or third-line chemotherapies that were administered after disease recurrence.
Our survival data in the NACT cohort are consistent with two other retrospective studies. Exploratory analyses from Canada revealed that the administration of 3–4 cycles of IP chemotherapy after optimal IDS was not predictive of improved PFS [12, 13]. In these studies, patients either received IP chemotherapy using the GOG 172 study regimen or IV chemotherapy every 3 weeks. The authors found a median PFS of 14.1 months in the IP group and 18.0 months in the IV group. Their PFS values are consistent with our results in this study.
A study of 120 patients who underwent NACT followed by IDS and then went on to receive either IP chemotherapy (47, 39.2%), dose-dense IV chemotherapy (17, 14.2%), or standard every 3 weeks IV chemotherapy (56, 46.7%) showed comparable PFS among the three groups: 9.9, 7.3 and 7.2 months, respectively [14].
Results from a prospective randomized multicenter phase II study on IP therapy after NACT (OV21/PETROC study) were recently presented. The IV arm of the trial consists of a divided-dose regimen with IV paclitaxel and IV carboplatin on day 1 and IV paclitaxel on day 8 repeating every 3 weeks. The IP arm that was used in the second stage of the study comprised of IV paclitaxel and IP carboplatin on day 1 and IP paclitaxel on day 8 repeating every 3 weeks. The study met its primary endpoint, and IP chemotherapy was associated with a decreased 9-month disease progression rate when compared to IV chemotherapy in a per-protocol analysis. However, there were no significant differences seen in PFS and OS [15]. The PFS and OS in the prospective study were similar to those found in our study, but we did not detect a difference in 9-month progression rate between the IP and IV groups.
Our experience and those of others in the NACT setting may be consistent with the hypothesis of platinum resistance developing when large numbers of potentially drug resistant cancer cells are exposed to front-line platinum chemotherapy, thereby preferentially selecting for these resistant cancer cells and allowing them to continue dividing. This idea was supported by an in vitro drug resistance assay demonstrating increased platinum resistance after NACT [16]. Platinum resistance will potentially decrease the response to platinum treatment regardless of the route of administration. In addition, the scheduling of IDS after 3–4 cycles of NACT with 3–4 cycles of postoperative chemotherapy creates an unavoidable delay in the administration of front-line platinum chemotherapy. These factors may change the tumor biology and subsequently blunt the survival advantage of IP chemotherapy seen after NACT and IDS.
In addition, the lack of survival benefit shown here in the NACT setting may be attributed to the fewer number of IP chemotherapy cycles administered to our NACT patients than had been reported in the previous GOG trials. It is our practice to prescribe 3–4 IP chemotherapy cycles after IDS to limit the total number of front-line platinum-based cycles to fewer than eight. In GOG 172, patients were assigned a total of six cycles, and although only 42% completed the six IP cycles, the study showed a survival benefit [8]. In our cohort, the median number of chemotherapy cycles after IDS was three, and 50 patients (82.0%) received 3–4 chemotherapy cycles after IDS. The number of IP chemotherapy cycles after PDS necessary to achieve a survival benefit is not yet known. GOG 172 suggested that the benefit of IP therapy occurred during initial cycles as 48% received three or fewer cycles of IP chemotherapy [8]. Tewari et al., from their analysis of GOG 114 and 172, reported that patients who completed more cycles of IP therapy after PDS had a lower risk of death; however, this finding was confounded by age, as younger patients were more likely to be able to tolerate more cycles [17]. In contrast, Suidan et al. did not detect a survival difference with additional IP chemotherapy cycles [18]. It is possible that receiving 3–4 IP cycles after NACT may be insufficient to derive a survival benefit.
Our study, comprised of patients from two tertiary academic centers, adds to the growing body of work demonstrating the difference in tumor response to IP chemotherapy after NACT and IDS. We were also able to compare our survival data after NACT with our survival data after PDS from the same time period. Consistent with prior literature, our data show that survival with IP chemotherapy after PDS was significantly improved compared to IV chemotherapy after PDS, but the survival benefit was not seen in the NACT group to the same extent.
The retrospective nature of this study lends itself to inherent selection biases and unavoidable confounding factors. The patients who received IP chemotherapy following PDS were younger than the patients who were given IV chemotherapy. It is possible that patients who were fitter and healthier were more likely to be offered IP chemotherapy. When evaluating survival outcomes, we controlled for age at time of diagnosis, and still IP therapy after PDS had improved PFS and OS when compared to IV therapy. In addition, although all of our patients received platinum-based chemotherapy before and after IDS, there was some heterogeneity among the chemotherapy regimens. This variability in IV regimens may have significance as the Japanese GOG 3016 study found a significant improvement in survival with dose-dense IV chemotherapy when compared to the conventional 3 weeks IV regimen cycle [19]. In addition, with our follow-up of 34 months, our overall survival data remain immature.
Another limitation is the number of patients included in this study. It is possible that with the 66 patients who received NACT followed by IDS, the study was underpowered to see a survival difference between the IP and IV groups. However, with over 250 patients, this remains a sizable cohort over a considerable 10-year time period.
In our analysis of our institutional IP experience since the NCI clinical statement, we demonstrated a survival advantage for IP chemotherapy following PDS as seen in the previous studies but were unable to show an advantage with when IP chemotherapy was given after NACT and optimal IDS. In light of the results of OV21/PETROC and GOG 252, there is ongoing discussion regarding the benefit of IP chemotherapy compared to dose-dense IV chemotherapy as well as many questions about the effects of bevacizumab, their dosing, and dosing schedules.
We found that the administration of IP chemotherapy after optimal PDS and a significant survival advantage over the administration of IV chemotherapy, but the use of IP chemotherapy after NACT and optimal IDS did not show the same survival benefit over IV chemotherapy. Our results suggest that IP chemotherapy may not result in a significant survival benefit after NACT and IDS as it does after PDS.
References
Hoskins WJ, McGuire WP, Brady MF et al (1994) The effect of diameter of largest residual disease on survival after primary cytoreductive surgery in patients with suboptimal residual epithelial ovarian carcinoma. Am J Obstet Gynecol 170(4):974–980
Winter WE III, Maxwell GL, Tian C et al (2007) Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol 25(24):3621–3627
Vergote I, Trope CG, Amant F et al (2010) Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med 363(10):943–953
Kehoe S, Hook J, Nankivell M et al (2015) Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomized, controlled, non-inferiority trial. Lancet 386:249–257
Hinchcliff EM, Melamed M, Clemmer JT et al (2016) Trends in the use of neoadjuvant chemotherapy for advanced-stage ovarian cancer: a National Cancer Data Base study. Gynecol Oncol 141(Suppl 1):28
Alberts DS, Liu PY, Hannigan EV et al (1996) Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med 335(26):1950–1955
Markman M, Bundy BN, Alberts DS et al (2001) Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol 19(4):1001–1007
Armstrong DK, Bundy B, Wenzel L et al (2006) Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med 354(1):34–43
National Cancer Institute NCI issues clinical announcement for preferred method of treatment for advanced ovarian cancer (2006). http://ctep.cancer.gov/highlights/docs/clin_annc_010506.pdf. Accessed 2017
Walker J, Brady MF, DiSilvestro PA et al (2016) A phase III trial of bevacizumab with IV versus IP chemotherapy for ovarian, fallopian tube, and peritoneal carcinoma: an NRG Oncology Study. Gynecol Oncol 141(Suppl 1):208
Wright A, Cronin A, Milne DE et al (2015) Use and effectiveness of intraperitoneal chemotherapy for treatment of ovarian cancer. J Clin Oncol 33(26):2841–2847
Le T, Latifah H, Jolicoeur L et al (2011) Does intraperitoneal chemotherapy benefit optimally debulked epithelial ovarian cancer patients after neoadjuvant chemotherapy? Gynecol Oncol 121(3):451–454
Al Mutairi NJ, Le T (2014) Does modality of adjuvant chemotherapy after interval surgical debulking matter in epithelial ovarian cancer? Int J Gynecol Cancer 24(3):461–467
Mueller JJ, Kelly A, Zhou Q et al (2016) Intraperitoneal chemotherapy after interval debulking surgery for advanced-stage ovarian cancer: feasibility and outcomes at a comprehensive cancer center. Gynecol Oncol 143(3):496–503
Mackay HJ, Gallagher CJ, Parulekar WR et al (2016) OV21/PETROC: a randomized gynecologic cancer intergroup (GCIG) phase II study of intraperitoneal (IP) versus intravenous (IV) chemotherapy following neoadjuvant chemotherapy and optimal debulking surgery in epithelial ovarian cancer (EOC). In: Oral presentation presented at 2016 ASCO annual meeting, Chicago, IL
Matsuo K, Eno ML, Im DD et al (2010) Chemotherapy time interval and development of platinum and taxane resistance in ovarian, fallopian, and peritoneal carcinoma. Arch Gynecol Obstet 281(2):325–328
Tewari D, Java JJ, Salani R et al (2015) Long-term survival advantage and prognostic factors associated with intraperitoneal chemotherapy treatment in advanced ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol 33(13):1460–1466
Suidan RS, Zhou Q, Iasonos A et al (2015) Prognostic significance of the number of postoperative intraperitoneal chemotherapy cycles for patients with advanced epithelial ovarian cancer. Int J Gynecol Cancer 25(4):599–606
Katsumata N, Yasuda M, Takahashi F et al (2009) Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open-label, randomised controlled trial. Lancet 374(9698):1331–1338
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose.
Ethical approval
This study was approved by the ethics committee of each participating hospital and was performed in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was not required for this study.
Rights and permissions
About this article
Cite this article
Lee, J., Curtin, J.P., Muggia, F.M. et al. Timing is everything: intraperitoneal chemotherapy after primary or interval debulking surgery for advanced ovarian cancer. Cancer Chemother Pharmacol 82, 55–63 (2018). https://doi.org/10.1007/s00280-018-3591-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-018-3591-y