Abstract
Purpose
SU5416 is a small, lipophilic synthetic molecule that selectively inhibits the tyrosine kinase activity of the VEGF receptor Flk-1/KDR. The role of this agent in brain tumors is currently being investigated. Pharmacokinetic studies of SU5416 have been performed in humans; however, there have been no studies of its penetration in the cerebrospinal fluid (CSF). We studied the pharmacokinetics of SU5416 in plasma and CSF after intravenous (i.v.) administration using a nonhuman primate model that is highly predictive of the CSF penetration in humans.
Experimental design
SU5416 (85 mg/m2, about 3.8 mg/kg) was administered i.v. over 20 min to four nonhuman primates. Serial plasma and CSF samples were obtained prior to, during, and after completion of the infusion for determination of SU5416 concentrations. SU5416 was measured in plasma and CSF using high-performance liquid chromatography (HPLC). Concentration-versus-time data were modeled using model-independent and model-dependent methods.
Results
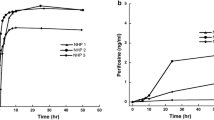
Peak plasma concentrations ranged from 6.3 to 14.5 μM and the mean plasma AUC was 620±180 μM·min. Disappearance of SU5416 from the plasma was best described by a one-compartment model with a half-life of 39±2.9 min. The volume of distribution was 36±11 l/m2 and the clearance was 0.62±0.2 l/min per m2. SU4516 was not quantifiable in the CSF.
Conclusions
There is minimal penetration of SU5416 into the CSF after i.v. administration. The very low CNS exposure to SU5416 after i.v. dosing suggests that this agent is not optimal for the treatment of leptomeningeal tumors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
SU5416 (Z-3-[(2,4-dimethylpyrrol-5-yl)methylidenyl]-indoline-2-one; Fig. 1) is a small, lipophilic synthetic molecule that selectively inhibits the tyrosine kinase activity of the VEGF receptor Flk-1/KDR [4, 8]. In preclinical studies, SU5416 has been shown to have no effect on tumor cells in vitro but causes dose-dependent inhibition of tumor growth in vivo [7]. SU5416 has shown activity in many different tumor xenografts in nude mice including melanoma, glioma, fibrosarcoma and lung, and in epidermoid, mammary, and prostate carcinomas [7]. In addition, Shaheen et al. studied the effect of SU5416 on tumor angiogenesis and metastasis in a human colon cancer xenograft model [12]. They demonstrated that SU5416 inhibits tumor metastases, microvessel formation, and cell proliferation. The findings show that SU5416 inhibition of the VEGF receptor system decreases tumor vascularity and vessel density and increases tumor cell apoptosis. The results indicate that targeting VEGF receptor is a rational approach to inhibiting tumor growth and prolonging survival [3].
Initial phase I and II clinical studies of SU5416 have been completed [1, 11] and phase III trials are in progress. Although the plasma pharmacokinetics of SU5416 have been well characterized, there are no detailed studies of the cerebrospinal fluid (CSF) disposition of SU5416. Therefore, we evaluated the plasma and CSF pharmacokinetics of SU5416 in a nonhuman primate model that is highly predictive of the CSF penetration of anticancer drugs in humans [6].
Materials and methods
Drug
SU5416 was supplied by SUGEN (South San Francisco, Calif.) in 25-ml vials at a concentration of 4.5 mg/ml in Cremophor EL, which includes polyethylene glycol 400/Polyoxyl 35 castor oil in addition to benzyl alcohol and dehydrated alcohol. Vials were stored at room temperature and protected from the light. The drug was further diluted to a final concentration of 1.5 mg/ml with normal saline and administered through non-PVC tubing. The bag was gently inverted to ensure complete mixing of the solution. Animals were premedicated with dexamethasone at a dose of 1 mg/kg. The drug was then administered at a rate of 100 ml/h.
Animals
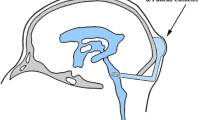
Five total doses of SU5416 were administered to four adult male rhesus monkeys (Macaca mulatta), ranging in weight from 12.3 to 13.6 kg, in these pharmacokinetic studies. The animals were fed NIH Open Formula Extruded Non-human Primate Diet twice daily and were group housed in accordance with the Guide for Care and Use of Laboratory Animals [9]. Blood samples were drawn through a catheter placed in either the femoral or saphenous vein contralateral to the site of drug administration. CSF samples were obtained from a chronically indwelling Pudenz catheter attached to a subcutaneously implanted Ommaya reservoir. The reservoir was pumped four times before and after each CSF sample collection to ensure adequate mixing with ventricular CSF.
Experiments
Four animals received a 3.8 mg/kg (about 85 mg/m2) dose of SU5416 administered via a central venous access device at a rate of 100 ml/h. The infusion durations ranged from 19 to 20.7 min. Blood was collected in heparinized tubes prior to the dose, at 10 min during the infusion, at the completion of the infusion, and at approximately 5, 15 and 30 min, and 1, 2, 3, 4, 5, 7, 8 and 24 h following completion of the infusion. Plasma was separated immediately by centrifugation at 3310 g for 10 min. The CSF samples were collected prior to the dose, at 10 min during the infusion, at the completion of the infusion, and at 15 and 30 min, and 1, 2, 3, 4, 5, 7 and 8 h following completion of the infusion. The plasma and CSF samples were stored at −80°C until the day of analysis.
Sample processing and analysis
SU5416 was measured using reverse-phase high performance liquid chromatography (HPLC). Plasma samples were thawed and then centrifuged for 10 min at 16,000 g prior to the solid-phase extraction. A Varian Bond-Elut 100 mg C8 solid-phase extraction column was conditioned with 1 ml methanol followed by 1 ml water. A 1-ml aliquot of the plasma was then placed on the cartridge. The cartridge was washed with 1 ml water followed by 1 ml 10% acetonitrile. The SU5416 was eluted with 1 ml 100% acetonitrile. The samples were then dried under a gentle flow of nitrogen. The residues were reconstituted in 500 μl of a solution of acetonitrile/DMSO/10 mM ammonium acetate (pH 2.6, ratio 4:2:3). A 25-μl aliquot was injected onto the HPLC system as described below. The recovery of SU5416 was 99.6±0.7%. Standard curves for both plasma and CSF were constructed by the addition of known amounts of SU5416 to plasma or phosphate-buffered saline, respectively. The CSF samples were thawed and centrifuged at 16,000 g for 10 min followed by direct injection of 50 μl onto the system without further processing. Standard curves were linear (r 2>0.995) over the range 0.04 to 210 μM for the plasma curve and 0.02 to 4.2 μM for the CSF curve. The lower limit of quantitation was 0.04 μM for plasma and 0.02 μM for CSF.
The HPLC system consisted of a Waters 600 Controller, a Waters 490E programmable multiwavelength UV detector with a reverse-phase NovaPak 4 μm C8 (3.9×150 mm) analytical column. SU5416 was eluted isocratically with a mobile phase of 50% acetonitrile/50% 10 mM ammonium acetate (pH 2.6) at a flow rate of 1 ml/min. SU5416 was monitored at a wavelength of 440 nm and had a retention time of 6.2 min.
Pharmacokinetic analysis
One- and two-compartment models were fitted to the plasma concentration-versus-time data from the individual SU5416 i.v. infusion experiments using Adapt II [13] (Fig. 2). Akaike’s information criterion were used to determine which equation best fitted the data. Other pharmacokinetic parameters (clearance, volume of distribution at steady state, and half-lives) were derived from the estimates of model parameters using standard techniques [13]. The areas under the concentration-time curve (AUC) were determined by the linear trapezoidal method [13] and extrapolated to infinity using the terminal rate constant.
Evaluation for toxicity
Clinical laboratory studies including complete blood counts, electrolytes, liver function tests, and renal function tests were obtained on a weekly basis for a minimum of 3 weeks each SU5416 infusion. Animals were also observed on a daily basis for a minimum of 3 weeks after infusion for any evidence of clinical toxicity.
Results
Pharmacokinetics
The elimination of SU5416 from plasma was best described by a one-compartment model. A representative plasma concentration-time profile is shown in Fig. 3. The concentration of SU5416 in the CSF was not quantifiable. The pharmacokinetic parameters for the i.v. infusion are listed in Table 1. AUC, Vc, Ke, and CL are expressed as the mean values ±SD; t1/2 is expressed as the harmonic mean.
The AUC of SU5416 in the plasma was 620±180 μM·min. SU5416 was rapidly eliminated from the plasma with a t1/2 of 39±2.9 min. The CLTB of SU5416 was 0.62±0.21 l/min per m2 and the volume of distribution was 36±11 l/m2.
Toxicity
SU5416 was well tolerated. There were no significant hematologic or other organ toxicities after a single i.v. infusion of drug. One animal developed mild, self-limited lethargy following administration of the drug. No other acute or chronic toxicity was observed.
Discussion
We studied the plasma and CSF kinetics of SU5416 in a nonhuman primate model that has been highly predictive of CSF drug penetration in humans [10]. Elimination of SU5416 from plasma was best described using a one-compartment model with a half-life of 39±2.9 min and a clearance of 0.62±0.21 l/min per m2. These plasma pharmacokinetic parameters are similar to previously reported preclinical and clinical results [1, 5, 11, 14]. Studies of SU5416 pharmacokinetics in humans at doses of 4.4 to 190 mg/m2 at the same infusion rate utilized in this study have shown an elimination half-life of approximately 50 min, a volume of distribution of approximately 22 l/m2, and a clearance of approximately 52 l/h (0.87 l/min) [11]. Pharmacokinetic studies of SU5416 reported in the literature have shown a two-compartment model for this drug [1, 5, 11].
In this study, SU5416 was not quantifiable in the CSF. SU5416 is more than 99% protein-bound (SUGEN Investigator Brochure). It is well established that protein-bound drug does not readily cross cell membranes and consequently has a restricted distribution [13]. Furthermore, because the protein content of the CSF is low relative to the amount of protein in the blood, CSF equilibrates with the free fraction of drug across a relatively impermeable membrane. Therefore minimal penetration of SU5416 into the CSF was not an unexpected finding.
SU5416 is currently being studied in clinical trials for the treatment of a variety of different types of malignancies including brain tumors. The ability of drugs to penetrate into the CSF has been used as a surrogate for penetration across the blood-brain barrier into the central nervous system. However, many brain tumors have a disrupted blood-brain barrier as evidenced by the fact that these tumors take up gadolinium, which does not cross the intact blood-brain barrier [2]. Thus the limited penetration of SU5416 into the CSF does not preclude antitumor activity against CNS tumors. Given the fact that there was no measurable penetration of SU5416 into the CSF following i.v. infusion, however, this agent may not be optimal for the treatment of leptomeningeal tumors.
References
Cropp G, Rosen L, Mulay M, Langecker P, Hannah A (1999) Pharmacokinetics and pharmacodynamics of SU5416 in a phase I, dose escalating trial in patients with advanced malignancies (abstract 619). Proc Am Soc Clin Oncol 18:161a
Donelli MG, Zucchetti M, D’Incalci M (1992) Do anticancer agents reach the tumor target in the human brain? Cancer Chemother Pharmacol 30:251–260
Ellis LM, Takahashi Y, Liu W, Shaheen RM (2000) Vascular endothelial growth factor in human colon cancer: biology and therapeutic implications. Oncologist 5 [Suppl 1]:11
Fong TAT, Shawver LK, Sun L, Tang C, App H, Powell TJ, Kim YH, Schreck R, Wang X, Risau W, Ullrich A, Hirth KP, McMahon G (1999) SU5416 is a potent and selective inhibitor of the vascular endothelial growth factor receptor that inhibits tyrosine kinase catalysis, tumor vascularization, and growth of multiple tumor types. Cancer Res 59:99
Kuenen BC, Rosen L, Smit EF, Parson MRN, Levi M, Ruijter R, Huisman H, Kedde MA, Noordhuis P, van der Vijgh WJF, Peters GJ, Cropp GF, Scigalla P, Hoekman K, Pinedo HM, Giaccone G (2002) Dose-finding and pharmacokinetic study of cisplatin, gemcitabine, and SU5416 in patients with solid tumors. J Clin Oncol 20:1657
McCully C, Balis F, Bacher J, Phillips J, Poplack D (1990) Rhesus monkey model for continuous infusion of drugs into cerebrospinal fluid. Lab Anim Sci 40:520
McMahon G (2000) VEGF receptor signaling in tumor angiogenesis. Oncologist 5 [Suppl 1]:3
Mendel DB, Laird AD, Smolich BD, Blake RA, Liang C, Hannah AL, Shaheen RM, Ellis LM, Weitman S, Shawver LK, Cherrington JM (2000) Development of SU5416, a selective small molecule inhibitor of the VEGF receptor tyrosine kinase activity, as an antiangiogenesis agent. Anticancer Drug Des 13:29
National Institutes of Health (1988) Guide for the care and use of laboratory animals. Department of Health, Education and Welfare (DHEW) publication (NIH) 85
Poplack DG, Bleyer WA, Wood JH, Kostolich M, Savitch JL, Ommaya AK (1977) A primate model for study of methotrexate pharmacokinetics in the central nervous system. Cancer Res 37:1982
Rosen L, Mulay M, Mayers A, Kabbinavar F, Rosen P, Cropp G, Hannah A (1999) Phase I dose-escalating trial of SU5416, a novel angiogenesis inhibitor in patients with advanced malignancies (abstract 618). Proc Am Soc Clin Oncol 18:161a
Shaheen RM, Davis DW, Liu W, Zebrowski BK, Wilson MR, Bucana CD, McConkey DJ, McMahon G, Ellis LM (1999) Antiangiogenic therapy targeting the tyrosine kinase receptor for vascular endothelial growth factor receptor inhibits the growth of colon cancer liver metastasis and induces tumor and endothelial cell apoptosis. Cancer Res 59:5412
Shargel L, Yu ABC (eds) (1999) Applied biopharmaceutics and pharmacokinetics. McGraw-Hill, New York
Sukbunterng J, Cropp G, Hannah A, Wagner GS, Shawver LK, Antonian L (2001) Pharmacokinetics and interspecies scaling of a novel VEGF receptor inhibitor, SU5416. J Pharm Pharmacol 53:1629
Acknowledgement
This work was supported in part by UOI HD37242 and the Glaser Pediatric Research Network.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Renbarger, J., Aleksic, A., McGuffey, L. et al. Plasma and cerebrospinal fluid pharmacokinetics of SU5416 after intravenous administration in nonhuman primates. Cancer Chemother Pharmacol 53, 39–42 (2004). https://doi.org/10.1007/s00280-003-0683-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-003-0683-z