Abstract
Purpose
As reported, epidermal growth factor receptor (EGFR) is over expressed in a variety of cancers including esophageal squamous cell carcinoma. Therefore, it becomes one of the potential targets for treating esophageal cancer. Pingyangmycin (PYM), a single A5 component of bleomycin, is currently used for the treatment of different types of cancers of epidermal origin, especially for head and neck cancers. In this report, the effect of PYM on EGFR expression in human esophageal cancer cells and the therapeutic efficacy of the combination of PYM and cetuximab on esophageal cancer xenograft were investigated.
Methods
The effects of PYM, cetuximab and the combination on EGFR signaling, proliferation, cell cycle, apoptosis were evaluated by using MTT, Western blotting, RT-PCR assays and flow cytometry assays, respectively, in vitro and the therapeutic efficacy by a xenograft model in athymic mice.
Results
Cell volume and nucleus were enlarged after PYM treatment. PYM showed potent cytotoxicity in both cell lines of Kyse-150 and Eca-109 in time and dosage-depended manner in MTT assay. PYM treatment induced G2/M phase arrest and apoptosis. Notably, the expression of EGFR was down-regulated by PYM in EGFR highly expression esophageal cancer cells. PYM plus cetuximab resulted in a potentiation of antiproliferative activity. PYM combined with cetuximab displayed a much higher therapeutic effect than that of the single agent on esophageal cancer xenograft in athymic mice.
Conclusions
PYM could down-regulate the expression of EGFR in esophageal cancer cells and potentiate the effects of cetuximab on esophageal cancer xenograft in nude mice. The combination of PYM and cetuximab, the EGFR-targeted combination of a chemotherapeutic agent and an antibody-based drug, might be useful in cancer therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antitumor antibiotic Pingyangmycin (bleomycin A5, PYM) is a single bleomycin A5 component with lower pulmonary toxicity than bleomycin (BLM), which is a multi-component complex [1]. PYM has been found highly effective against a wide spectrum of experimental tumors and has been applied in clinical chemotherapy in China since 1979 [2]. PYM shares the same mechanism with the BLM. The cytotoxicity of BLM is mainly due to direct DNA damage, as it causes single- and double-strand DNA breaks that are seen as chromosomal gaps, deletions and DNA fragmentation [3, 4]. It has been particularly effective in different types of cancer of epidermal origin (testicle, skin, lung, head and neck tumors) and lymphomas [5].
Esophageal cancer is one of the most common gastrointestinal cancers and a major cause of cancer-related mortality world widely. There are two major histological subtypes of esophageal cancer: squamous cell carcinoma and adeno-carcinoma. Approximately two-thirds of new cases of squamous cell carcinoma are detected in China (47%) and Central Asia (19%) [6]. For the patients with loco-regional disease, surgical resection is the best treatment option for cure [7]. However, overall survival after resection is relatively poor with 5-year survival rates remain 35%. Neoadjuvant chemotherapy may improve the survival of esophageal cancer patients [8]. Targeted therapy could bring out further benefit by selectively destroying tumor cells.
Epidermal growth factor receptor (EGFR) is commonly over expressed in many solid tumors and such over expression frequently correlates with a poor prognosis. Thus, EGFR is an attractive molecular target, and agents have been developed to specifically target it. The EGF family of receptor tyrosine kinases consists of four members: EGFR (human EGFR HER-1), HER-2, HER-3 and HER-4. EGFR/HER family-related signaling has been reported to play a role in modulating cell proliferation, survival, migration and differentiation including breast cancer, and head and neck cancer [9]. It has been reported that EGFR is frequently over expressed in various squamous cell carcinomas, including esophageal carcinomas [10–12]. The positive rates of over expressed EGFR protein are 40–80% in esophageal cancer [13–16]. These data also revealed that no EGFR expression or only focal staining was present in normal esophageal epithelium. EGFR over expression is exclusively confined to cancer cells. Over expression of EGFR protein was associated with EGFR gene amplification. EGFR protein over expression was significantly correlated with the depth of tumor invasion.
In this study, two EGFR highly expression esophageal squamous cell carcinoma cells lines of Kyse-150 and Eca-109 were chosen; we observed the effect of PYM on cell growth, cell cycle distribution, induction of apoptosis and inhibition of the activation of EGFR in esophageal cancer cells. Further, we examined whether PYM had significant synergy with cetuximab both in cell culture and in vivo.
Materials and methods
Chemicals and reagents
Pingyangmycin was purchased from Tianjin Taihe Pharmaceutical Co. Ltd. (Tianjin, China). The monoclonal antibody EGFR—cetuximab (Erbitux) was purchased from Merck (NJ, USA). The followed antibodies were purchased from Santa Cruz: EGFR (sc-03), phospho-EGFR (sc-101668), Actin (sc-1616). Antibodies of AKT and phospho-AKT were from Cell Signaling Technology (Danvers, MA, USA). MTT were purchased from Sigma (St. Louis, MO, USA). EGF was obtained from Roche (Shanghai, China).
Cell culture
Human esophageal squamous cancer carcinoma cells Kyse-150 and Eca-109 were obtained from the Cell Center of the Cancer Institute, Chinese Academy of Medical Science and Peking Union Medical College. Both of cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum, 2 mmol/l glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin. Cultures were maintained at 37°C in humidified 95% air and 5% carbon dioxide atmosphere.
Cell viability assay
Cell viability was examined by MTT assays, according to the instructions of the manufacturer. Cells were seeded in 96-well plates with 3,000 cells/well. After overnight incubations, triplicate wells were treated with varying concentrations of drugs for indicated times. All assays were done in triplicate. The inhibition rate was calculated according to the formula: inhibition rate (%) = (absorbency of control − absorbency of treated cells)/absorbency of control × 100.
Protein extraction and Western blot analysis
Before the cells were harvested, the EGF (100 ng/ml) was added. Total cellular protein was extracted using a lysis buffer (50 mmol/l Tris–HCl, pH 7.4, 1% NP-40, 0.25% sodium deoxycholate, 150 mmol/l NaCl, 1 mmol/l EGTA, 1 mmol/l EDTA, 0.1% SDS) with protease inhibitor (1 mmol/l PMSF, 10 μg/ml aprotinin, 10 μg/ml leupeptin and 10 μg/ml pepstatin). The proteins were quantified by using a BCA protein assay kit (Pierce, Rockford, IL, USA). An equal amount of protein samples were loaded onto 7.5% SDS-PAGE gel and transferred to PVDF. Membranes were then incubated in a blocking buffer (1% bovine serum albumin in 20 mmol/l Tris–HCl, 150 mmol/l NaCl, 0.1% Tween-20), followed by incubated overnight at 4°C with the primary antibodies and then with HRP-conjugated secondary antibody. Detection was carried out using an enhanced chemiluminescence agent (Millipore Corporation, Billerica, MA). β-actin served as an internal control.
FITC-annexin V/PI apoptosis assay
Cells were harvested and resuspended in 200 μl binding buffer. Then 10 μl FITC-labeled enhanced annexin V (Baosai Biotechnology Ltd., Beijing, China) and 100 ng/ml propidium iodide were added. The percentage of annexin V-positive cells was counted on FACScan flow cytometer (Beckman Coulter).
Cell cycle analysis
Cells were harvested and centrifuged at 600g for 5 min. Then cells were fixed in ice-cold 70% ethanol and stored at −20°C for 24 h before analysis. For cell cycle analysis, cells were washed twice in PBS and stained with 50 μg/ml propidium iodide and 200 μg/ml RNase A for 30 min. The samples were analyzed with FACScan flow cytometer (Beckman Coulter).
Staining with Hoechst 33342
Cells were treated with PYM at 1, 5 and 10 μM for 48 h. The cells were washed with PBS and fixed with 4% paraformaldehyde for 10 min at room temperature and then washed with PBS. Hoechst 33342 (5 μg/mL) was added to the fixed cells, and incubated for 10 min at room temperature. Then cells were imaged using an Olympus IX-70 inverted fluorescent microscope.
Fluorescence-activated cell sorting (FACS) analysis EGFR expression
Expression rate of EGFR on cells was assessed by FACS as follows: Kyse-150 and Eca-109 cells were detached by treatment with trypsin–EDTA, washed and resuspended in PBS. 10 × 106 cells was treated with 2% rabbit serum and anti-EGFR antibody and incubated for 2 h on ice. After washing with PBS, the cells were incubated with goat anti-rabbit-FITC antibody for 1 h on ice, washed and analyzed by fluorescence-activated cell sorting.
Determination of EGFR mRNA levels by reverse transcription-PCR
Total cellular RNA was isolated using Trizol reagent according to the manufacturer’s instructions. RNA was reverse transcribed and amplified by PCR. RNA was subjected to reverse transcription reaction, and PCR amplifications were performed with the following sense and antisense primers: 5′-CACCTGCGTGAAGAAGTGTC-3′ and 5′-TCCTTGAGGGAGCGTAATCC-3′. These amplifications yielded a 498-bp EGFR product. The constitutive gene β-actin was also amplified as control, using the following sense and antisense primers: 5′-CCCAGGCACCAGGGCGTGATGGT-3′ and 5′-GGACTCCATGCCCAGGAAGGAA-3′, respectively, which yielded a 714-bp product. PCR was carried out according to the following program: 25 cycles at 94°C for 40 s, 65°C for 40 s and 72°C for 40 s. Amplified DNA was separated on a 1% agarose gel and visualized with ethidium bromide.
Evaluation of the antitumor efficacy of PYM, cetuximab alone and the combination in athymic nude mice bearing esophageal carcinoma Kyes-150 xenograft
Kyse-150 human esophageal caner cells (5 × 106 cell per animal) were injected subcutaneously into the armpit of 6- to 8-week-old BALB/c female athymic mice. When the tumor volume reached approximately 1 × 1 × 1 cm3, the mice were killed. After removing the necrotic portion of the tumor mass, the tumor was cut into 2 × 2 × 2 mm3 blocks, and the blocks were implanted subcutaneously into the armpit of female athymic mice. One week later, when the solid tumors were palpable, the mice were divided into groups and treated by intraperitoneal injection with saline, PYM, cetuximab and PYM plus cetuximab, respectively, twice a week for 3 weeks. Mice were weighed, and tumor sizes were measured with a caliper and recorded every 3 days. The tumor volume was determined using the formula (length × width2) × 0.5.
Analysis of combination effect of PYM and cetuximab in vitro and in vivo
Drug interactions in vitro were analyzed according to coefficient of drug interaction (CDI) [17, 18]. CDI was calculated according to the formula: CDI = AB/(A × B), where A and B are the ratio of the survival values of respective single agent and the control; AB is the ratio of survival values of two drugs combination and the control. A synergistic effect was considered to be a two-drug combination for CDI < 1 and significantly synergistic effect of a two-drug combination for CDI < 0.7. Combination analysis in vivo was performed by the fraction tumor volume [19, 20]. The effects of two combined drugs can be calculated by multiplying the fractional tumor volume by each single drug. The combination was defined as synergistic, additive or antagonistic, respectively, if the effect of the drugs was smaller than, equal to, or larger than the number of calculation.
Statistics analysis
Results are indicated as the means ± SD. Treatment effects were compared using the Student’s t test and, differences between the means were considered to be significant when P < 0.05.
Results
Effects of PYM on cell morphology and proliferation
The Hoechst 33342 staining was used to assess the changes in nuclear morphology following the treatment of PYM. Enlarged cells having a single giant nucleus were induced after 48 h treatment, especially eminent in the Kyse-150 cells (Fig. 1a). The nuclei of untreated cells were normal and exhibited diffused staining of the chromatin. After exposure to PYM for 48 h, some condensed nuclei were observed when cells were exposed to higher concentrations of PYM. Dose–response and time–response growth inhibitory effects were observed in antiproliferative assays (Fig. 1b, c). Kyse-150 and Eca-109 cells were treated with PYM of different concentrations for indicated times. The IC50 values of PYM for the Kyse-150 cells were 113.69, 12.06 and 2.76 μM. The IC50 values of PYM for the Eca-109 cells were 91.37, 17.94 and 3.53 μM for 24, 48 and 72 h, respectively.
Effect of PYM on cell proliferation of Kyse-150 and Eca-109 cells. a Kyse-150 and Eca-109 cells were treated with the indicated concentrations of PYM for 48 h and then stained with the DNA-binding dye Hoechst 33342 (200×). b, c Cells were treated with various concentrations of PYM for 24, 48 and 72 h in Kyse-150 and in Eca-109 cells, respectively. The effects on cell proliferation were examined by the MTT assay, and cell proliferation was calculated as the percentage of control. All assays were done in triplicate
Effects of PYM on cell cycle progression and apoptosis induction
After treatment with PYM, the cells significantly accumulated in the G2/M phase in a concentration-dependent manner. PYM could eminently induce G2/M cell cycle arrest in esophageal cells at lower concentration. The rate of G2/M cell cycle arrest in the Kyse-150 cells (Fig. 2a) was much more remarkable than that in the Eca-109 cells (Fig. 2b). Flow cytometry combined with FITC-Annexin V/PI staining showed that PYM at 1 μM induced less apoptosis in both cell lines. The ratio of apoptosis was significantly enhanced when cells were incubated with 10 μM PYM for 48 h (Fig. 2c).
Cell cycle analysis and apoptosis of Kyse-150 and Eca-109 cells after treated with PYM. a After a 48 h exposure to different concentrations of PYM, Kyse-150 cells were stained with PI. Percentages of the total cell population in the different phases of the cell cycle were determined and included. b The same method as a in Eca-109 cells. c Cells were stained with FITC-Annexin V/PI. Apoptosis was determined by the percentages of FITC-Annexin V + cells. Data represent the mean ± SD of three independent experiments. Results were derived from three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 versus control
Downregulation of EGFR expression by PYM
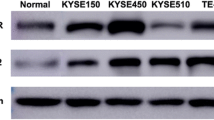
The EGFR was highly expressed in both of esophageal cell lines. Phospho-EGFR protein cannot be detected on standard condition and can be detected stimulated by EGF (Fig. 3a). Positive expression rates of EGFR on esophageal cancer cells were analyzed by immunofluorescence assay. The percentage of EGFR-positive cells is 99.8% in Kyse-150 cells and 99.9% in Eca-109 cells (Fig. 3b). A dose-dependent decrease in the EGFR protein expression in both of esophageal cell lines was observed after exposure of the cells to different concentrations of PYM (1, 5, or 10 μM) (Fig. 4a). Moreover, the levels of phospho-EGFR also decreased in a dose-dependent manner in cells after EGF stimulation. PYM reduced the expression level of EGFR and the phosphorylation of EGFR; however, it had no influence on the expression of AKT and phosphorylation AKT. Time-dependent down-regulation of EGFR was also observed in Kyse-150 cells exposed to 10 μM PYM. Western blotting showed that there was a marked down-regulation of EGFR at 72 h after PYM exposure (Fig. 4b). Furthermore, as evaluated by semiquantitative RT-PCR, EGFR mRNA was downregulated after the cells exposed 10 μM PYM (Fig. 4c). These results clearly indicated that PYM decreased EGFR expression via downregulation of EGFR mRNA levels. In addition, the influence of the cetuximab and PYM on EGFR protein and the special downstream protein-AKT was evaluated. The results showed that the cetuximab did not affect the expression of EGFR and AKT but rather reduced EGFR and AKT-phosphorylation (Fig. 5).
Expression of EGFR proteins in esophageal cancer cells. a EGFR and p-EGFR proteins were detected by Western blotting. The cells were grown to 90% confluence and then incubated with serum-free medium overnight. Before harvested, the EGF was added to the medium. Then cells were harvested 15 min after EGF treatment, and lysates were prepared for Western blot analysis of protein expression. β-actin was served as inter-control. b Positive expression rate of EGFR on esophageal cancer cells was analyzed by immunofluorescence assay. 1 Cells treated with isotype control; 2 cells treated with treated with anti-EGFR antibody
Effects on proteins and gene expression of PYM in esophageal cancer cell. a Cells were treated with the drugs for 72 h. Before harvested, the EGF was added to the medium. Cells were harvested 15 min after EGF treatment, and lysates were prepared for Western blot analysis of protein expression. β-actin was served as inter-control. The figure is representative of three independent experiments. b Cells were treated with the 10 μM PYM at 24, 48 and 72 h, respectively. EGFR proteins were detected by Western blotting. c EGFR gene was analyzed from cDNA obtained after RNA extraction of untreated cells, and cells treated with PYM for 72 h, respectively
Effects combination of PYM and cetuximab alone or in combination with proteins in esophageal cancer cells. The cells were treated with the drugs for 72 h. Before harvested, the EGF was added to the medium. Then cells were harvested 15 min after EGF treatment, and lysates were prepared for Western blot analysis of protein expression. β-actin was served as inter-control. The figure is representative of three independent experiments
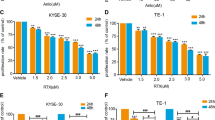
The combination of PYM and cetuximab synergistically inhibited the proliferation of both the esophageal carcinoma Kyse-150 and Eca-109 cells
To evaluate the nature of the interaction between PYM and cetuximab (additive or synergism), combination analyses were performed. The data in Fig. 6 illustrate the slight-to-moderate synergistic growth inhibitory effects (CDI < 1) against Kyse-150 cells and Eca-109 cells when 1 μM PYM was combined with various concentrations of cetuximab.
Effect of PYM on tumor growth of Kyse-150 xenografts in athymic mice and the synergism with cetuximab
To characterize the in vivo effects of the combination, Kyse-150 human tumor xenografts were established. In the Kyse-150 human tumor xenografts, the doses of PYM (15 mg/kg) and cetuximab (20 mg/kg) resulted in higher inhibition of tumor growth. The combination therapy of PYM and cetuximab resulted in a significant inhibition of tumor growth compared with cetuximab or PYM given alone (P < 0.001) (Fig. 7; Table 1). All the treatments were well tolerated, and there were no signs of toxicity or body weight loss during therapy. Analysis of the in vivo interaction between both drugs was performed. Combination therapy with PYM (15 mg/kg) and cetuximab (20 mg/kg) showed a synergistic effect on tumor growth inhibition (Table 1). The combination of lower doses of PYM and cetuximab resulted in 88.7% inhibition of tumor growth.
Mean tumor volumes (a) and mean body weights of mice (b) in each group are shown. Inhibitory effects of PYM on the growth of esophageal carcinoma Kyse-150 xenografts in nude mice. Mice were treated with PYM or cetuximab twice weekly for 3 weeks. Tumors were measured twice weekly and volumes calculated as described in “Materials and methods”
Discussion
EGFR signaling pathway is reported to be involved in modulating cell proliferation, survival, migration and differentiation of a range of tumors including breast cancer, and head and neck cancer. Approximately 50–70% of esophageal cancers show an overexpression of EGFR protein, which provides a good reason for targeting this signal pathway [10, 21, 22]. The most widely studied EGFR targeting agent is cetuximab, a monoclonal antibody that is used to treat metastatic colorectal cancer or head and neck cancer that over expressed EGFR. Cetuximab has been shown to have antitumor activity in preclinical studies and demonstrated synergistic effects together with chemotherapy and radiotherapy in other types of cancer. As reported, cetuximab plus radiotherapy improved locoregional control and reduces mortality in a Phase III trial for head and neck cancer [23]. Several recent studies combined cetuximab with radiotherapy, paclitaxel, irinotecan, fluorouracil or cisplatin for patients with esophageal cancer. During the clinical studies, no matter cetuximab as monotherapy or cetuximab combined with other chemotherapy, these results are encouraging although the combination with paclitaxel, carboplatin and radiation therapy is not standard care yet [24–26]. It has been reported that RAS/RAF mutations are negatively associated with response to cetuximab. Ras gene and Raf gene mutations occur commonly in some tumors and lead to abnormal signaling, but the incidence of Ras and Raf mutation is very low in esophageal carcinoma [27–29]. In light of the relatively low frequency of Ras and Raf mutations and overexpression of EGFR, cetuximab would be appropriate as a combined monoclonal antibody for esophageal carcinoma.
For enhancement of the therapeutic effect by drug combination, it is of interest to consider the co-targeted strategy. Cetuximab is an antibody-based drug targeting EGFR. It is of importance to combine cetuximab with a chemotherapeutic agent that shows action on the same molecular target—EGFR and investigate the antitumor effect of this combination. In this regard, BLM or its analog may be one of the most interesting chemotherapeutic agents as a candidate to combine with cetuximab.
Pingyangmycin, a chemotherapeutic agent of single BLM component A5, has been used for treatment of head and neck cancer, in some occasions, for esophageal cancer. For decades, PYM has been used for clinical cancer therapy in China. In the present study, we have observed that PYM could effectively inhibit cell growth and cell cycle progression and induce apoptosis in esophageal cancer cells. Notably, PYM down-regulates EGFR and accordingly p-EGFR. There is evidence that PYM reduced EGFR expression mainly at the transcription level. The decrease in EGFR mRNA levels was comparable to the decrease found at the receptor level. However, the EGFR family downstream signaling pathway proteins, AKT and p-AKT were not changed. To our knowledge, no data on BLM down-regulation of EGFR pathway have been reported. DNA damage by BLM could induce many signaling pathways that affect cell growth and death. The response to DNA damage was first seen by nuclear proteins, including ATM and ATR [30], which in turn stimulate the activation of Chk1, Chk2 and p53 [31]. In addition to changes of nuclear proteins, the DNA damage response also involves cytoplasmic pathways, such as those involving MAPKs [32]. These nuclear and cytoplasmic proteins control the activation of cell cycle checkpoints as well as apoptosis. Furthermore, the mechanisms linking DNA damage to changes of EGFR requires further experimentation. Our study provides rational evidence that PYM is an interesting candidate for combination with cetuximab or other EGFR-targeted antibody-based drugs. In the present study, we found that PYM and cetuximab inhibited the growth of Kyse-150 tumors at the low doses of PYM and cetuximab. Because the 50 mg/kg dose of cetuximab alone effectively inhibited the growth of A431 xenografts undergoing complete remission [19], we choice 20 mg/kg dose of cetuximab in order to observe the synergistic effect. The combination of lower doses of PYM and cetuximab resulted in 88.7% inhibition of tumor growth. The combination of PYM and cetuximab designed by us focused the same target-EGFR, although they may have different mechanisms involving the interaction with EGFR. As known, cetuximab competitively inhibits the binding of EGF and other ligands, such as TNF-α, to EGFR. The binding of cetuximab to the EGFR blocks phosphorylation and activation of receptor-associated kinases, resulting in inhibition of cell growth, induction of apoptosis and other effects. BLM acts by induction of DNA strand breaks. The cytotoxicity of PYM is to mediate both single-stranded and double-stranded DNA damage. So, the different pattern of targeted EGFR induced by cetuximab and PYM involved in the EGFR pathway induced by the two drugs provided a strong rationale to investigate the biological activity of the combination of the drugs.
Targeted therapy might offer an alternative treatment and the combination of antibody-based drug, and chemotherapeutic agent may improve results than therapy alone. A study by Oliveras-Ferraros C demonstrated that CDDP interacts with cetuximab at decreasing EGFR protein expression in MDA-MB-468 basal-like breast cancer cells. The cetuximab, CDDP alone and the combination of cetuximab at the optimal concentration of CDDP could inhibit the EGFR expression by 48, 44 and 97% [33]. Our results also showed that the antibody-based drug cetuximab showed low antiproliferative activity. As known, the action mechanism of cetuximab is to bind to the EGFR and prevent other ligands binding and activating of EGFR-phophorylation and the downstream signaling pathway. PYM as traditionally chemotherapeutic agents was effective in esophageal cancer cells. We first demonstrated that PYM could reduce significantly the expression of EGFR both at the mRNA and protein levels. Thus, the simultaneous targeting of cell surface membrane receptor with monoclonal antibodies and chemotherapy agents would be an effective anticancer therapy. This strategy could be called target-oriented combination. The target-oriented combination, especially the combination of a therapeutic antibody and a traditional chemotherapy drug both act on the same target, is a promising research strategy.
In summary, EGFR is the one of most important target in cancer-targeted therapy and cetuximab with chemotherapy is a promising combination toward esophageal cancer. Results of this study indicated that PYM could downregulate EGFR expression and potentiate the efficacy of cetuximab, an EGFR targeting antibody therapeutic, against EGFR highly expressed esophageal cancer. Further studies are needed to investigate the exact downstream events in this target-oriented combination.
References
Zhen YS, Li DD, Li Q et al (1981) Studies on antitumor effect and pharmacology of pingyangmycin. Topics on cancer chemotherapy. In: Proceedings of international symposium on adriamycin and other drugs in antitumor chemotherapy, China Academic Publishers, pp 211–224
Zhen YS, Li DD (2009) Antitumor antibiotic pingyangmycin: research and clinical use for 40 years. Chin J Antibiot 34:577–580
Byrnes RW, Templin J, Sem D, Lyman S, Petering DH (1990) Intracellular DNA strand scission and growth inhibition of Ehrlich ascites tumor cells by bleomycins. Cancer Res 50:5275–5286
Chen J, Stubbe J (2005) Bleomycins: towards better therapeutics. Nat Rev Cancer 5:102–112
Williams ED, Merrick MV, Lavender JP (1975) The distribution and dosimetry of 111In-Bleomycin in man. Br J Radiol 48:275–278
Homs MY, Voest EE, Siersema PD (2009) Emerging drugs for esophageal cancer. Expert Opin Emerg Drugs 14:329–339
Mariette C, Piessen G, Triboulet JP (2007) Therapeutic strategies in esophageal carcinoma: role of surgery and other modalities. Lancet Oncol 8:545–553
Gebski V, Burmeister B, Smithers BM, Foo K, Zalcberg J, Simes J (2007) Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in esophageal carcinoma: a metaanalysis. Lancet Oncol 8:226–234
Schlessinger J (2000) Cell signaling by receptor tyrosine kinases. Cell 103:211–225
Gibault L, Metges JP, Conan-Charlet V, Lozac’h P, Robaszkiewicz M, Bessaguet C, Lagarde N, Volant A (2005) Diffuse EGFR staining is associated with reduced overall survival in locally advanced oesophageal squamous cell cancer. Br J Cancer 93:107–115
Itakura Y, Sasano H, Shiga C, Furukawa Y, Shiga K, Mori S, Nagura H (1994) Epidermal growth factor receptor overexpression in esophageal carcinoma. An immunohistochemical study correlated with clinicopathologic findings and DNA amplification. Cancer 74:795–804
Yano H, Shiozaki H, Kobayashi K, Yano T, Tahara H, Tamura S, Mori T (1991) Immunohistologic detection of the epidermal growth factor receptor in human esophageal squamous cell carcinoma. Cancer 67:91–98
Hanawa M, Suzuki S, Dobashi Y, Yamane T, Kono K, Enomoto N, Ooi A (2006) EGFRprotein overexpression and gene amplification in squamous cell carcinomas of the esophagus. Int J Cancer 118:1173–1180
Miyawaki M, Hijiya N, Tsukamoto Y, Nakada C, Kawahara K, Moriyama M (2008) Enhanced phosphorylation of the epidermal growth factor receptor at the site of tyrosine 992 in esophageal carcinomas. APMIS 116:1097–1106
Carneiro A, Isinger A, Karlsson A, Johansson J, Jönsson G, Bendahl PO, Falkenback D, Halvarsson B, Nilbert M (2008) Prognostic impact of array-based genomic profiles in esophageal squamous cell cancer. BMC Cancer 11:98–106
Boone J, van Hillegersberg R, Offerhaus GJ, van Diest PJ, Borel Rinkes IH, Ten Kate FJ (2009) Targets for molecular therapy in esophageal squamous cell carcinoma: an immunohistochemical analysis. Dis Esophagus 22:496–504
Cao SS, Zhen YS (1989) Potentiation of antimetabolite: antitumor activity in vivo by dipyridamole and amphotericin B. Cancer Chemother Pharmacol 24:181–186
Zhen YS, Taniki T, Weber G (1992) Azidothymidine and dipyridamole as biochemical response modifiers: synergism with methotrexate and 5-fluorouracil in human colon and pancreatic carcinoma cells. Oncol Res 4:73–88
Matar P, Rojo F, Cassia R, Moreno-Bueno G, Di Cosimo S, Tabernero J, Guzmán M, Rodriguez S, Arribas J, Palacios J, Baselga J (2004) Combined epidermal growth factor receptor targeting with the tyrosine kinase inhibitor gefitinib (ZD1839) and the monoclonal antibody cetuximab (IMC-C225): superiority over single-agent receptor targeting. Clin Cancer Res 10:6487–6501
Yokoyama Y, Dhanabal M, Griffioen AW, Sukhatme VP, Ramakrishnan S (2000) Synergy between angiostatin and endostatin: inhibition of ovarian cancer growth. Cancer Res 60:2190–2196
Wei Q, Chen L, Sheng L, Nordgren H, Wester K, Carlsson J (2007) EGFR, HER2 and HER3 expression in esophageal primary tumours and corresponding metastases. Int J Oncol 31:493–499
Langer R, Von Rahden BH, Nahrig J, Von Weyhern C, Reiter R, Feith M, Stein HJ, Siewert JR, Höfler H, Sarbia M (2006) Prognostic significance of expression patterns of c-erbB-2, p53, p16INK4A, p27KIP1, cyclin D1 and epidermal growth factor receptor in oesophageal adenocarcinoma: a tissue microarray study. J Clin Pathol 59:631–634
Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, Ove R, Kies MS, Baselga J, Youssoufian H, Amellal N, Rowinsky EK, Ang KK (2006) Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 354:567–578
Safran H, Suntharalingam M, Dipetrillo T, Ng T, Doyle LA, Krasna M, Plette A, Evans D, Wanebo H, Akerman P, Spector J, Kennedy N, Kennedy T (2008) Cetuximab with concurrent chemoradiation for esophagogastric cancer: assessment of toxicity. Int J Radiat Oncol Biol Phys 70:391–395
Gold PJ, Goldman B, Iqbal S, Leichman LP, Lenz HJ, Blanke CD (2010) Cetuximab as second-line therapy in patients with metastatic esophageal cancer: a phase II Southwest Oncology Group Study. J Thorac Oncol 5:1472–1476
Ku GY, Shah MA, Tang LH, Miron B, Kelsen DP, Ilson DH (2008) Cetuximab (C225) plus irinotecan/cisplatin (CPT/Cis) for CPT/Cis-refractory esophageal cancer. J Clin Oncol 26(Supplement; ASCO Meeting Abstracts):15580
Hollstein MC, Smits AM, Galiana C, Yamasaki H, Bos JL, Mandard A, Partensky C, Montesano R (1988) Amplification of epidermal growth factor receptor gene but no evidence of ras mutations in primary human esophageal cancers. Cancer Res 48:5119–5123
Hollstein MC, Peri L, Mandard AM, Welsh JA, Montesano R, Metcalf RA, Bak M, Harris CC (1991) Genetic analysis of human esophageal tumors from two high incidence geographic areas: frequent p53 base substitutions and absence of ras mutations. Cancer Res 51:4102–4106
Lyronis ID, Baritaki S, Bizakis I, Krambovitis E, Spandidos DA (2008) K-ras mutation, HPV infection and smoking or alcohol abuse positively correlate with esophageal squamous carcinoma. Pathol Oncol Res 14:267–273
Nakayama Y, Igarashi A, Kikuchi I, Obata Y, Fukumoto Y, Yamaguchi N (2009) Bleomycin-induced over-replication involves sustained inhibition of mitotic entry through the ATM/ATR pathway. Exp Cell Res 315:2515–2528
Zhou BB, Elledge SJ (2000) The DNA damage response: putting checkpoints in perspective. Nature 408:433–439
Benhar M, Engelberg D, Levitzki A (2002) ROS, stress-activated kinases and stress signaling in cancer. EMBO Rep 3:420–425
Oliveras-Ferraros C, Vazquez-Martin A, López-Bonet E, Martín-Castillo B, Del Barco S, Brunet J, Menendez JA (2008) Growth and molecular interactions of the anti-EGFR antibody cetuximab and the DNA cross-linking agent cisplatin in gefitinib-resistant MDA-MB-468 cells: new prospects in the treatment of triple-negative/basal-like breast cancer. Int J Oncol 33:1165–1176
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gong, Jh., Liu, Xj., Li, Y. et al. Pingyangmycin downregulates the expression of EGFR and enhances the effects of cetuximab on esophageal cancer cells and the xenograft in athymic mice. Cancer Chemother Pharmacol 69, 1323–1332 (2012). https://doi.org/10.1007/s00280-012-1827-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-012-1827-9