Abstract
Purpose
Paclitaxel-based chemoradiotherapy was proven to be efficacious in treating patients with advanced esophageal cancer. However, the toxicity and the development of resistance limited its anticancer efficiency. The present study was to evaluate the antitumor effects of lapatinib, a dual tyrosine inhibitor of both epidermal growth factor receptor (EGFR) and human epidermal growth factor receptor 2 (HER2), combined with paclitaxel on the esophageal squamous cancer.
Methods
MTT assays were used to evaluate the effects of the combination of lapatinib and paclitaxel on the growth of esophageal squamous cancer cell lines (KYSE150, KYSE450, KYSE510 and TE-7). The activity of the combination of two agents on cell invasion, migration and apoptosis was measured by wound healing assay, transwell assay and Annexin V-FITC/PI stain assay. Western blot assay was used to analyze the effects of the two agents on the EGFR/HER2 signaling. The in vivo efficacy was evaluated in KYSE450 xenograft nude mouse model.
Results
The combination of lapatinib and paclitaxel was highly synergistic in inhibiting cell growth with a combination index of < 1, and suppressed significantly the invasion and migration capability of esophageal squamous cancer cells. Esophageal squamous cancer cells displayed increased rates of apoptosis after treatment with lapatinib plus paclitaxel. The phosphorylated EGFR and HER2 as well as the activation of downstream molecules MAPKs and AKT significantly decreased when exposed to lapatinib and paclitaxel. In vivo studies showed that the combination of two agents had greater antitumor efficacy than either agent alone.
Conclusions
The combination of lapatinib with paclitaxel showed synergistic antitumor activity, suggesting their potential in treating patients with esophageal squamous cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Esophageal cancer, one of the most common malignant tumors, is the sixth leading cause of cancer-related death in the word [1]. Esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC) are the two histopathologically different types of esophageal cancer. EAC mainly occurs in western countries, whereas the incidence of ESCC is predominant in Asian countries and southeastern Africa with an estimation of more than 100 cases/100,000 person-years [2, 3]. Treatments for esophageal cancer include combinations of surgery, radiotherapy and chemotherapy. However, many cases of esophageal cancer were diagnosed at an inoperable stage and complete resection was rarely performed because esophageal cancer spreads rapidly. Therefore, 5-year survival rates for esophageal cancer are poor, ranging from 15 to 25% [4]. Neoadjuvant therapy with chemotherapy or radiotherapy has supplemented surgery as standard treatment of locally advanced esophageal cancer. The optimal regimen of chemoradiotherapy has not yet been established. Standard combinations consisting of cisplatin with 5-fluorouracil or paclitaxel are widely used [5,6,7]. Several clinical studies revealed that these combinations have significant therapeutic effects on advanced ESCC [8,9,10]. However, the toxicity and the development of resistance limited their anticancer efficiency. As a result, the development of novel chemosensitizer or new drug combination therapies against ESCC is imperative.
Epidermal growth factor receptor (EGFR) and human epidermal growth factor receptor 2 (HER2/ErbB2) are members of the HER/ErbB tyrosine kinase receptor family. Both of them play crucial roles in cell proliferation, survival, migration, and metastasis [11]. EGFR and HER2 overexpression have been observed in over 30% of esophageal cancer, and their expression was correlated with increased invasion, poor differentiated histology, and worse prognosis [12,13,14]. These data provided the rationale for targeting EGFR and HER2 in the treatment of esophageal cancer.
Lapatinib (GW572016) is a small-molecule dual tyrosine kinase inhibitor of both EGFR and HER2. In 2007, lapatinib was first approved by FDA for combination with capecitabine in the treatment of advanced breast cancer patients with HER2 positive [15]. In addition to breast cancer, lapatinib has proven efficacy in a range of malignancies in preclinical models [16,17,18,19]; however, in clinical trials, lapatinib used alone resulted only in minimal activity [20,21,22]. Studies from our group and others revealed that lapatinib was effective in treating esophageal, gastroesophageal junction and gastric cancers when in combination with chemotherapy agents [23,24,25]. Paclitaxel, also called taxol, is an anti-microtubule agent that stabilizes microtubule structure within the cells, causing mitotic arrest and apoptosis [26]. It has been used for the treatment of various types of solid cancers, showing response rates of around 30% when used alone and around 40% when used in combination with cisplatin in ESCC [10, 27,28,29]. Moreover, paclitaxel may increase the sensitivity of tumor cells to radiation [30]. In the present study, we evaluate the antitumor activity of lapatinib combined with paclitaxel on esophageal squamous cancer in vitro and in vivo. Our objective was to identify the potential interactions between lapatinib and paclitaxel, and provide the essential data for the next clinical trials of the combination on patients with esophageal squamous cancer.
Materials and methods
Ethics statement
BALB/c nude mice (female, 6–8 weeks, 18–20 g) used in the study were purchased from Vital River Laboratory Animal Technology Co. Ltd. (Beijing, China), and maintained under specific pathogen-free (SPF) conditions. All experimental procedures were approved by the Ethics Committee of Xinxiang Medical University (No. XXMU-2016-050) and were performed in accordance with international and national guidelines.
Cell lines and culture
Human esophageal squamous carcinoma cell (ESCC) lines KYSE150, KYSE450, KYSE510 and TE-7 were obtained from Cell Center of Peking Union Medical College, China, and cultured in RPMI 1640 media supplemented with 10% fetal bovine serum (FBS, Life Technologies, CA, USA), 100 unit/ml penicillin and 100 mg/ml streptomycin (Beyotime Biotechnology, Jiangsu, China). The cells were cultured at 37 °C in a 5% CO2 environment.
Reagents and antibodies
Lapatinib (GW572016) was purchased from MedChem Express (MCE, USA), dissolved in dimethyl sulfoxide (DMSO) and stored at the concentration of 10 mmol/l. Paclitaxel injection (5 mg/ml) was purchased from the Beijing SL PHARM (Beijing, China), and diluted with PBS just before use. ((3-(4, 5-Dimethyl-thiazol-2-yl)-2, 5-diphenyltetrazolium bromide) (MTT) was obtained from Sigma-Aldrich Chemical Inc. (St. Louis, MO, USA). The primary antibodies [anti-EGFR, -HER2, -AKT, -p38MAPK, -ERK, -SAPK/JNK, -β-actin and phosphorylated EGFR, -HER2, -AKT (Ser473), -p38MAPK (Thr180/Thr182), -ERK (Thr202/Thr204), -SAPK/JNK (Thr183/Thr185)] were provided by Cell Signaling Technology (MA, USA). Annexin V-FITC Apoptosis Detection Kit was supplied by Beyotime Biotechnology (Jiangsu, China). Matrigel invasion chamber 24-well plate 8.0 micron was purchased from Corning (NY, USA).
Cell viability assay
Cell viability was measured by MTT assays. ESCC cells were seeded in 96-well plates for 24 h. After treatment with lapatinib, paclitaxel alone or the combination at different concentrations for 48 h, 20 µl MTT (5 mg/ml) was added to each well, and incubated for 4 h at 37 °C. The supernatant was removed and 150 µl DMSO were added to each well. After shaking for 5 min, the absorbance at 570 nm was measured using an ELISA reader. Growth inhibition was calculated as a percentage of the untreated controls. Combination drug index (CI) of lapatinib plus paclitaxel was calculated using the median effect principle (Talalay–Chou method) [31]. The CI < 1, CI = 1 and CI > 1 indicate the synergistic, additive and antagonistic effect, respectively.
Wound healing assay
Cells were seeded in six-well plates at a density of 4 × 105 cells per well and cultured for 24 h to yield confluent monolayers for wounding. A sterile 10-µl pipette tip was used to gently and solely scratch the monolayer at the centre of each well. The cell debris was removed by washing with PBS then cells were treated with vehicle (0.5% DMSO), lapatinib (5 µmol/l), paclitaxel (5 ng/ml) alone or in combination for another 24 h. The cells were photographed using a light microscope. Experiments were performed three times.
Transwell migration and invasion assay
Cell migration and invasion was measured using a two-chamber transwell system (8-µm pore size). For migration assay, esophageal squamous cancer cells (2 × 104) suspended in 200 µl of serum-free 1640-RPMI were directly planted in the upper chambers of the transwell. For invasion assay, the upper chambers were first coated with matrigel according to the manufacturer’s instruction, and then the cells were planted in the upper chambers. The vehicle (0.5% DMSO), lapatinib (5 µmol/l), paclitaxel (5 ng/ml) alone or combination of lapatinib and paclitaxel was added to the upper chambers in both assays. Then 500 µl of 1640-RPMI medium containing 10% serum was added to the lower chambers. The transwell chambers were incubated at 37 °C in 5% CO2 for 48 h (KYSE150 cells) or 72 h (TE-7 cells). Cells that did not migrate or invade through the filter and/or matrigel were gently wiped with a cotton swab. Migrated or invaded cells beneath the filter were fixed with 4% paraformaldehyde for 10 min and stained with crystal violet for 30 min. The migrated or invaded cells were observed under inverted microscope and were quantified by counting the number of cells from five random fields of vision.
Cell apoptosis assays
The apoptotic effect of lapatinib and/or paclitaxel on esophageal squamous cancer cells were tested using an Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) staining. Cells were seeded in six-well plates and incubated for 24 h, then treated with vehicle (0.5% DMSO), lapatinib (5 µmol/l), paclitaxel (5 ng/ml), or lapatinib combined with paclitaxel for 48 h. According to the manufacturer’s instruction, cells were collected, washed twice with PBS, and resuspended in 200 µl binding buffer containing Annexin V-FITC and PI (Beyotime Biotechnology, Jiangsu, China). Cells were incubated for 15 min in the dark. After adding 400 µl binding buffer, cells were analyzed for fluorescence with a flow cytometer (BD Corp).
Western blot assay
Esophageal squamous cancer cells (KYSE150, KYSE450, KYSE510 and TE-7) were plated into 100-mm dishes at the density of 1 × 106 and cultured for 24 h. Then, the cells were incubated with vehicle (0.5% DMSO), lapatinib (5 µmol/l) or paclitaxel (5 ng/ml) or the two drugs combination for 48 h. The cells were lysed on ice for 30 min in cell lysis buffer (Beyotime biotechnology, Jiangsu, China) containing 1% phenylmethanesulfonyl fluoride (PMSF). For proteins extracted from tumor tissues, 100 mg tumor tissues were put in 1 ml RIPA lysis buffer (Beyotime biotechnology, Jiangsu, China) supplemented with 1 mmol/l PMSF, homogenized on ice, and lysed on ice for 30 min. Cell or tissue extracts were clarified by centrifugation at 10,000g for 15 min at 4 °C, and the total protein was quantitated using bicinchoninic acid kit (Beijing Dingguo Biotechnology, Beijing, China). Each protein (30 µg) was applied on a 10% SDS-PAGE and transferred onto polyvinylidene fluoride (PVDF) membranes (Millipore, MA, USA). The membranes were blocked with 5% BSA for 2 h at room temperature, and then incubated with primary antibodies overnight at 4 °C (diluted 1:1000, Cell Signaling Technology). After washing with TBST buffer three times, membranes were incubated with secondary HRP-conjugated antibodies for 1 h at room temperature (diluted 1:4000, Cell Signaling Technology). The bands were visualized by reacting with the Immobilon Western Chemiluminescent HRP Substrate (Millipore) and captured by Amersham Imager 600 system (GE Healthcare, UT, USA).
In vivo efficacy assay
In vivo efficacy of lapatinib, paclitaxel alone or the combination was measured by KYSE450 xenograft nude mice model. Female BALB/c nude mice were purchased from Vital River Laboratory Animal Technology Co. Ltd., and hosted under specific pathogen-free conditions. 1 × 107 KYSE450 cells resuspended in 200 µl PBS were injected into right armpit of the nude mice subcutaneously. When the average tumor volume reached 100 mm3 (about 10 days later), the mice were randomly divided into four groups (n = 8): control group, lapatinib treatment group, paclitaxel treatment group and lapatinib plus paclitaxel treatment group. Lapatinib was dissolved in 0.5% carboxymethyl cellulose and 0.1% Tween-80 vehicle and was given once daily at dosage of 100 mg/kg by oral gavage. Paclitaxel was administered at dosage of 7.5 mg/kg every 7 days by intraperitoneal injection. The control group received 0.5% carboxymethyl cellulose/0.1% Tween-80 vehicle and PBS treatment at the same schedule as lapatinib and paclitaxel. Tumor size and animal body weight were measured every 3 days and tumor volume was determined by length × width2/2. The inhibition rates were calculated by 1 − tumor volume (treated)/tumor volume (control) × 100%. The mice were killed on day 35, and the tumors were removed from the mice and weighed.
Statistical analysis
Each experiment was repeated at least three times. Results are presented at mean ± standard deviation (SD) and the data were analyzed using the GraphPad Prism 5 software. Statistical significance was evaluated using one-way analysis of variance (ANOVA). P < 0.05 was considered statistically significant.
Results
Effects of lapatinib in combination with paclitaxel on the growth of esophageal squamous cancer cells
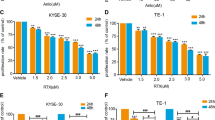
First, the EGFR and HER2 expression levels on four ESCC cell lines and normal epithelium cells were analyzed. The four ESCC cell lines showed EGFR and HER2 expression in high levels (except that the KYSE510 cells have a relatively low expression of EGFR). But in normal esophageal epithelial cells, EGFR and HER2 levels were much lower than that of ESCC cells (Fig. 1). The cytotoxicity of lapatinib and paclitaxel alone in different ESCC cells is shown in Fig. 2a and Table 1. Four ESCC cell lines (KYSE150, KYSE450, KYSE510 and TE-7) showed similar sensitivity to lapatinib with IC50 values about 5 µmol/l. Therefore, there were no correlations between the IC50 values and the EGFR and HER2 expression levels. The sensitivity of TE-7 cells to paclitaxel was much lower than that of the other three cells. To determine the combination effects, cells were treated with both agents at fixed ratios (lapatinib:paclitaxel = 100:1) spanning the IC50 of each agent. Combination treatment with lapatinib and paclitaxel significantly decreased the growth of cells compared to either agent alone (Fig. 2b). To determine the interactions between lapatinib and paclitaxel were antagonistic, additive or synergistic, the Chou–Talalay method was used to determine the combined index. Clear synergy was noted between lapatinib and paclitaxel in the KYSE450, KYSE510 and TE-7 cells (CI < 1). The exception to this was KYSE150 cells treated with higher concentrations of lapatinib and paclitaxel in which the effects were additive or antagonistic (Fig. 2c).
Growth inhibition effects of lapatinib, paclitaxel alone and in combination on ESCC cells. Cells were treated with lapatinib or paclitaxel alone (a) or the combination of two drugs mixed at concentration ratio of 100:1 (b) for 48 h, then the cell viability was measured by MTT assays. c The combination index (CI) values of lapatinib and paclitaxel against ESCC cells were calculated using Chou–Talalay method. The CI values of < 1 which indicated the synergistic interactions between lapatinib and paclitaxel
Effects of the combination of lapatinib with paclitaxel on the invasion and migration of esophageal squamous cancer cells
The effects of lapatinib and paclitaxel on the migration and invasion of esophageal squamous cancer cells were measured using wound healing assay and transwell assay. The migration assays showed that 5 µmol/l lapatinib and 5 ng/ml paclitaxel could significantly decrease the migration of KYSE150 cells and TE-7 cells. And combined treatment of lapatinib with paclitaxel reduced the migration capability more significantly than either agent alone (Fig. 3a–d). After exposure to lapatinib and paclitaxel, the ESCC cells that invade through the matrigel membrane decreased markedly compared with vehicle, and lapatinib or paclitaxel treatment alone (P < 0.05, Fig. 3e, f). These indicated that combination of the two agents synergistically inhibit the invasion and migration of ESCC cells.
The effects of lapatinib in combination with paclitaxel on the invasion and migration of ESCC cells. The migration capability of KYSE150 cells (a) or TE-7 cells (b) after exposure to lapatinib (5 µmol/l), paclitaxel (5 ng/ml) or lapatinib plus paclitaxel for 24 h was measured by wound healing assays. c, d KYSE150 cells or TE-7 cells were planted in the upper chambers of transwell system, and incubated with indicated drugs [vehicle refers to 0.5% DMSO, lap refers to lapatinib (5 µmol/l), and pac refers to paclitaxel (5 ng/ml)] for 48 h (KYSE150) or 72 h (TE-7). The migrated cells from five random fields of vision (× 200) were counted and analyzed using one-way ANOVA and Tukey post hoc test. **P < 0.01 vs vehicle, ***P < 0.001 vs vehicle. ###P < 0.001, lapatinib + paclitaxel vs lapatinib or paclitaxel. e KYSE150 or TE-7 cells invaded through the matrigel membrane were measured by matrigel-coated transwell assay (× 200). f The invaded cells from five random fields of vision were counted and analyzed using two-tailed paired t test. **P < 0.01 vs vehicle, ***P < 0.001 vs vehicle. #P < 0.05, lapatinib + paclitaxel vs lapatinib or paclitaxel. ##P < 0.01, lapatinib + paclitaxel vs lapatinib or paclitaxel
Synergistic proapoptotic activity of lapatinib and paclitaxel on esophageal squamous cancer cells
To confirm the synergistic interaction of lapatinib with paclitaxel, we evaluated the induction of cell apoptosis in four ESCC cell lines. Lapatinib or paclitaxel alone induced TE-7 cell apoptosis of 15.75 and 21.52%, respectively (P < 0.001 vs vehicle). The combination of the two agents induced cell apoptosis of 38.8%, which is significantly higher than that of lapatinib or paclitaxel alone (P < 0.001). Similar results were also obtained from KYSE150, KYSE450 and KYSE510 cell lines (Fig. 4a, b). As one of the main cleavage targets of caspase 3, the level of cleaved PARP increased markedly after treatment with the combination of lapatinib and paclitaxel (Fig. 4c). This result confirmed that lapatinib combined with paclitaxel demonstrated enhanced proapoptotic activity on esophageal squamous cancer cells.
Effects of lapatinib, paclitaxel alone or in combination on apoptosis of ESCC cells. a Cells were treated with single lapatinib (5 µmol/l), paclitaxel (5 ng/ml) or both drugs for 48 h, and the apoptotic cells were stained by Annexin V-FITC and PI. The lower left quadrants (FITC−/PI−) indicated the viable cells, and the lower right quadrants (FITC+/PI−) indicated the early apoptotic cells. The upper right quadrants (FITC+/PI+) indicated the late apoptotic cells, and the upper left quadrants (FITC−/PI+) indicated the dead cells. b Apoptosis ratios are the sum of early apoptotic cells and late apoptotic cells. Vehicle refers to 0.5% DMSO, lap refers to lapatinib, and pac refers to paclitaxel. *P < 0.05 vs vehicle, **P < 0.01 vs vehicle, ***P < 0.001 vs vehicle. ##P < 0.01 lapatinib + paclitaxel vs lapatinib or paclitaxel alone, ###P < 0.001 lapatinib + paclitaxel vs lapatinib or paclitaxel. c The cleaved PARP levels of ESCC cells after exposure to indicated drugs for 48 h were detected by western blot analysis. Actin was used as a loading control
Effects of the combination of lapatinib with paclitaxel on the EGFR/HER2 signaling pathways
To discuss the mechanisms of synergistic cell growth inhibition and enhanced apoptosis after treatment with lapatinib and paclitaxel, ESCC cells were treated with either drug alone or the combination for 48 h followed by assessment of HER signaling pathways using western blot analysis. The signaling pathways induced by activated EGFR and HER2 include the MAPK and the phosphatidylinositol 3-kinase (PI3K)/AKT pathway, both of which play a key role in the proliferation and cell survival responses. As shown in Fig. 5, phosphorylation of EGFR and HER2 was activated at detectable levels under normal culture conditions with serum. Downstream signaling molecules, such as AKT, ERK, p38MAPK, and JNK, were also activated. Paclitaxel alone shows negligible effects on the EGFR/HER2 activation as well as the activation of downstream molecules. Lapatinib treatment alone significantly inhibited the EGFR and HER2 phosphorylation, and then blocked the activation of ERK, AKT and p38MAPK. But the combination of lapatinib with paclitaxel showed further enhanced inhibitory effects on the EGFR/HER2 signaling. Phosphorylation of JNK was not affected by the lapatinib alone in the KYSE150, KYSE450 and TE-7 cells, whereas it decreased significantly in the presence of both lapatinib and paclitaxel in four ESCC cell lines. Lapatinib and paclitaxel alone or in combination did not show any effects on the amount of total EGFR, HER2, ERK, p38MAPK, JNK and AKT protein.
Effects of lapatinib, paclitaxel alone or in combination on the EGFR/HER2 signal pathway. KYSE150, KYSE450, KYSE510 and TE-7 cells were incubated with lapatinib (5 µmol/l), paclitaxel (5 ng/ml) or both drugs for 48 h, the phosphorylation of EGFR, HER2, p42/44MAPK (ERK), p38MAPK, JNK and AKT as well as their total expression levels were detected by western blot analysis. Actin was used as a loading control
In vivo efficacy and mechanisms of the combination of lapatinib with paclitaxel
The in vivo antitumor effects of lapatinib and paclitaxel in combination were tested in a mouse xenograft model bearing KYSE450 tumors. They administrated alone or in combination both demonstrated significant inhibition in the xenograft growth (Fig. 6a). Single lapatinib treatment at 100 mg/kg for 24 days (from day 12 to day 35) yielded tumor growth inhibition rate of 46.3% (P < 0.01 vs control). Paclitaxel alone at 7.5 mg/kg in once-a-week regimen (injection i.p. at day 12, 19, 26 and 33) yielded tumor growth inhibition rate of 40.7% (P < 0.05 vs control). The combination of two agents yielded a significantly greater inhibition in tumor growth than either agent alone (inhibition rate of 64.2%, P < 0.05 compared with lapatinib-treated group; P < 0.01 compared with paclitaxel-treated group). All treatments were well tolerated, and no animals death, body weight loss, or any toxic signs were observed during the course of the treatment (Fig. 6b).
In vivo antitumor efficacy of lapatinib, paclitaxel alone or in combination. Nude mice bearing the KYSE450 xenografts were treated with carboxymethyl cellulose/Tween-80 vehicle, lapatinib (100 mg/kg), paclitaxel (7.5 mg/kg), or lapatinib plus paclitaxel as shown in Materials and Methods. The mean tumor volume (a) and mean animal weight (b) in each group during the experiment were shown. **P < 0.01 vs vehicle, ***P < 0.001 vs vehicle. ##P < 0.01, lapatinib + paclitaxel vs lapatinib or paclitaxel. c The inhibitory effects of lapatinib, paclitaxel alone or in combination on EGFR/HER2 signaling in xenograft tumors. Mice bearing KYSE450 xenografts were killed on day 35. The tumors were isolated from the mice and immediately frozen in liquid nitrogen. Total proteins were extracted from tumor tissues and the phosphorylation and total expression of EGFR, HER2, AKT and ERK were analyzed by western blot
To further investigate the in vivo mechanisms of antitumor efficacy, the phosphorylation of EGFR and HER2 as well as the downstream molecules AKT and ERK in the xenograft tumor tissues after treatment with lapatinib, paclitaxel alone or in combination were detected by western blot analysis. The results demonstrated that phosphorylation of EGFR, HER2, AKT and ERK decreased slightly in the single lapatinib or paclitaxel treatment group. But their phosphorylation levels markedly reduced after treatment by lapatinib in combination with paclitaxel. The total EGFR, HER2, AKT and ERK protein levels also remained unchanged after exposure to the single drug or combination (Fig. 6c).
Discussion
For advanced or metastatic esophageal cancer, chemotherapy is the main stay of treatment options. Cisplatin-based regimen (cisplatin and fluorouracil) and taxane-based regimen (paclitaxel and docetaxel) represented the most used chemotherapy backbone [8]. However, none of the clinical trials reported a statistically significant difference in overall survival when compared with surgery alone [32, 33]. Therefore, recent clinical trials have focused on the addition of targeted therapies to a chemotherapy backbone, and most work has focused on anti-vascular endothelial growth factor (VEGF) and anti-EGFR/HER2 therapies. Bevacizumab (Avastin), a humanized monoclonal antibody that targets the VEGF, was evaluated in combination with capecitabine–cisplatin in the first-line treatment of advanced gastric cancer in a phase III trial. The results revealed that adding bevacizumab to chemotherapy was associated with significant increases in progression-free survival (6.7 vs 5.3 months; P = 0.0037) and overall response rate (46.0 vs 37.4%; P = 0.0315) [34]. Trastuzumab (Herceptin) is a humanized monoclonal antibody that targets the HER2. Combination with chemotherapy vs chemotherapy alone for the treatment of HER2-positive advanced gastric or gastrio-esophageal junction cancer was evaluated in a phase 3, open-label, randomised controlled trial (ToGA), and the results showed that median overall survival was significantly increased in the trastuzumab plus chemotherapy group [35]. However, the patients with esophageal cancer did not benefit from the anti-EGFR agents including the monoclonal antibodies (e.g. cetuximab) and tyrosine kinase inhibitors (e.g. gefitinib and erlotinib) [36, 37]. This was probably due to the mutations of KRAS and EGFR is rare in esophageal cancer [38], and the crosstalk between EGFR and HER2 was another reason. Studies have shown that HER2 gene amplification may have implications in predicting response to EGFR inhibition [39]. Lapatinib is a tyrosine kinase inhibitor (TKI) of both EGFR and HER2, its application in treating various cancers including esophageal cancer and gastric cancer when combined with chemotherapies has been reported. The TRIO-013/LOGiC, a randomized phase III trial showed that addition of lapatinib to capecitabine and oxaliplatin did not increase overall survival in patients with HER2-amplified gastroesophageal adenocarcinoma. For the esophageal squamous cell carcinoma, studies from our team and other teams revealed that lapatinib synergistically interacts with 5-fluorouracil in inhibiting the growth of ESCC cells [40, 41]. In present study, we evaluated the therapeutic potential of lapatinib alone or in combination with another standard chemotherapeutic drug, paclitaxel, for the treatment of esophageal squamous cancer, and to understand the mechanisms of action of these drugs.
The effects of lapatinib alone or in combination with paclitaxel on cell viability were measured on four ESCC cells that express differential levels of EGFR and HER2. Lapatinib showed similar cytotoxicity to ESCC cells. There was no correlation between the IC50 values of lapatinib and the EGFR and HER2 expression level. When combined with paclitaxel, the cell growth was further inhibited, and the combination index (CI) values were less than 1 which indicated a synergistic effect between lapatinib and paclitaxel. The synergy was seen when the ratio of concentrations of two drugs was 100:1 (lapatinib:paclitaxel). In vivo, the combination of lapatinib with paclitaxel also showed an enhanced inhibition in the growth of esophageal squamous carcinoma xenografts with no increase in toxicity. Furthermore, the dose of paclitaxel in our experiment was 7.5 mg/kg (about 25 mg/m2) for once a week. This dosage was significantly lower than the clinical dose of paclitaxel (135–175 mg/m2). These data were very meaningful for the clinical application because the strategies based on chemosensitisation using decreased doses of toxic agents in combination with a low-toxic tyrosine kinase inhibitors to complement the efficacy of the treatment are the trends of recent studies.
Metastasis is a key biological characteristic of malignant tumors; we also tested the effects of lapatinib combined with paclitaxel on the invasion and migration capability of ESCC cells. Wound healing assay and transwell assay both revealed that lapatinib acted synergistically with paclitaxel in inhibiting the metastasis of ESCC cells. In addition, the two agents also showed synergistic proapoptotic activity on the ESCC cells when they used together.
We found that the synergistic antitumor effects of lapatinib in combination with paclitaxel were probably mediated by changes in cell signaling. As a potent pan-ErbB tyrosine kinase inhibitor, lapatinib (5 µmol/l) treatment alone exhibited significant inhibiting activity on the phosphorylation of EGFR and HER2, whereas the paclitaxel (5 ng/ml) alone showed negligible effect on the EGFR and HER2 activation. However, the levels of phosphorylated EGFR and HER2 were much lower following treatment with lapatinib in combination with paclitaxel. Ras/MAPK and PI3K/AKT are the two main downstream signal pathways that are mediated by EGFR/HER2. Treatment with the combination of lapatinib and paclitaxel resulted in marked decrease in the phosphorylation levels of MAPKs (ERK, p38MAPK and JNK) and AKT, and this reduction was more significant than that of treatment with either agent alone. Our data suggest that inhibition of the EGFR/HER2 signaling pathway by combining a chemotherapeutic drug and a TKI may augment the effects of both agents on the downstream signaling pathways.
A study from McHugh et al. [42] demonstrated that antagonism was observed when lapatinib and chemotherapy agents (capecitabine and cisplatin) were given together, but the synergy was seen when lapatinib was given before and during the chemotherapy agents. As a result, the identification of optimal schedules is also important for the successful clinical evaluation of cancer therapeutics, especially when the two drugs are combined. In the present study, the synergy was obtained by lapatinib administered concomitant with paclitaxel. Further experiments are required to test whether lapatinib given before or after the paclitaxel have some effects on the two drugs interaction.
In summary, lapatinib combined with cytotoxic agent, paclitaxel, have synergistic effects on inhibiting the growth and proliferation of esophageal squamous cancer cell both in vitro and in vivo. Their combination also showed enhanced activity on the cell apoptosis, invasion and migration. These efficacies were achieved by inhibiting the EGFR/HER2 signaling pathways. Our study will provide essential data for the next clinical investigations of lapatinib plus paclitaxel on esophageal squamous cancer.
References
Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Dicker D, Pain A, Hamavid H et al (1984) The global burden of cancer 2013. JAMA Oncol 1:505–527. https://doi.org/10.1001/jamaoncol.2015.0735
Wang AH, Liu Y, Wang B, He YX, Fang YX, Yan YP (2014) Epidemiological studies of esophageal cancer in the era of genome-wide association studies. World J Gastrointest Pathophysiol 5:335–343. https://doi.org/10.4291/wjgp.v5.i3.335
Domper Arnal MJ, Ferrández Arenas Á, Lanas Arbeloa Á (2015) Esophageal cancer: risk factors, screening and endoscopic treatment in Western and Eastern countries. World J Gastroenterol 21:7933–7943. https://doi.org/10.3748/wjg.v21.i26.7933
Bystricky B, Okines AF, Cunningham D (2011) Optimal therapeutic strategies for resectable oesophageal or oesophagogastric junction cancer. Drugs 71:541–555. https://doi.org/10.2165/11585460-000000000-00000
Mariette C, Piessen G, Triboulet JP (2007) Therapeutic strategies in oesophageal carcinoma: role of surgery and other modalities. Lancet Oncol 8:545–553. https://doi.org/10.1016/S1470-2045(07)70172-9
Lagergren J, Smyth E, Cunningham D, Lagergren P (2017) Oesophageal cancer. Lancet 390:2383–2396. https://doi.org/10.1016/S0140-6736(17)31462-9
Neuner G, Patel A, Suntharalingam M (2009) Chemoradiotherapy for esophageal cancer. Gastrointest Cancer Res 3:57–65. https://doi.org/10.1080/17512433.2017.1313112
Tomasello G, Ghidini M, Barni S, Passalacqua R, Petrelli F (2017) Overview of different available chemotherapy regimens combined with radiotherapy for the neoadjuvant and definitive treatment of esophageal cancer. Expert Rev Clin Pharmacol 10:649–660. https://doi.org/10.1080/17512433.2017.1313112
Imai H, Komine K, Takahashi S et al (2016) Efficacy and safety assessment of paclitaxel in patients with docetaxel-resistant esophageal squamous cell carcimona. Chemotherapy 61:262–268. https://doi.org/10.1159/000444122
Liu Y, Ren Z, Yuan L, Xu S, Yao Z, Qiao L, Li K (2016) Paclitaxel plus cisplatin vs. 5-fluorouracil plus cisplatin as first-line treatment for patients with advanced squamous cell esophageal cancer. Am J Cancer Res 6:2345–2350
Holbro T, Civenni G, Hynes NE (2003) The ErbB receptors and their role in cancer progression. Exp Cell Res 284:99–110
Gibault L, Metges JP, Conan-Charlet V, Lozac’h P, Robaszkiewicz M, Bessaguet C, Lagarde N, Volant A (2005) Diffuse EGFR staining is associated with reduced overall survival in locally advanced esophageal squamous cell cancer. Br J Cancer 93:107–115. https://doi.org/10.1038/sj.bjc.6602625
Brien TP, Odze RD, Sheehan CE, McKenna BJ, Ross JS (2000) Her-2/neu gene amplification by FISH predicts poor survival in Barrett’s esophagus-associated adenocarcinoma. Hum Pathol 31:35–39
Reddy D, Wainberg ZA (2011) Targeted therapies for metastatic esophagogastric cancer. Curr Treat Options Oncol 12:46–60. https://doi.org/10.1007/s11864-011-0138-4
Geyer CE, Forster J, Lindquist D et al (2006) Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 355:2733–2743. https://doi.org/10.1056/NEJMoa064320
Kondo N, Tsukuda M, Ishiguro Y, Kimura M, Fujita K, Sakakibara A, Takahashi H, Toth G, Matsuda H (2010) Antitumor effects of lapatinib (GW572016), a dual inhibitor of EGFR and HER2, in combination with cisplatin or paclitaxel on head and neck squamous cell carcinoma. Oncol Rep 23:957–963
Wainberg ZA, Anghel A, Desai AJ, Ayala R, Luo T, Safran B, Fejzo MS, Hecht JR, Slamon DJ, Finn RS (2010) Lapatinib, a dual EGFR and HER2 kinase inhibitor, selectively inhibits HER2-amplified human gastric cancer cells and is synergistic with trastuzumab in vitro and in vivo. Clin Cancer Res 16:1509–1519. https://doi.org/10.1158/1078-0432.CCR-09-1112
McHugh LA, Sayan AE, Mejlvang J, Griffiths TR, Sun Y, Manson MM, Tulchinsky E, Mellon JK, Kriajevska M (2009) Lapatinib, a dual inhibitor of ErbB-1/-2 receptors, enhances effects of combination chemotherapy in bladder cancer cells. Int J Oncol 34:1155–1163
Shiraishi K, Mimura K, Izawa S et al (2013) Lapatinib acts on gastric cancer through both antiproliferative function and augmentation of trastuzumab-mediated antibody-dependent cellular cytotoxicity. Gastric Cancer 16:571–580. https://doi.org/10.1007/s10120-012-0219-5
Powles T, Huddart RA, Elliott T et al (2017) Phase III, double-blind, randomized trial that compared maintenance lapatinib versus placebo after first-line chemotherapy in patients with human epidermal growth factor receptor 1/2-positive metastatic bladder cancer. J Clin Oncol 35: 48–55. https://doi.org/10.1200/JCO.2015.66.3468
Gomez HL, Doval DC, Chavez MA et al (2008) Efficacy and safety of lapatinib as first-line therapy for ErbB2-amplified locally advanced or metastatic breast cancer. J Clin Oncol 26:2999–3005. https://doi.org/10.1200/JCO.2007.14.0590
Lorenzen S, Riera Knorrenschild J, Haag GM et al (2015) Lapatinib versus lapatinib plus capecitabine as second-line treatment in human epidermal growth factor receptor 2-amplified metastatic gastro-oesophageal cancer: a randomised phase II trial of the Arbeitsgemeinschaft Internistische Onkologie. Eur J Cancer 51:569–576. https://doi.org/10.1016/j.ejca.2015.01.059
Guo XF, Zhu XF, Zhong GS, Deng BG, Gao ZT, Wang H (2012) Lapatinib, a dual inhibitor of EGFR and HER2, has synergistic effects with 5-fluorouracil on esophageal carcinoma. Oncol Rep 27:1639–1645. https://doi.org/10.3892/or.2012.1659
Tanizaki J, Okamoto I, Takezawa K, Tsukioka S, Uchida J, Kiniwa M, Fukuoka M, Nakagawa K (2010) Synergistic antitumor effect of S-1 and HER2-targeting agents in gastric cancer with HER2 amplification. Mol Cancer Ther 9:1198–1207. https://doi.org/10.1158/1535-7163.MCT-10-0045
Okines A, Cunningham D, Chau I (2011) Targeting the human EGFR family in esophagogastric cancer. Nat Rev Clin Oncol 8:492–503. https://doi.org/10.1038/nrclinonc.2011.45
Kavallaris M (2010) Microtubules and resistance to tubulin binding agents. Nat Rev Cancer 10:194–204. https://doi.org/10.1038/nrc2803
Ajani JA, Ilson DH, Daugherty K, Pazdur R, Lynch PM, Kelsen DP (1994) Activity of taxol in patients with squamous cell carcinoma and adenocarcinoma of the esophagus. J Natl Cancer Inst 86:1086–1091
Kato K, Tahara M, Hironaka S, Muro K, Takiuchi H, Hamamoto Y, Imamoto H, Amano N, Seriu T (2011) A phase II study of PTX biweekly 1-h infusion for advanced or recurrent esophageal cancer in patients who had previously received platinum based chemotherapy. Cancer Chemother Pharmacol 67:1265–1272. https://doi.org/10.1007/s00280-010-1422-x
Polee MB, Eskens FA, van der Burg ME, Splinter TA, Siersema PD, Tilanus HW, Verweij J, Stoter G, van der Gaast A (2002) Phase II study of bi-weekly administration of paclitaxel and cisplatin in patients with advanced oesophageal cancer. Br J Cancer 86:669–673. https://doi.org/10.1038/sj.bjc.6600166
Tishler RB, Schiff PB, Geard CR, Hall EJ (1992) Taxol: a novel radiation sensitizer. Int J Radiat Oncol Biol Phys 22:613–617
Chou TC, Talalay P (1984) Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 22:27–55
Ando N, Iizuka T, Ide H et al (2003) Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus: a Japan Clinical Oncology Group Study—JCOG9204. J Clin Oncol 21:4592–4596. https://doi.org/10.1200/JCO.2003.12.095
Ilson DH (2008) Esophageal cancer chemotherapy: recent advances. Gastrointest Cancer Res 2:85–92
Ohtsu A, Shah MA, Van Cutsem E et al (2011) Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol 29:3968–3976. https://doi.org/10.1200/JCO.2011.36.2236
Bang YJ, Van Cutsem E, Feyereislova A et al (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376:687–697. https://doi.org/10.1016/S0140-6736(10)61121-X
Suntharalingam M, Winter K, Ilson D et al (2017) Effect of the addition of cetuximab to paclitaxel, cisplatin, and radiation therapy for patients with esophageal cancer: the NRG oncology RTOG 0436 phase 3 randomized clinical trial. JAMA Oncol 3:1520–1528. https://doi.org/10.1001/jamaoncol.2017.1598
Dragovich T, McCoy S, Fenoglio-Preiser CM et al (2006) Phase II trial of erlotinib in gastroesophageal junction and gastric adenocarcinomas: SWOG 0127. J Clin Oncol 24: 4922–4927. https://doi.org/10.1200/JCO.2006.07.1316
Kwak EL, Jankowski J, Thayer SP et al (2006) Epidermal growth factor receptor kinase domain mutations in esophageal and pancreatic adenocarcinomas. Clin Cancer Res 12:4283–4287. https://doi.org/10.1158/1078-0432.CCR-06-0189
Cappuzzo F, Varella-Garcia M, Shigematsu H et al (2005) Increased HER2 gene copy number is associated with response to gefitinib therapy in epidermal growth factor receptor-positive non-small-cell lung cancer patients. J Clin Oncol 23:5007–5018. https://doi.org/10.1200/JCO.2005.09.111
Hecht JR, Bang YJ, Qin SK et al (2016) Lapatinib in combination with capecitabine plus oxaliplatin in human epidermal growth factor receptor 2-positive advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma: TRIO-013/LOGiC—a randomized phase III trial. J Clin Oncol 34:443–451. https://doi.org/10.1200/JCO.2015.62.6598
Hou W, Qin X, Zhu X et al (2013) Lapatinib inhibits the growth of esophageal squamous cell carcinoma and synergistically interacts with 5-fluorouracil in patient-derived xenograft models. Oncol Rep 30:707–714. https://doi.org/10.3892/or.2013.2500
McHugh LA, Kriajevska M, Mellon JK, Griffiths TR (2007) Combined treatment of bladder cancer cell lines with lapatinib and varying chemotherapy regimens—evidence of schedule-dependent synergy. Urology 69:390–394. https://doi.org/10.1016/j.urology.2006.12.003
Acknowledgements
This study was supported by the Grant from National Natural Science Foundation of China (no. 81202447 and no. 81373135), the Programs for Science and Technology Development of Henan (no. 172102310502), and the Support Project for Talents of Science and Technology Innovation in Universities of Henan (no. 15HASTIT040).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Guo, Xf., Li, Ss., Zhu, Xf. et al. Lapatinib in combination with paclitaxel plays synergistic antitumor effects on esophageal squamous cancer. Cancer Chemother Pharmacol 82, 383–394 (2018). https://doi.org/10.1007/s00280-018-3627-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-018-3627-3