Abstract
Purpose
Osteosarcoma is the most common primary bone tumour appearing in children and adolescents. Recent studies demonstrate that osteosarcoma possesses a stem-like cell subset, so-called cancer stem-like cells, refractory to conventional chemotherapeutics and pointed out as responsible for relapses frequently observed in osteosarcoma patients. Here, we explored the therapeutic potential of Metformin on osteosarcoma stem-like cells, alone and as a chemosensitizer of doxorubicin.
Methods
Stem-like cells were isolated from human osteosarcoma cell lines, MNNG/HOS and MG-63, using the sphere-forming assay. Metformin cytotoxicity alone and combined with doxorubicin were evaluated using MTT/BrdU assays. Protein levels of AMPK and AKT were evaluated by Western Blot. Cellular metabolic status was assessed based on [18F]-FDG uptake and lactate production measurements. Sphere-forming efficiency and expression of pluripotency transcription factors analysed by qRT-PCR were tested as readout of Metformin effects on stemness features.
Results
Metformin induced a concentration-dependent decrease in the metabolic activity and proliferation of sphere-forming cells and improved doxorubicin-induced cytotoxicity. This drug also down-regulated the expression of master regulators of pluripotency (OCT4, SOX2, NANOG), and decreased spheres’ self-renewal ability. Metformin effects on mitochondria led to the activation and phosphorylation of the energetic sensor AMPK along with an upregulation of the pro-survival AKT pathway in both cell populations. Furthermore, Metformin-induced mitochondrial stress increased [18F]-FDG uptake and lactate production in parental cells but not in the quiescent stem-like cells, suggesting the inability of the latter to cope with the energy crisis induced by metformin.
Conclusions
This preclinical study suggests that Metformin may be a potentially useful therapeutic agent and chemosensitizer of osteosarcoma stem-like cells to doxorubicin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteosarcoma is the most frequent primary malignant bone tumour that appears mainly in childhood and adolescence, comprising 20% of all bone tumours and 5% of paediatric tumours overall. It represents the sixth most common cause of cancer in children older than 15 years and is considered an aggressive neoplasia [1]. In recent years, a significant improvement in the 5-year survival rates to approximately 65%–75% was achieved with the introduction of high-dose multiagent neoadjuvant chemotherapy, followed by surgical resection and adjuvant chemotherapy [2, 3]. Despite that, 20–40% of patients with non-metastatic osteosarcoma at diagnosis still relapse and die, mostly due to the development of resistance to current treatments [4, 5].
Nowadays, there is substantial evidence that many tumours, including osteosarcoma, contain a small subset of cells with stem-like properties, referred as cancer stem-like cells (CSCs) that like normal stem cells have the ability to self-renew and to generate differentiated progeny with limited proliferative capacity [5,6,7]. These cells have crucial therapeutic implications in cancer progression, since they possess mechanisms underlying both chemo- and radioresistance and are associated with aggressiveness and metastatic abilities [8,9,10,11,12]. The high rates of metastatic recurrences observed in osteosarcoma have been associated with the presence of CSCs, which underlies the need of novel therapeutic strategies focused on their eradication [10, 13].
Recently, cancer cell metabolism has become an exciting and promising field for therapeutic intervention. It is recognised that tumour cells rely on metabolic changes to support their growth and survival that involves a shift in nutrient metabolism towards biosynthesis [14, 15]. Cancer cell metabolism is characterised by an enhanced glucose uptake and a persistent activation of aerobic glycolysis even in the presence of oxygen, a widely accepted phenomenon known as Warburg effect [16, 17]. With respect to the metabolic requirements of CSCs, there is no consensus, with some studies indicating that they rely primarily in aerobic glycolysis, in clear contrast with others suggesting that CSCs adopt oxidative phosphorylation as their principal source of energy. For instance, the studies of Palorini et al., performed in an osteosarcoma stem-like cell line (3AB-OS) suggest that CSCs rely mostly on glycosis to sustain their proliferation, survival and ATP production [18], while Issaq et al. observed that they are differentially sensitive to inhibition of glycolysis, and even more sensitive to combined inhibition of glycolysis and mitochondrial respiration by different drug combinations [19].
The anti-diabetic semi-synthetic biguanide Metformin (MET) has been extensively used in the treatment of type-2 diabetes patients [20, 21], but recently has also been associated with reduced cancer risk and improved cancer prognosis [22]. The anticancer beneficial effect of MET is attributed to the activation of AMP-activated protein kinase/mammalian target of rapamycin (AMPK/mTOR) pathway [21, 23]. Metformin blocks the complex I of mitochondrial oxidative phosphorylation, which provokes an increase in NADH/NAD+ ratio with subsequent imbalance in the AMP/ATP ratio. The activation of AMPK, which is a central regulator of metabolic pathways, leads to the inhibition of anabolic processes while inducing stimulation of catabolism. Moreover, AMPK activation leads to the inhibition of mTOR by TSC2 phosphorylation with consequent decrease in cell growth, proliferation and protein synthesis. Furthermore, several studies demonstrated that MET potentiates the cytotoxic effects of chemotherapeutics in osteosarcoma [24] and also in breast [25] and colon cancer [26]. Besides, other studies demonstrated a preferential cytotoxicity of MET to CSCs relative to their differentiated counterparts in several tumours, based on its ability to inhibit mitochondrial activity [27,28,29]. Despite that most studies attribute the anti-tumoural effects of MET to the indirect activation of AMPK and subsequent suppression of mTOR, the exact mechanism of MET operating in CSCs remains largely unknown.
In this study, we provide evidence that MET exert a preferential cytotoxicity against stem-like spheres by dysregulating the signalling machinery that safeguards self-renewal ability, as a result of their inability to deal with the subsequent energy crisis induced by mitochondrial inhibition. This high dependency on mitochondrial bioenergetics can be envisaged as a metabolic weakness and exploited as a potential target for osteosarcoma stem-like cells.
Materials and methods
Cell culture and sphere-forming assay
Human MNNG/HOS and MG-63 osteosarcoma cell lines were obtained from the American Type Culture Collection (ATCC, Rockville, MD). These cells were cultured in monolayer with RPMI-1640 medium (Sigma–Aldrich®, St. Louis, USA) supplemented with 10% (v/v) heat inactivated fetal bovine serum (FBS, Gibco®, Waltham, USA) at 37 °C in a humidified atmosphere with 5% CO2 and 95% air. The isolation of stem-like cells was performed using the sphere-forming assay as previously described [9]. In brief, cells were harvested and seeded at a density of 60 × 103 cells/well on 6-well poly-HEMA-coated plates (Sigma) and cultured for 7–10 days in DMEM-F12 medium (Sigma) with 1% methylcellulose (Sigma) supplemented with 20 nM progesterone (Sigma), 100 µM putrescine (Sigma), 1% insulin-transferrin-selenium-A supplement (Gibco®), 1% antibiotic/antimicotic (Gibco®), 10 ng/mL human epidermal growth factor (EGF, Sigma) and 10 ng/mL human basic fibroblast growth factor (bFGF, Prepotech, EC, London, UK). Fresh aliquots of growth factors were added twice a week. For assessment of self-renewal, dissociated cells from primary spherical colonies were re-seeded in serum-free medium in non-adherent conditions for secondary sphere formation.
Drug cytotoxicity assays
Both parental MNNG/HOS and MG-63 cells and corresponding spheres were treated with MET (Sigma) as a single agent or in combination with doxorubicin [DOX (DOXO-cell®, Portugal)], which is currently used as a first-line therapy for osteosarcoma. Cells were seeded in 96-well plates overnight and then incubated with increasing concentrations of MET (0.1, 1, 2 and 5 mM) for a period of 48 h. The effects of MET on metabolic activity and cell proliferation were measured using the MTT [3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide] assay (MTT, M2128, Sigma) and the 5-bromo-2′-deoxyuridine (BrdU) incorporation assay (Cell Proliferation ELISA, BrdU colorimetric, Roche®, Germany), respectively, and were performed according to the manufacturer instructions. Absorbance values were expressed as percentages relatively to untreated controls.
The cytotoxicity of MET in combination with DOX was assessed using the MTT colorimetric assay. Cells were treated with three DOX concentrations (0.25, 0.5 and 1 µM) in combination with increasing concentrations of MET from 0.1 to 5 mM.
Effects of Metformin on sphere formation and self-renewal ability
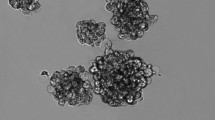
Next, we evaluated whether MET caused a depletion of the stem-like pool within parental cells or a dysregulation in the self-renewal ability of spheres. The former was assessed by measuring the sphere-forming efficiency (SFE) in dissociated parental cells plated in serum-free medium in non-adherent conditions (first generation). The later was assessed using sphere-dissociated cells from the first-generation untreated spheres, and cultured under the same conditions. In both cases, the sphere-forming assay was performed in the presence of MET at concentrations varying from 0.1 to 5 mM during 48 h. The formed spherical colonies were observed and counted in an inverted microscope (Nikon, Eclipse TS 100). Sphere-forming efficiency was calculated as the number of spheres formed divided by the initial number of single cells seeded and expressed as percentage. Spheres’ size and morphological appearance were also evaluated. Images of floating spherical colonies were acquired in a fluorescence microscope (Leica DFC350 FX, Leica Microsystems, USA) operating in brightfield mode.
Analysis of mRNA expression by quantitative RT-PCR
Total RNA was extracted using TRIzol® reagent (Invitrogen). First-strand cDNA was synthetised from 1 µg of purified RNA using NZY First-Strand cDNA Synthesis kit (NZYTech, Lisbon, Portugal) in a reaction volume of 20 µl according to the manufacturer instructions in a GeneAmp® PCR system 9700 thermal cycler (Applied Biosystems). Primer sequences used on quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR) are listed in Table 1 and were purchased from NZYTech. The qRT-PCR was performed in duplicate using a PerfeCTA SYBR Green FastMix (GRISP, Porto, Portugal) in a CFX—96™ Real-Time PCR Detection System (BioRad Laboratories, Inc) in a final volume of 12.5 µl containing 2.5 µl of 25× diluted cDNA, according to the following protocol: starting with an initial denaturation step at 95 °C during 5 min, where the TAQ polymerase was activated, followed by 39 cycles of amplification in thermal cycler conditions of 10 s at 95 °C where DNA was denaturated, 10 s at 60 °C to allow the primer annealing, 10 s at 72 °C for the elongation process and a final extension cycle at 72 °C during 10 min. mRNA expression data was normalised to three housekeeping genes and analysed using the ΔΔCt method and Bio-Rad CFX Manager™ 3.0 software.
Western blot
Protein samples were collected from untreated MNNG/HOS and MG-63 cells and corresponding spheres and following exposure to MET in the concentrations ranging from 0.1 to 5 mM. Cells were lysed with a RIPA buffer containing proteases and phosphatases inhibitors (Roche®). Protein concentration was determined using the bicinchoninic acid assay (BCA, Sigma), according to the manufacturer instructions. About 40–60 µg of total protein extract were separated by SDS–PAGE electrophoresis and then electrotransferred onto methanol-activated hydrophobic polyvinylidenedifluoride membranes. Non-specific protein interaction was blocked by incubating the membranes with 5% non-fat dry milk in 0.1% TBS-T during 1 h at room temperature. Membranes were incubated overnight at 4 °C with primary antibodies at a dilution of 1:1000 against Sox2 (Cell Signalling Technology, Danvers, MA, USA), AMPKα (Cell Signalling), pAMPKα (Thr172) (Cell Signalling), Glut1 (Millipore), Akt (Cell Signalling), pAkt (Ser473) (Cell Signalling) and at a dilution of 1:5000 for human β-actin (Sigma). The membranes were washed with PBS-T and then incubated with alkaline phosphatase-conjugated secondary anti-rabbit or anti-mouse antibodies, at a dilution of 1:20,000 during 1 h at room temperature. The reactive bands were visualised by chemifluorescence (ECF™ western blotting Reagent Pack, GE Lifesciences, Pittsburg, PA) using Typhoon™ FLA 9000 (GE Healthcare Bioscience, Sweden). The band intensities were estimated using ImageJ software (National Institutes of Health, Bethesda, MD) and normalised to that of corresponding loading controls (β-actin or total AMPKα). Data obtained from treated conditions was normalised to corresponding controls.
Cellular metabolic activity
The metabolic activity of parental adherent cells and corresponding spheres was assessed based on [18F]-fluoro-2-deoxyglucose ([18F]-FDG) uptake, which is an analogue of glucose and routinely used in Positron Emission Tomography (PET) imaging studies. This radiopharmaceutical was provided by the Institute for Nuclear Sciences Applied to Health (ICNAS, University of Coimbra, Coimbra, Portugal). Cells were treated with MET (0.1–5 mM) during 48 h and then incubated with [18F]-FDG (0.75 MBq/mL) for 1 h in a humidified incubator at 37 °C with 5% CO2. Afterwards, the culture medium was collected into glass tubes and the cells were washed with PBS, scraped and collected into glass tubes. Both tubes containing the collected culture medium and the scraped cells were assayed for radioactivity in a Radioisotope Calibrator Counter (CRC-15W Capintec, USA) within the 18F sensitivity energy window of 400–600 keV. After reading radioactivity, cells were lysed with 1% SDS (m/v) in PBS (pH 7.0) and the total protein was quantified using the bicinchoninic acid assay (BCA, B9643, Sigma–Aldrich®). The cellular uptake of [18F]-FDG was expressed as a ratio to the levels found in untreated cells and normalised to total protein content.
Lactate production
Lactate production by adherent parental and sphere-forming cells was measured using the colorimetric lactate assay kit (Sigma), after 48 h exposure to MET at the same concentrations used in [18F]-FDG uptake studies (0.1–5 mM). The supernatant of treated cells was collected and deproteinised with a 10 kDa spin filter (Amicon®) to remove lactate dehydrogenase. Lactate dehydrogenase concentration in the soluble fraction was determined by an enzymatic-based assay (Sigma) according to the manufacturer instructions. Cells were lysed in 1% (m/v) SDS solution in PBS (pH 7.0) and the total protein was determined using the bicinchoninic acid assay. Lactate dehydrogenase produced by the cells was normalised to total protein content.
Statistical analysis
All the results were presented as mean ± standard error mean (SEM) of the indicated number of experiments performed. The Mann–Whitney non-parametric test was used to perform statistical analysis. P ≤ 0.05 was assumed as statistical significant. Statistical analysis and graphical illustrations were prepared using GraphPad Prism software version 6 (GraphPad Software, San Diego, CA, USA). Artwork was prepared in Adobe Illustrator CS5.1 (Adobe Systems Incorporated, USA).
Results
Human osteosarcoma cell lines form spherical colonies exhibiting stem-like features
The presence of stem-like cells in human osteosarcoma cell lines MNNG/HOS and MG-63 was identified through the sphere formation assay as described elsewhere [9]. Adherent cells cultured under anchorage-independent conditions and serum-free medium grew in suspension and formed compact spherical colonies that were further termed as spheres or stem-like sphere cells (Fig. 1a). These cells maintained the ability to generate new spherical colonies in subsequent generations demonstrating their self-renewal capacity (data not shown). The stemness nature of these cells was confirmed by the expression of pluripotency-related transcription factors OCT4, NANOG and SOX2, which are known to play a key role in the maintenance of self-renewal and pluripotency of embryonic stem cells (ESCs). The mRNA expression analysis showed a 5.5- and 20-fold increase in SOX2 expression in spheres relatively to parental cell lines MNNG/HOS and MG-63, respectively (Fig. 1b), that was confirmed at protein level by Western Blot (Fig. 1c). The other transcription factors were also more amplified in spheres relatively to adherent cells but at less extension. Moreover, sphere-forming cells were also characterised by a variable expression pattern of drug resistance related genes including the aldehyde dehydrogenase (ALDH) isoforms ALDH1A1, ALDH2 and ALDH7A1 and the ATP-binding cassette transporters ABCB1 and ABCG2 (Fig. 1b), also recognised as stem-like markers.
Osteosarcoma sphere-forming cells express embryonic pluripotency markers. a MNNG/HOS and MG-63 parental cell lines grow in adherent conditions (left panel) and form spheres after 7 days in serum-free medium under non-adherent conditions (right panel). b Expression of pluripotency-related markers (OCT4, NANOG and SOX2), isoforms of ALDH (ALDH1A1, ALDH2 and ALDH7A1) and drug resistance-related genes (ABCB1 and ABCG2) by qRT-PCR. The graph shows the fold change in gene expression in spheres relative to corresponding parental cell line that was set as 1. c Analysis of Sox2 protein levels by Western blot in parental cells and corresponding spheres. Bar graphs represent fold-difference of protein levels after normalisation to β-actin. Data represents mean + SEM of three (n = 3) independent experiments. # p ≤ 0.05, ## p ≤ 0.01 compared to parental cells
Metformin decreases cell metabolic activity and proliferation of osteosarcoma stem-like cells
The effects of MET on the metabolic activity were estimated by the MTT assay, a colorimetric method based on the mitochondrial succinate dehydrogenase activity in viable cells. This drug induced a progressive and concentration-dependent decrease in the metabolic activity in the stem-like subpopulations of both cell lines. This effect was less pronounced in parental cells, as depicted in Fig. 2a, b. For concentrations above 1 mM the percentage of metabolically active sphere-derived cells was significantly lower (p < 0.05) than that in corresponding parental cells exposed to equal concentration of MET. The anti-proliferative effects of MET were also more pronounced in the stem-like subset (Fig. 2c, d) than in corresponding parental cells, based on the cellular BrdU incorporation. Spheres derived from the MNNG/HOS cell line seem to be more susceptible to the anti-proliferative effect of MET, as indicated by the lower percentage of proliferating cells comparatively to MG-63-derived spheres exposed to the same concentration of MET (Fig. 2c, d).
Metformin induces preferentially a decrease in the metabolic activity and proliferation rate of osteosarcoma spheres. Effects of different concentrations of MET in metabolic activity (a, b) and proliferation (c, d) assessed using the MTT and BrdU assays, respectively, in MNNG/HOS and MG-63 parental cells and corresponding spheres. Data were expressed as mean + SEM of three (n = 3) independent experiments performed in triplicate. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, compared to untreated cells; # p ≤ 0.05, ## p ≤ 0.01 compared to parental cells exposed to the same MET concentrations
Altogether, these results showed that MET exerts a preferential cytotoxic effect in stem-like sphere cells by regulating proliferation and metabolic activity, and a less and moderate effect in parental cells.
Metformin decreases the sphere-formation and self-renewal abilities of stem-like cells and the expression of pluripotency-related transcription factors
After verifying the higher susceptibility of spheres to MET, we then examined the effect of MET on sphere-forming and self-renewal abilities. The former was assessed by measuring the SFE of adherent cells plated in serum-free medium in non-adherent conditions (first generation). The later was calculated based on the number of spheres formed using dissociated cells from the first sphere generation.
No significant alterations were observed in the SFE of MNNG/HOS and MG-63 cells (Fig. 3a, left panel) treated with MET in the range of tested concentrations, with the exception of the MG-63 cells treated with 5 mM MET. However, the size of spheres decreased progressively with the increasing MET concentrations and their structure was less compact compared with untreated cells (Fig. 3a, right panel). A more noticeable effect of MET was observed on the self-renewal ability of dissociated first generation spheres mainly those derived from the MNNG/HOS cell line, as showed by the progressive and significant reduction in the SFE as well on the size and morphology of spheres. For 2 and 5 mM of MET the spheres revealed a structure more similar to cellular aggregates with irregular contours and not as compact and round as the ones derived from control untreated cells (Fig. 3c). MET also compromised the self-renewal ability of MG-63 cells, although with a more pronounced effect on the spheres size and loss of colony morphology for concentrations immediately above 0.1 mM MET (Fig. 3b, d, right panels). Alterations on the SFE were only significant for the highest MET concentration (Fig. 3b, d, left panels).
Metformin decreases the sphere-forming and self-renewal abilities of CSCs-enriched spheres by downregulation of pluripotency-related transcription factors. a, b Adherent MNNG/HOS and MG-63 cells were treated with different concentrations of MET (0.1–5 mM) during 1 week and then plated in the sphere-forming assay conditions. c, d First-generation sphere-forming cells were treated with a range of concentrations of MET (0.1–5 mM) during 1 week and then plated in the sphere-forming assay conditions. In a–d left panels represent total number of spheres per total number of cells plated and right panels represent the diameter of spheres in µm. Lower panels in a–d show representative images of morphology and size of spheres after treatment with the indicated doses of MET, scale bars = 100 µm. e, f mRNA expression of SOX2, OCT4 and NANOG tested by qRT-PCR in spheres treated with 2 and 5 mM of MET for 1 week. Bar graphs represent fold-change mRNA expression + SEM in MET versus untreated cells, after normalisation to three housekeeping genes (GAPDH, 18S and HPRT-1). *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, significantly different from untreated cells
To further understand the growth inhibitory effects of MET on sphere-forming cells, we analysed the mRNA expression levels of pluripotency stem cell markers SOX2, OCT4, and NANOG in spheres after treatment with the two highest MET concentrations (2 and 5 mM). At these drug concentrations it was observed a significant decrease in the mRNA expression levels of these pluripotency transcription factors, relatively to untreated cells, except for OCT4 and NANOG levels in MNNG/HOS spheres at 2 mM that remained unchanged or increased, respectively (Fig. 3e, f), suggesting that higher concentrations of MET are needed for the simultaneous downregulation of these three transcription factors in this cell line. This might reflect some degree of heterogeneity between sphere-forming cells and differences in the cellular response to MET-induced metabolic stress, with consequent impact on the expression of core pluripotency markers. Even so, these results suggest that MET disrupts the core transcriptional regulatory circuit required to maintain the self-renewal and pluripotency of stem-like spheres.
Metformin induces activation of sensors of energetic status (pAMPKThr172) and interferes with survival pathways (pAktSer473)
The central mechanism of the anti-carcinogenic effect of MET has been attributed to the activation of the energy sensor AMPKα, a key factor in the regulation of metabolic and energy status, which is crucial for cell survival. Once activated, AMPKα downregulates anabolic processes and upregulates catabolic processes that lead to ATP synthesis [21]. Based on this hypothesis, we evaluated the protein levels of the activated form of AMPKα (pAMPKThr172) after 48 h exposure to MET by Western Blot analysis. We observed a progressive increase in AMPKα phosphorylation in Thr172 residue, in both parental cells and spheres, which became significant for concentrations above 1 mM (Fig. 4a).
Metformin activates AMPK and the pro-survival PI3K/Akt pathway in osteosarcoma cancer cells. a, b Western Blot results show a MET concentration-dependent phosphorylation of AMPKα (Thr172) and Akt (Ser473) in parental cells and spheres after 48 h of treatment with MET (0.1–2 mM). Relative protein levels were quantified using ImageJ software, by dividing the optical density of the specific protein band by that of the corresponding non-phosphorylated forms and then normalised to untreated (CTR) cells. Data represents mean + SEM of four (n = 4) or three (n = 3) independent experiments. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 compared to untreated cells # p ≤ 0.05 compared to parental cells in the same conditions
Since tumour cells are also capable of activating survival-related pathways in response to cytotoxic insults, we analysed the effect of MET on Akt phosphorylation, a key readout of molecular activation of the survival PI3K/Akt pathway. Exposure to increasing concentrations of MET induced an increase in pAktSer473 becoming significant for concentrations above 1 mM in spheres compared to untreated cells, with no apparent differences to parental cells (Fig. 4b).
Metformin interferes with the metabolic activity of osteosarcoma cell lines
Subsequently to AMPKα activation, MET upregulates processes that lead to ATP synthesis, including glucose uptake. Therefore, we measured the cellular uptake of [18F]-FDG, a glucose analogue radiotracer, in both cell populations following treatment with MET. This is an established method for assessing the metabolic activity in tumour cells that reflects both transport and phosphorylation of glucose by viable cells. Under basal conditions, sphere-forming cells displayed lower accumulation of [18F]-FDG in relation to parental cells (Fig. 5a), which can be explained by their quiescent nature or slow-proliferating rate and less energy demands. Accordingly, although not significant, there is a trend towards a downregulation of the glucose transporter Glut 1 expression in spheres in relation to parental cells (Fig. 5b).
Metformin increases cellular uptake of [18F]-FDG and lactate production in parental cells but not in spheres. a Cellular uptake of [18F]-FDG in parental cells and corresponding spheres without treatment with MET. b Protein levels of Glut1 in parental cells and spheres subpopulations. c Cellular uptake of [18F]-FDG in MNNG/HOS and MG-63 cells and spheres following treatment with increasing concentrations of MET during 48 h. [18F]-FDG uptake was measured after 1 h incubation with the radiotracer and was expressed as a ratio to the levels found in untreated cells and normalised to protein content. d Lactate production by MNNG/HOS and MG-63 cells and respective spheres following treatment with increasing concentrations of MET during 48 h. Relative protein levels were quantified using ImageJ software, by dividing the optical density of the specific protein band by that of the loading control (β-actin). Data represents mean + SEM of four (n = 4) or three (n = 3) independent experiments. # p ≤ 0.05, ## p ≤ 0.01, ### p ≤ 0.001, compared to corresponding parental cells; *p ≤ 0.05, **p ≤ 0.01, compared to untreated cells
The treatment with MET induced a progressive increase in the cellular uptake of [18F]-FDG and lactate production in parental cells but not in spheres, whose uptake levels remained similar to those observed in untreated controls (Fig. 5c, d). Contrarily to parental cells, the activation of AMPK induced by MET in spheres was not accomplished by an increase in [18F]-FDG uptake (Fig. 5c) or lactate production (Fig. 5d), suggesting the inability of these cells to co-opt for aerobic glycolysis to compensate the energetic stress in response to MET-mediated mitochondria complex I inhibition. Altogether these results suggest that osteosarcoma stem-like spheres rely more on oxidative phosphorylation for survival compared with their parental differentiated counterparts, and display less ability to cope with metabolic mitochondrial stress.
Metformin potentiates DOX-induced cytotoxicity preferentially in stem-like spheres
Cancer stem-like cells are widely known for their increased chemoresistance. As already demonstrated by our group [9, 12], the isolated spheres of either MNNG/HOS or MG-63 cell lines display a drug concentration–response consistent with enhanced chemoresistance to DOX relatively to their differentiated counterparts (Fig. 6a). Given the preferential cytotoxicity of MET to sphere-forming cells, as previously observed with the MTT and BrdU assays (Fig. 2), we tested whether MET potentiates the DOX cytotoxic effects towards spheres. We conducted a MET-based combinatorial therapy using three fixed concentrations of DOX within the therapeutic range (0.25, 0.5 and 1 µM) in combination with varying concentrations of MET (0.1–5 mM). Both parental cells and corresponding spheres were incubated with each drug alone and in combination for 48 h. Cell viability was assessed using the MTT colorimetric assay. The combination of varying concentrations of MET with 0.25 µM DOX, which per se showed relatively low cytotoxic effects on spheres of both cell lines, sensitised the cells to DOX in a dose-dependent way (Fig. 6b, c). Metformin also enhanced the cytotoxicity of 0.5 and 1 µM DOX, mainly in the stem-like subset. In parental cells this effect was less pronounced and mainly observed when used at 2 or 5 mM, which falls out of the clinical range used in the treatment of diabetes and with potentially life-threatening toxicity. These observations suggest that MET in combination with DOX could contribute for the reduction of chemotherapeutic doses of DOX used in the therapy with consequent reduction of its side effects.
Metformin potentiates the cytotoxic effect of doxorubicin (DOX) in resistant osteosarcoma spheres. a Sphere-forming cells are less susceptible to DOX, within the therapeutic range, as compared to their parental cells. b, c MNNG/HOS and MG-63 cells, and respective spheres, were treated with DOX (0.25–1 µM) with or without MET (0.1–5 mM) for 48 h and the combinatorial effects in cell viability were measured by MTT assay. Data were expressed as mean + SEM of three (n = 3) independent experiments performed in triplicate. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, compared to cells treated only with DOX or to control; # p ≤ 0.05, ## p ≤ 0.01 and ### p ≤ 0.001, compared to parental cells exposed to the same treatment
Discussion
In the present study, we investigated the potential use of MET as co-adjuvant of DOX in the treatment of osteosarcoma, aiming to target stem-like cells, which are considered to play a major role in several aspects of tumorigenesis, including the clinical failure of the majority of available conventional therapies and consequent appearance of metastatic disease.
In previous studies, we demonstrated that osteosarcoma cell lines harbour a distinct cell subpopulation of cells exhibiting stem-like properties and chemoradio-resistance compared to their more differentiated counterparts [9]. An extensive characterisation of these cells demonstrated they express a core of transcription markers known to regulate the pluripotency and self-renewal of ESCs, being Sox2 one of the most regularly expressed [30], a trait that was confirmed in this new set of experiments. Additionally, the increased expression of the ALDH isoforms ALDH1A1, ALDH2 and ALDH7A1 together with the drug resistance-related genes ABCB1 and ABCG2 observed in spheres are likely to contribute to their enhanced resistance to doxorubicin as well as to other drugs [31].
The anti-diabetic drug Metformin has received particular attention for its anticancer properties due to anti-proliferative and anti-tumour growth effects in mouse xenografts, suggesting the possibility of its use as an anticancer agent in non-diabetic contexts [20]. Our data showed that MET induced a decrease in metabolic activity and proliferation in both parental and stem-like populations with the latter being more susceptible to MET, in opposite to the classical chemotherapeutic drug DOX that demonstrated enhanced cytotoxicity against parental cells. These results were in agreement with those observed in other tumour types including thyroid [27, 32], pancreatic [27, 32], breast and fibrosarcoma [33] stem-like populations relatively to their differentiated counterparts.
The potential benefits of MET, as an anticancer agent, are believed to result from the activation of AMPKα, which plays a major role in the regulation of energetic metabolism and proliferation in tumour cells. This drug activates the AMPKα signalling pathway in response to the inhibition of mitochondrial complex I and drop of intracellular ATP levels, which from a metabolic point of view, promotes the conservation of ATP under metabolic stress by activating pathways of catabolic metabolism [14, 34], and suppression of the mTOR activity, a signalling pathway involved in protein synthesis, tumour cells growth and pathogenesis [21]. Some studies showed that mTOR inhibition, although being an important target in cancer treatment, can be circumvented by the activation of the compensatory PI3K/Akt survival pathway leadind to drug resistance [35, 36]. Our results showed that MET activated AMPKα in both parental cells and spheres, as indicated by the phosphorylation of AMPKα, alongside with a progressive increase in Akt phosphorylation in both cell populations. Despite the activation of this survival pathway, MET still exerted pronounced anti-proliferative effects in spheres, suggesting that other AMPK-independent mechanisms are underlying the citotoxicity of MET in these cells,. As mentioned above the activation of AMPKα and subsequent modulation of downstream pathways would lead to stimulation of glycolysis for the production of ATP. This effect was evidenced in parental cells as indicated by the progressive increase in the [18F]-FDG uptake and lactate production paralleled to the increased pAMPKThr172 levels, suggesting these cells increase aerobic glycolysis to compensate the limitation of ATP production driven by MET-induced inhibition of mitochondrial metabolism. The stem-like fraction demonstrated less ability to deal with the metabolic stress, since neither [18F]-FDG uptake or lactate production changed with MET concentrations. It has been recognised that tumour cells rely mostly on glycolysis for their energy demands, even in the presence of oxygen, a metabolic profile of tumour cells known as the Warburg effect that constitute the basis for the use of PET imaging with [18F]-FDG in clinical diagnosis of solid tumours [37, 38]. Some studies suggest that stem-like cells are even more glycolytic than their normal counterparts for the maintenance of stemness properties [39]. Studies from Loureiro et al. and Vega-Naredo et al. suggest that stem-like cells from embryonal carcinoma cells, suppress their metabolism to allow the maintenance of stemness as well as their proliferative rate. These embryonal stem-like cells seem to possess immature and inactive mitochondria avoiding the complex oxidative metabolism processes that occur in these organelles [40, 41]. Contrarily, others sustain that CSCs rely mainly on oxidative phosphorylation as their main source of energy, since CSCs are rather quiescent in terms of proliferation [9]. Our previous data showed a low level of Ki-67 staining in a few number of cells in paraffin-embedded spheres, which is indicative of slowly proliferating cells suggesting a quiescent phenotype in opposite to the strong Ki-67 staining intensity in proliferating parental cells [30]. Here, we demonstrated that sphere-forming cells consumed less glucose compared to their differentiated counterparts, as indicated by the lower accumulation of [18F]-FDG and produce less lactate, indicating a preference for mitochondrial oxidative metabolism rather glycolysis. This oxidative phenotype has been observed in CSCs isolated from breast cancer [42] and ovarian cancer patients [43], and seems to be related to the capacity to resist apoptosis. The preferred dependency of CSCs on oxidative phosphorylation for energy production might explain the vulnerability of spheres to the inhibition of the mitochondrial respiratory complex I by MET, which is crucial for oxidative phosphorylation in mammalian cells [44]. Würth et al. in a study performed in glioblastoma cell lines observed similar markedly different responses to MET regarding glucose uptake, with differentiated cells showing significant increases in [18F]-FDG uptake, while CSCs showed virtually no response with increasing concentrations of MET [29]. Together, these findings suggest that CSCs are unable to switch to a glycolytic metabolism to compensate their bioenergetics when the mitochondrial respiration is impaired [45, 46]. Moreover, treatment with MET, induced a partial suppression of stemness traits, namely an impairment in the sphere-forming efficiency and self-renewal capacity of spheres and downregulation of pluripotency markers, particularly noticeable at the highest MET concentration tested. These results are in accordance with the recent published work of Chen et al., who found a decrease in the stemness properties in MG-63 cells following exposure to MET [47]. Similar effects were observed in CSCs of pancreatic [27], breast [48] and thyroid cancer [32], suggesting that MET creates a metabolic imbalance that compromise the stemness properties of CSCs. Vazquez-Martin et al., demonstrated that MET-driven activation of AMPK creates a metabolic barrier that impairs the reprogramming of somatic cells into a pluripotent state, by preventing the transcriptional activation of OCT4, which is a master regulator of the pluripotent state [49]. This might explain the preventive effect of MET in the initiation of several cancers in MET-treated patients, considering that dedifferentiation might predispose a normal cell to become tumorigenic.
Metformin has been shown to potentiate the cytotoxic effects of some classic chemotherapeutic agents including DOX and cisplatin in a variety of cancer cell lines [24, 50, 51]. We provided evidence that MET potentiated the cytotoxicity of DOX in osteosarcoma stem-like cells, mainly when combined with a low concentration of DOX (0.25 µM, below the IC50) that per se did not affect significantly the viability of spheres. These results are largely in line with those reported previously by Kevin Struhl’s group, in which they observed that the administration of MET together with a reduced dosage of DOX is highly effective in blocking tumour growth and preventing relapse in a variety of cancer cell types [25, 50].
On the basis of our observations, we conclude that MET exerted a preferential cytotoxicity against osteosarcoma spheres and improved DOX-induced cytotoxicity, via repression of the signalling machinery that safeguards self-renewal and pluripotency, as a result of their inability to deal with the subsequent energy crisis induced by MET-mediated mitochondrial inhibition. Although our results suggest that stem-like spheres rely more on oxidative phosphorylation for energy supply, further studies are needed to elucidate the complex mechanism of metabolism regulation in osteosarcoma stem-like cells.
References
Demiralp B, Sarkar G, Okuno SH, Yaszemski MJ, Maran A (2011) Osteosarcoma—an evaluation of current diagnosis, treatment and chemotherapy. Eur Musculoskelet Rev 6:18–23
Marina N, Gebhardt M, Teot L, Gorlick R (2004) Biology and Therapeutic Advances for Pediatric Osteosarcoma. Oncologist 9:422–441. doi:10.1634/theoncologist.9-4-422
Federman N, Bernthal N, Eilber FC, Tap WD (2009) The multidisciplinary management of osteosarcoma. Curr Treat Options in Oncol 10:82–93. doi:10.1007/s11864-009-0087-3
Chan HSL, Haddad G, DeBoer G, Ling V, Grogan TM (1997) P-glycoprotein expression: critical determinant in the response to osteosarcoma chemotherapy. J Natl Cancer Inst 89:1706–1715. doi:10.1093/jnci/89.22.1706
Siclari V, Qin L (2010) Targeting the osteosarcoma cancer stem cell. J Orthop Surg Res 5:78. doi:10.1186/1749-799X-5-78
Lorico A, Rappa G (2011) Phenotypic Heterogeneity of Breast Cancer Stem Cells. J Oncol 2011. doi:10.1155/2011/135039
Matchett KB, Lappin TR (2014) Concise reviews: cancer stem cells: from concept to cure. STEM CELLS 32:2563–2570. doi:10.1002/stem.1798
Tirino V, Desiderio V, Paino F, De Rosa A, Papaccio F, Fazioli F, Pirozzi G, Papaccio G (2011) Human primary bone sarcomas contain CD133+ cancer stem cells displaying high tumorigenicity in vivo. FASEB J 25:2022–2030. doi:10.1096/fj.10-179036
Martins-Neves SR, Lopes A, do Carmo A, Paiva A, Simoes P, Abrunhosa A, Gomes C (2012) Therapeutic implications of an enriched cancer stem-like cell population in a human osteosarcoma cell line. BMC Cancer 12:139. doi:10.1186/1471-2407-12-139
Basu-Roy U, Basilico C, Mansukhani A (2013) Perspectives on cancer stem cells in osteosarcoma. Cancer Lett 338:158–167. doi:10.1016/j.canlet.2012.05.028
Pattabiraman DR, Weinberg RA (2014) Tackling the cancer stem cells—what challenges do they pose? Nat Rev Drug Discov 13:497–512. doi:10.1038/nrd4253
Gonçalves C, Martins-Neves SR, Paiva-Oliveira D, Oliveira VEB, Fontes-Ribeiro C, Gomes CMF (2015) Sensitizing osteosarcoma stem cells to doxorubicin-induced apoptosis through retention of doxorubicin and modulation of apoptotic-related proteins. Life Sci 130:47–56. doi:10.1016/j.lfs.2015.03.009
Diaz A, Leon K (2011) Therapeutic approaches to target cancer stem cells. Cancers 3:3331–3352. doi:10.3390/cancers3033331
Vander Heiden MG, Cantley LC, Thompson CB (2009) Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324:1029–1033. doi:10.1126/science.1160809
Hanahan D, Weinberg R (2011) Hallmarks of cancer: the next generation. Cell 144:646–674
Warburg O (1923) Metabolism of tumours. Biochem Z 142:317–333
Weinhouse S, Warburg O, Burk D, Schade AL (1956) On respiratory impairment in cancer cells. Science 124:267. doi:10.1126/science.124.3215.267
Palorini R, Votta G, Balestrieri C, Monestiroli A, Olivieri S, Vento R, Chiaradonna F (2014) Energy metabolism characterization of a novel cancer stem cell-like line 3AB-OS. J Cell Biochem 115:368–379. doi:10.1002/jcb.24671
Issaq SH, Teicher BA, Monks A (2014) Bioenergetic properties of human sarcoma cells help define sensitivity to metabolic inhibitors. Cell Cycle 13:1152–1161. doi:10.4161/cc.28010
Del Barco S, Vazquez-Martin A, Cufí S, Oliveras-Ferraros C, Bosch-Barrera J, Joven J, Martin-Castillo B, Menendez JA (2011) Metformin: Multi-faceted protection against cancer. Oncotarget 2:896–917. doi:10.18632/oncotarget.387
Kourelis TV, Siegel RD (2012) Metformin and cancer: new applications for an old drug. Med Oncol 29:1314–1327. doi:10.1007/s12032-011-9846-7
Foretz M, Guigas B, Bertrand L, Pollak M, Viollet B (2014) Metformin: from mechanisms of action to therapies. Cell Metabolism 20:953–966. doi:10.1016/j.cmet.2014.09.018
Ashinuma H, Takiguchi Y, Kitazono S, Kitazono-Saitoh M, Kitamura A, Chiba T, Tada Y, Kurosu K, Sakaida E, Sekine I, Tanabe N, Iwama A, Yokosuka O, Tatsumi K (2012) Antiproliferative action of metformin in human lung cancer cell lines. Oncol Rep 28:8–14. doi:10.3892/or.2012.1763
Quattrini I, Conti A, Pazzaglia L, Novello C, Ferrari S, Picci P, Benassi MS (2014) Metformin inhibits growth and sensitizes osteosarcoma cell lines to cisplatin through cell cycle modulation. Oncol Rep 31:370–375. doi:10.3892/or.2013.2862
Hirsch HA, Iliopoulos D, Tsichlis PN, Struhl K (2009) Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res 69:7507–7511. doi:10.1158/0008-5472.CAN-09-2994
Nangia-Makker P, Yu Y, Vasudevan A, Farhana L, Rajendra SG, Levi E, Majumdar APN (2014) Metformin: a potential therapeutic agent for recurrent colon cancer. PLoS One 9:e84369. doi:10.1371/journal.pone.0084369
Bao B, Wang Z, Ali S, Ahmad A, Azmi AS, Sarkar SH, Banerjee S, Kong D, Li Y, Thakur S, Sarkar FH (2012) Metformin inhibits cell proliferation, migration and invasion by attenuating CSC function mediated by deregulating miRNAs in pancreatic cancer cells. Cancer Prev Res 5:355. doi:10.1158/1940-6207.CAPR-11-0299
Hirsch HA, Iliopoulos D, Struhl K (2013) Metformin inhibits the inflammatory response associated with cellular transformation and cancer stem cell growth. Proc Natl Acad Sci USA 110:972–977. doi:10.1073/pnas.1221055110
Würth R, Pattarozzi A, Gatti M, Bajetto A, Corsaro A, Parodi A, Sirito R, Massollo M, Marini C, Zona G, Fenoglio D, Sambuceti G, Filaci G, Daga A, Barbieri F, Florio T (2013) Metformin selectively affects human glioblastoma tumor-initiating cell viability: a role for metformin-induced inhibition of Akt. Cell Cycle 12:145–156. doi:10.4161/cc.23050
Martins-Neves SR, Corver WE, Paiva-Oliveira DI, van den Akker BEWM, Briaire-de-Bruijn IH, Bovée JVMG, Gomes CMF, Cleton-Jansen A-M (2016) Osteosarcoma stem cells have active Wnt/b-catenin and overexpress SOX2 and KLF4. J Cell Physiol 231:876–886. doi:10.1002/jcp.25179
Moreb JS, Ucar D, Han S, Amory JK, Goldstein AS, Ostmark B, Chang LJ (2012) The enzymatic activity of human aldehyde dehydrogenases 1A2 and 2 (ALDH1A2 and ALDH2) is detected by Aldefluor, inhibited by diethylaminobenzaldehyde and has significant effects on cell proliferation and drug resistance. Chem Biol Interact 195:52–60. doi:10.1016/j.cbi.2011.10.007
Chen G, Xu S, Renko K, Derwahl M (2012) Metformin inhibits growth of thyroid carcinoma cells, suppresses self-renewal of derived cancer stem cells, and potentiates the effect of chemotherapeutic agents. J Clin Endocrinol Metab 97:E510-E520. doi:10.1210/jc.2011-1754
Song CW, Lee H, Dings RPM, Williams B, Powers J, Santos TD, Choi BH, Park HJ (2012) Metformin kills and radiosensitizes cancer cells and preferentially kills cancer stem cells. Sci Rep 2:362. doi:10.1038/srep00362
Hardie DG (2011) AMP-activated protein kinase-an energy sensor that regulates all aspects of cell function. Genes Dev 25:1895–1908. doi:10.1101/gad.17420111
Wan X, Harkavy B, Shen N, Grohar P, Helman LJ (2007) Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene 26:1932–1940. doi:10.1038/sj.onc.1209990
Lane HA, Breuleux M (2009) Optimal targeting of the mTORC1 kinase in human cancer. Curr Opin Cell Biol 21:219–229. doi:10.1016/j.ceb.2009.01.016
Zheng J (2012) Energy metabolism of cancer: glycolysis versus oxidative phosphorylation (Review). Oncol Lett 4:1151–1157. doi:10.3892/ol.2012.928
Challapalli A, Aboagye EO (2016) Positron emission tomography imaging of tumor cell metabolism and application to therapy response monitoring. Front Oncol 6:44. doi:10.3389/fonc.2016.00044
Peiris-Pagès M, Martinez-Outschoorn UE, Pestell RG, Sotgia F, Lisanti MP (2016) Cancer stem cell metabolism. Breast Cancer Res 18:55. doi:10.1186/s13058-016-0712-6
Loureiro L, Mesquita KA, Oliveira PJ, Vega-Naredo I (2013) Mitochondria in cancer stem cells: a target for therapy. Recent Pat Endocr Metab Immune Drug Discov 7:102–114. doi:10.2174/18722148113079990006
Vega-Naredo I, Loureiro R, Mesquita KA, Barbosa IA, Tavares LC, Branco AF, Erickson JR, Holy J, Perkins EL, Carvalho RA, Oliveira PJ (2014) Mitochondrial metabolism directs stemness and differentiation in P19 embryonal carcinoma stem cells. Cell Death Differ 21:1560–1574. doi:10.1038/cdd.2014.66
Farnie G, Sotgia F, Lisanti MP (2015) High mitochondrial mass identifies a sub-population of stem-like cancer cells that are chemo-resistant. Oncotarget 6:30472–30486. doi:10.18632/oncotarget.5401
Pastò A, Bellio C, Pilotto G, Ciminale V, Silic-Benussi M, Guzzo G, Rasola A, Frasson C, Nardo G, Zulato E, Nicoletto MO, Manicone M, Indraccolo S, Amadori A (2014) Cancer stem cells from epithelial ovarian cancer patients privilege oxidative phosphorylation, and resist glucose deprivation. Oncotarget 5:4305–4319. doi:10.18632/oncotarget.2010
Sancho P, Burgos-Ramos E, Tavera A, Bou Kheir T, Jagust P, Schoenhals M, Barneda D, Sellers K, Campos-Olivas R, Graña O, Viera CR, Yuneva M, Sainz B Jr, Heeschen C (2015) MYC/PGC-1a; balance determines the metabolic phenotype and plasticity of pancreatic cancer stem cells. Cell Metab 22:590–605. doi:10.1016/j.cmet.2015.08.015
Lonardo E, Cioffi M, Sancho P, Sanchez-Ripoll Y, Trabulo SM, Dorado J, Balic A, Hidalgo M, Heeschen C (2013) Metformin Targets the metabolic achilles heel of human pancreatic cancer stem cells. PLoS One 8:e76518. doi:10.1371/journal.pone.0076518
Andrzejewski S, Gravel SP, Pollak M, St-Pierre J (2014) Metformin directly acts on mitochondria to alter cellular bioenergetics. Cancer Metab 2:12. doi:10.1186/2049-3002-2-12
Chen X, Hu C, Zhang W, Shen Y, Wang J, Hu F, Yu P (2015) Metformin inhibits the proliferation, metastasis, and cancer stem-like sphere formation in osteosarcoma MG63 cells in vitro. Tumor Biol 36:9873–9883. doi:10.1007/s13277-015-3751-1
Jung JW, Park SB, Lee SJ, Seo MS, Trosko JE, Kang KS (2011) Metformin represses self-renewal of the human breast carcinoma stem cells via inhibition of estrogen receptor-mediated OCT4 expression. PLoS One 6:e28068. doi:10.1371/journal.pone.0028068
Vazquez-Martin A, Vellon L, Quirós PM, Cufí S, Ruiz de Galarreta E, Oliveras-Ferraros C, Martin AG, Martin-Castillo B, López-Otín C, Menendez JA (2012) Activation of AMP-activated protein kinase (AMPK) provides a metabolic barrier to reprogramming somatic cells into stem cells. Cell Cycle 11:974–989. doi:10.4161/cc.11.5.19450
Iliopoulos D, Hirsch HA, Struhl K (2011) Metformin decreases the dose of chemotherapy for prolonging tumor remission in mouse xenografts involving multiple cancer cell types. Cancer Res 71:3196–3201. doi:10.1158/0008-5472.CAN-10-3471
Cooper AC, Fleming IN, Phyu SM, Smith TAD (2015) Changes in [18F]Fluoro-2-deoxy-d-glucose incorporation induced by doxorubicin and anti-HER antibodies by breast cancer cells modulated by co-treatment with metformin and its effects on intracellular signalling. J Cancer Res Clin Oncol 141:1523–1532. doi:10.1007/s00432-015-1909-2
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was funded by the Portuguese Foundation for Science and Technology (FCT, Pest-UID/NEU/04539/2013) and FEDER-COMPETE (FCOMP-01-0124-FEDER-028417 and POCI-01-0145-FEDER-007440). Sara Martins-Neves received a PhD individual grant from FCT (SFRH/BD/69603/2010).
Conflict of interest
Daniela I. Paiva-Oliveira declares that she has no conflict of interest. Sara R. Martins-Neves declares that she has no conflict of interest. Antero J. Abrunhosa declares that he has no conflict of interest. Carlos Fontes-Ribeiro declares that he has no conflict of interest. Célia M. F. Gomes declare that she has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Paiva-Oliveira, D.I., Martins-Neves, S.R., Abrunhosa, A.J. et al. Therapeutic potential of the metabolic modulator Metformin on osteosarcoma cancer stem-like cells. Cancer Chemother Pharmacol 81, 49–63 (2018). https://doi.org/10.1007/s00280-017-3467-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-017-3467-6