Abstract
With the increasing prevalence of comorbidity in an ageing population, it is crucial to better understand the impact of comorbidity on health-related quality of life (HRQoL) after lymphoma or multiple myeloma (MM) diagnosis. We included 261 newly diagnosed patients (67% response rate) diagnosed with lymphoma or MM between October 2020 and March 2023 in a longitudinal survey. The European Organisation for Research and Treatment of Cancer (EORTC) questionnaires were used to measure generic and disease-specific HRQoL. Evidence-based guidelines for interpretation of the EORTC questionnaires were used to identify clinical importance. Patients were classified as having ‘no comorbidity’, ‘mild comorbidity’ (e.g. arthrosis or rheumatism), or ‘moderate-severe comorbidity’ (e.g. heart or lung disease), using the adapted self-administered comorbidity questionnaire. At diagnosis, the mean age was 64 years, 63% were male and 38% reported no comorbidity, 33% mild comorbidity, and 29% moderate-severe comorbidity. Patients with mild or moderate-severe comorbidity reported clinically relevant worse HRQoL at diagnosis than patients without comorbidity. One year post-diagnosis most outcomes showed clinically relevant improvement, irrespective of comorbidity. However, outcomes of physical functioning (β=-7.9, p < 0.05), global health status (β=-7.6, p < 0.05), bone pain (β = 8.1 to 9.1, p < 0.05), muscle/joint pain (β = 14.5 to 18.8, p < 0.01) and muscle weakness (β = 10.4 to 15.6, p < 0.05) improved less among those with comorbidity, and clinically relevant differences between comorbidity groups persisted over time. With clinically relevant worse HRQoL at diagnosis and less recovery of HRQoL during the first year after diagnosis in patients with comorbidity, consideration of both prognosis and HRQoL is important when making treatment decisions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients with lymphoma or multiple myeloma (MM) have a median age of 69 years at diagnosis [1, 2]. The incidence of these malignancies increases with age, except for Hodgkin’s lymphoma, and most patients suffer from one or more comorbidities [1]. These comorbidities are mostly mild such as hypertension or arthrosis, but 10–15% have a severe comorbidity (e.g. heart disease) at diagnosis [3,4,5]. With a higher prevalence of (multiple) comorbidities in an ageing population [6], a better understanding of the impact of comorbidity on patients with lymphoma or MM is needed.
Research shows that from 6 to 12 months after diagnosis, cancer survivors with comorbidity experience higher (persistent) symptom burden and worse functioning than those without comorbidity [7,8,9,10]. Comorbidity is an important factor in the treatment decisions made in daily clinical practice [11, 12]. Patients with (more) severe comorbidity are more likely to receive less toxic treatment, and are often excluded from stem cell transplantation (SCT) or clinical trials [13,14,15,16,17]. Over time, treatment decisions will increasingly prioritize preserving health-related quality of life (HRQoL) in these patients [17, 18].
Knowledge of HRQoL during the diagnostic phase of newly diagnosed patients with lymphoma or MM, treated in daily clinical practice, is limited. Most HRQoL studies during the first year after diagnosis of lymphoma or MM have been conducted in clinical trial settings, with a selection of specific treatment regimens and often excluding patients with severe comorbidity [5, 15, 19,20,21,22], or they have not started earlier than six months after diagnosis [23, 24]. Therefore, a better understanding of the impact of comorbidity is needed. This will help to improve treatment decisions, early symptom management, and develop interventions to improve (long-term) HRQoL outcomes in patients with comorbidity [15, 25, 26].
The aims of this study were to investigate if (1) there are clinically relevant differences in HRQoL at diagnosis and during the first year after diagnosis among patients with lymphoma or MM with no, mild or moderate-severe comorbidity in a real-world setting, (2) the HRQoL of patients with comorbidity deteriorates more over time than those without, (3) whether higher toxicity treatment or SCT would increase deterioration of HRQoL, and (4) patients return to similar HRQoL levels as an age- and sex-matched normative population 12 months after diagnosis. We hypothesized that (1) patients with more severe comorbidity would report worse HRQoL, (2) that comorbidity would have a negative impact on HRQoL over time, with either accelerated deterioration and/or less recovery up to 12 months after diagnosis, and (3) that high toxicity treatment is associated with worse HRQoL, irrespective of comorbidity.
Methods
Setting and population
Data collection was done within PROFILES (Patient Reported Outcomes Following Initial treatment and Long term Evaluation of Survivorship), a registry for the study of the physical and psychosocial impact of cancer and its treatment [27]. PROFILES is linked directly to clinical data from the population-based Netherlands Cancer Registry (NCR).
Patients diagnosed with lymphoma or MM between October 2020 and March 2023, as defined by the International Classification of Diseases for Oncology-3 codes (ICD-O-3) [28], and aged 18 years and older at the time of diagnosis, were invited to participate in a longitudinal survey. At the time of diagnosis, their treating hematologist or nurse practitioner gave them the patient information sheet and asked if they agreed to be contacted by a researcher. After receiving consent, the hematologist/nurse practitioner gave the research team the patients’ name and telephone number. Within 2–4 days after notification the patient was contacted by the research team to discuss the study content and their willingness to participate. If patients wanted to participate, they could choose to receive a paper or online questionnaire, after giving informed consent. Patients completed questionnaires between diagnosis and the start of treatment, and at three, six and twelve months after diagnosis. Patients with severe cognitive impairment (e.g. dementia), or who were transitioning to end-of-life care were excluded. To compare participating patients with the total population of newly diagnosed patients in participating hospitals, clinical information on group-level was obtained from the NCR.
Patients were categorized into three groups: (1) indolent lymphoma (low grade Non-Hodgkin Lymphoma (LG-NHL) or Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma (CLL/SLL)), (2) aggressive lymphoma (Hodgkin Lymphoma (HL) or high grade Non-Hodgkin lymphoma (HG-NHL)), and (3) Multiple Myeloma (MM). Ethical approval for the study was obtained from a certified Medical Ethics Committee (of the Maxima Medical Centre in Veldhoven, the Netherlands; NL20.011 and NL78561.015.21).
Study measures
Comorbidity was measured using the adapted Self-administered Comorbidity Questionnaire (SCQ) [29]. Patients were categorized into groups according to whether they reported ‘no comorbidity’, ‘mild comorbidity’, or ‘moderate-severe comorbidity’, at time of the first questionnaire. Similar to categorization of comorbidity in the Charlson Comorbidity Index (CCI), patients were classified as having moderate-severe comorbidity if they reported heart disease, stroke, diabetes, kidney disease, liver disease, chronic pulmonary disease, or a previous solid malignancy [11]. Patients who reported hypertension, anemia or other blood disorders, arthrosis, rheumatism, thyroid disease, ulcer, depression or COVID-19 were classified as having mild comorbidity.
The Dutch validated versions of the European Organisation for Research and Treatment of Cancer (EORTC) questionnaires were used to measure HRQoL. The EORTC Quality of Life Questionnaire Core 30 (QLQ-C30) was used to measure 15 scales of generic HRQoL, among all patients in this cohort. Disease-specific items were obtained from the EORTC disease-specific questionnaires (EORTC Hodgkin Lymphoma 27 (HL27), Non-Hodgkin Lymphoma High-Grade 29 (NHL-HG29), Non-Hodgkin Lymphoma Low-Grade 20 (NHL-LG20), Chronic Lymphocytic Leukemia 17 (CLL17), and Multiple Myeloma 20 (MY20)) [30,31,32]. Disease-specific items were selected based on their corresponding content in the different questionnaires per subtype. Nine disease-specific items were selected for patients with indolent or aggressive lymphoma, and eleven disease-specific items for patients with MM. Answer categories of the EORTC questionnaires range from one (not at all) to four (very much). After linear transformation, all scales and single-item measures range in score from 0 to 100. A higher score on a functioning scale indicates better functioning, whereas a higher score for symptoms indicates higher symptom burden [30]. Evidence-based guidelines for interpretation of the EORTC QLQ-C30 were used to identify clinically relevant differences between groups of comorbidity [33], and clinically relevant changes over time [34] for generic symptoms and functioning. For disease-specific symptoms, the recommendations of Osoba et al. were used to identify clinically relevant differences between groups of comorbidity and clinically relevant changes over time [35, 36].
Clinical information about hematologic subtype, stage of disease, time since diagnosis, initial treatment regimen, and number of treatment cycles was obtained from the NCR. The initial treatment was categorized as ‘no systemic therapy’ (no treatment/active surveillance/radiotherapy), ‘systemic therapy’, or ‘systemic therapy + SCT’. An overview of the treatment regimens according to hematologic malignancy group and comorbidity is provided in Online Resource 1.
Patients’ age, sex, marital status and educational level were also assessed in the questionnaire.
Comparison with a normative population without cancer
A normative population was selected from a reference cohort of 2040 individuals from the general Dutch population (CentER panel) to compare outcomes one year after diagnosis between patients and people without cancer [37]. This cohort is considered representative for the Dutch-speaking population in the Netherlands. Norm participants were matched based on the frequency distribution by stratum (defined by age categories and sex) per hematologic malignancy group. The normative population received a questionnaire in 2017, including the EORTC QLQ-C30, neuropathy and sociodemographic data. Other than neuropathy, no disease-specific items were available in the normative population.
Statistical analyses
Statistical analyses were carried out using IBM SPSS Statistics for Windows, version 29 (IBM Corp., Armonk, N.Y., USA). P values of < 0.05 were considered statistically significant. Sociodemographic and clinical differences between questionnaire participants and the total population of newly diagnosed patients were analyzed with a chi-square or t-test, where appropriate. To identify potential confounding between comorbidity and age, the Spearmann rank correlation test was used.
Mixed models were performed to analyze the course of HRQoL during the first year after diagnosis. Main effects of the a priori determined clinical characteristics (comorbidity and initial treatment category, and for aggressive lymphoma also the hematologic malignancy HL vs. HG-NHL) and sociodemographic characteristics (age, sex, educational level, having a partner) were included in the models. To avoid overfitting, likelihood ratio tests were used to assess and determine the inclusion of main effects for the number of treatment cycles. The final model was obtained based on significant likelihood ratio tests and improved model fit based on a smaller Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC) value.
Analyses were carried out per hematologic malignancy subgroup (1) indolent lymphoma, (2) aggressive lymphoma and (3) multiple myeloma.
Results
Characteristics of the study population
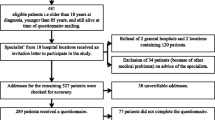
Of the 389 patients referred by their treating hematologist/nurse practitioner, 82% (N = 319 patients) were willing to participate, 70% (N = 271 patients) completed at least one questionnaire, and clinical data were available for 67% (N = 261 patients). Questionnaire data for the 24 (lymphoma) and 26 (MM) HRQoL outcomes were complete for 96-100%. Patients completed on average three measurements. Response rates at T1, T2 and T3 were 63%, 57% and 38% respectively. 2% (N = 7) of patients were lost to follow-up due to death. A flow-chart of the data-collection is shown in Fig. 1.
At inclusion, mean age was 64 years, 63% were male. 38% of patients reported no comorbidity (LG-NHL 32%, HL 67%, HG-NHL 42% and MM 26%), 33% mild comorbidity (LG-NHL 41%, HL 21%, HG-NHL 27% and MM 38%), and 29% reported moderate-severe comorbidity (LG-NHL 27%, HL 13%, HG-NHL 31% and MM 35%) at inclusion. Most commonly reported comorbidities were hypertension (27%), anemia or other blood diseases (20%) and arthrosis (20%). The most commonly reported moderate-severe comorbidities were heart disease (12%), chronic pulmonary disease (10%) and diabetes (10%). Age and comorbidity had a weak correlation (0.03, p < 0.001), meaning there was no confounding.
Characteristics of all participants, per subgroup, are presented in Table 1.
Questionnaire participants compared with the total population of newly diagnosed patients
Participating patients were more likely to have an aggressive subtype (LG-NHL 30% vs. 39%, HL 9% vs. 6%, HG-NHL 39% vs. 34%; p = 0.01), and were more likely to receive active treatment (chemo/immunotherapy 45% vs. 30%, chemo/immunotherapy + radiotherapy 12% vs. 4%, SCT 9% vs. 5%; p < 0.01) compared to the total population of newly diagnosed patients in the participating hospitals. In addition, participants were significantly younger (63 vs. 68 years; p < 0.01). No significant differences were observed for sex and stage of disease (data not shown).
HRQoL during the first year after diagnosis
Indolent lymphoma
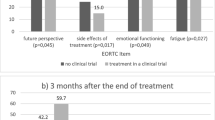
At baseline, among patients with indolent lymphoma, both those with mild and moderate-severe comorbidity reported clinically relevant worse scores on 6 of the 15 QLQ-C30 scales, and 4 of the 9 disease-specific items, compared to those without comorbidity (Table 2 and Online Resource 2). The differences were the largest for physical functioning, global health status/quality of life (QoL), bone pain, muscle/joint pain and muscle weakness (Figs. 2, 3 and 4). Patients with moderate-severe comorbidity also reported clinically relevant better scores on 4 of the 15 QLQ-C30 scales, and 1 of the 9 disease-specific items, compared to patients without comorbidity.
Over time, most of the HRQoL outcomes showed small to medium clinically relevant improvements, irrespective of comorbidity. However, symptoms of diarrhea, neuropathy and financial problems worsened over time in patients with no or mild comorbidity. Compared to patients without comorbidity more deterioration was observed in the course of symptoms for bone pain, muscle/joint pain and muscle weakness in patients with mild and or moderate-severe comorbidity (Online Resource 2).
With respect to treatment, patients who received systemic therapy reported statistically significantly more neuropathy symptoms over time than patients without systemic therapy (β=-21.5, p < 0.05). Furthermore, irrespective of their comorbidity, patients who received 5–6 cycles reported worse cognitive functioning during the first year compared to patients who received 1–4 cycles of systemic therapy (β=-21.0, p = 0.01).
Compared to the age- and sex-matched normative population one year after diagnosis, patients with mild or moderate-severe comorbidity reported clinically relevant worse scores on 10 of the 15 QLQ-C30 scales and for neuropathy. Better levels of HRQoL than the normative population were observed on 2 of the 15 QLQ-C30 scales (Online Resource 3).
Aggressive lymphoma
At baseline, among patients with aggressive lymphoma, those with moderate-severe comorbidity reported clinically relevant worse scores on 11 of the 15 QLQ-C30 scales, and 7 of the 9 disease-specific items, compared to patients without comorbidity. The largest differences were reported for physical and role functioning, and symptoms of fatigue, pain, and feeling sick (Table 2 and Online Resource 2). Patients with mild comorbidity however, reported better scores on 6 of the 15 QLQ-C30 scales, and 2 of the 9 disease-specific items at diagnosis, compared to patients without comorbidity.
Over time, similar to patients with indolent lymphoma, almost all HRQoL outcomes showed clinically relevant improvement one year after diagnosis, irrespective of comorbidity. However, clinically relevant differences persisted between patients with moderate-severe comorbidity and those without, with worse HRQoL in patients with moderate-severe comorbidity. Moreover, patients with moderate-severe comorbidity, had worse HRQoL on 4 of the 15 QLQ-C30 scales, and 5 of the 9 disease-specific items, compared to those without comorbidity (Figs. 2, 3 and 4; Table 2 and Online Resource 2).
With respect to treatment, patients who received 5–6 cycles of systemic therapy, compared to 1–4 cycles, reported significantly lower physical functioning (β=-8.6, p = 0.01), more fatigue (β = 8.8, p < 0.05), insomnia (β = 10.8, p < 0.05), neuropathy (β = 7.3, p < 0.05), lack of energy (β = 9.1, p < 0.05) and sudden tiredness (β = 12.3, p < 0.05) during the first year.
Compared to the age- and sex-matched normative population one year after diagnosis, patients with mild or moderate-severe comorbidity reported clinically relevant worse scores on 11 of the 15 QLQ-C30 scales and neuropathy. Better levels of HRQoL than the normative population were observed on 1 of the 15 QLQ-C30 scales.
Multiple myeloma
At baseline, patients with MM with mild or moderate-severe comorbidity reported clinically relevant worse scores on 9 of the 15 QLQ-C30 scales, and 7 of the 11 disease-specific items, compared to those without comorbidity. The largest differences were observed for fatigue, pain, appetite loss, bone pain, hip pain and drowsiness (Table 2 and Online Resource 2). In contrast, patients with moderate-severe comorbidity also reported better scores on 1 of the 15 QLQ-C30 scales, and 3 of the 11 disease-specific items at diagnosis.
Over time, irrespective of comorbidity, most outcomes had a medium clinically relevant improvement. Symptoms of neuropathy did not improve but became clinically relevant worse (Figs. 2, 3 and 4). Patients with moderate-severe comorbidity reported more deterioration of drowsiness and future perspective. Other HRQoL outcomes did not recover less in patients with comorbidity than in those without comorbidity.
With respect to treatment, almost half of MM patients received ‘systemic therapy + SCT’ (45.5%), and these were relatively more likely to be patients without comorbidity (no 71%, mild 33% and moderate-severe 37%). To better understand the HRQoL outcomes for comorbidity and the relation with SCT, we additionally stratified the corrected means among patients with MM for SCT (Figs. 2, 3 and 4). Patients with MM who underwent SCT, reported clinically relevant worse role functioning, global health status/QoL, more fatigue and pain, as well as more symptoms of bone pain, hip pain and neuropathy, compared to patients with MM who did not undergo SCT (data not shown).
Finally, compared to patients with lymphoma, functional impairment and symptom burden among patients with MM were considerably, and clinically relevant worse (Figs. 2, 3 and 4).
Compared to the age- and sex-matched normative population one year after diagnosis, patients with mild or moderate-severe comorbidity reported clinically relevant worse scores on 12 of the 15 QLQ-C30 scales and neuropathy. Similar or better HRQoL scores than the normative population were observed on 2 of the 15 QLQ-C30 scales.
Discussion
This study found that patients with mild or moderate-severe comorbidity had clinically relevant worse HRQoL at diagnosis and during the first year after diagnosis, compared to patients without comorbidity. One year after diagnosis most outcomes of HRQoL showed clinically relevant improvement compared to baseline, irrespective of comorbidity. However, patients with mild or moderate-severe comorbidity showed less improvement in HRQoL than those without comorbidity. Across all hematologic malignancy groups, patients who received more systemic therapy cycles or who underwent SCT reported worse HRQoL, irrespective of comorbidity. One year after diagnosis most patients with lymphoma or MM reported worse HRQoL than the age- and sex-matched normative population.
These findings align with existing literature on HRQoL differences between patients with and without comorbidity from 6 to 12 months after diagnosis [7,8,9,10]. In addition, our findings show that HRQoL differences between patients with or without comorbidity present themselves at time of diagnosis, and persist over time. Across all hematologic malignancy groups, patients with moderate-severe comorbidity reported consistently lower physical functioning, worse global health status/QoL, and a higher symptom burden (especially for fatigue and/or drowsiness) compared to those without comorbidity. Similar problems and clinically relevant improvements have been reported in newly diagnosed patients with lymphoma or MM and comorbidity in clinical trials [5, 19,20,21,22]. When patients with moderate-severe comorbidity were excluded, HRQoL mean scores were slightly higher and symptoms of fatigue and pain showed better recovery over time than in our study [5, 21]. In clinical trials that included patients with moderate-severe comorbidity, HRQoL means and recovery over time were similar to our study [22]. Over time, the absolute HRQoL difference between patients with and without comorbidity remains consistent. Accounting for the lower HRQoL in patients with moderate-severe comorbidity, this may suggest that HRQoL outcomes from clinical trials are useful for predicting HRQoL outcomes in a larger population, despite the exclusion of patients with more severe comorbidity.
The largest HRQoL differences were observed in disease-specific items. Although most outcomes had clinically relevant improved one year after diagnosis, symptoms of bone pain, muscle/joint pain and neuropathy worsened. These symptoms might benefit from (better) symptom monitoring and/or pain management during and after treatment, in order to improve (long-term) HRQoL and patient expectations [26, 38]. Regular individualized HRQoL assessment and using patient-reported outcomes in clinical practice allow for timely intervention or referral to supportive care if needed [39]. This will enable providing more personalized care and informed treatment decisions while preserving HRQoL as much as possible.
With respect to hematologic malignancy group, patients with MM had persistently and clinically relevant worse HRQoL than patients with indolent or aggressive lymphoma. This may be due to the fact that MM is an incurable subtype with a relatively longer treatment duration of high toxicity treatment [40, 41]. Prolonged systemic treatment negatively affects HRQoL in patients with MM [5], and it is important that the focus of treatment is not only on improving overall survival but also on optimizing treatment duration and maintaining HRQoL [18]. Recovery of HRQoL to similar levels as the age- and sex-matched normative population was only observed in 3–5 QLQ-C30 scales. In particular, patients’ global health status/quality of life scores are similar or better one year after diagnosis, which may be the result of adjusted internal standards, values, or conceptualization of HRQoL (response-shift) [42]. The lower HRQoL in patients compared to the normative population one year after diagnosis are comparable to outcomes in studies that investigated HRQoL of patients from 6 to 12 months after diagnosis [4, 23, 24, 43].
Due to the real-world data setting, treatment regimens were highly heterogeneous. As a result specific regimens were represented by small proportions of patients, leading to a more general categorization of treatment and systemic therapy. Nonetheless, results showed that patients who received more systemic therapy cycles had statistically significant lower physical functioning, increased fatigue, and more persistent neuropathy symptoms, irrespective of comorbidity or hematologic malignancy. Patients who underwent SCT reported the lowest role functioning, global health status/QoL, and an increased symptom burden compared to those who did not undergo SCT. More detailed information on specific treatment regimen, and possibly related treatment decisions based on comorbidity and expected toxicity, may provide further insight into which patient characteristics or treatment regimens are specifically associated with poor HRQoL.
Our results indicate that in aggressive subtypes of hematologic malignancies, the impact of mild or moderate-severe comorbidity at diagnosis appears to be somewhat less relevant for HRQoL. Namely, patients with aggressive lymphoma or MM with moderate-severe comorbidity had statistically significantly and clinically relevant worse HRQoL, while in patients with indolent lymphoma worse HRQoL was also observed in patients with mild comorbidity. Both the more aggressive subtype of the disease, as well as the more toxic treatment, may have a greater impact on HRQoL over time, which may outweigh the impact of comorbidity at diagnosis.
The strengths of our study include HRQoL measures at diagnosis, the real-world data setting including patients with more severe comorbidity, and the disease-specific symptoms that were measured. However, some limitations must be acknowledged. First, although the SCQ has moderately strong correlations with a standard medical record-based measure of comorbidity, the use of patient self-reported comorbidity may have led to under- or overestimation of comorbidity [29]. Second, although we have aligned the classification of mild or moderate-severe comorbidity with the CCI, the 12 conditions in the SCQ do not provide detailed information on severity. Without information on severity, it is difficult to compare the burden of one or more comorbidities. The severity and the total number of comorbidities were not taken into account when classifying patients, and the classification may not always be accurate enough [11]. Third, follow-up data are likely to be affected by patient drop-out, especially between 6 and 12 months after diagnosis. Patient drop-out may have led to possible under- or overestimation of HRQoL at 12 months post-diagnosis, potentially affecting the clinically relevant differences and/or the differences with the normative population [44, 45].
In conclusion, newly diagnosed patients with lymphoma or MM who have mild or moderate-severe comorbidity have clinically relevant worse HRQoL during the first year after diagnosis than patients without comorbidity. Regardless of comorbidity, patients treated with more cycles of systemic therapy and/or SCT reported worse HRQoL than those treated with less toxic treatment. Recognition and awareness of comorbidity and HRQoL at diagnosis may be an important prognostic factor for long-term HRQoL. We strongly recommend that clinicians consider both prognosis and the presence of comorbidity and HRQoL at diagnosis when making treatment decisions. This will improve symptom management in patients with comorbidity and help maintain HRQoL over time.
Data availability
Since 2011, PROFILES registry data is freely available according to the FAIR (Findable, Accessible, Interoperable, Reusable) data principles for non-commercial (international) scientific research, subject only to privacy and confidentiality restrictions. Data is made available through Questacy (DDI 3.x XML) and can be accessed by our website (www.profilesregistry.nl). In order to arrange optimal long-term data warehousing and dissemination, we follow the quality guidelines that are formulated in the ‘Data Seal of Approval’ (www.datasealofapproval.org) document, developed by Data Archiving and Networked Services (DANS).
References
Cordoba R et al (2021) A comprehensive approach to therapy of haematological malignancies in older patients. Lancet Haematol 8(11):e840–e852
The Netherlands Comprehensive Cancer Organisation (IKNL). Netherlands Cancer Registry application (2022) ; www.iknl.nl/nkr-cijfers
Thurmes P et al (2008) Comorbid conditions and survival in unselected, newly diagnosed patients with chronic lymphocytic leukemia. Leuk Lymphoma 49(1):49–56
Ekels A et al (2023) The course of self-perceived cognitive functioning among patients with lymphoma and the co-occurrence with fatigue and psychological distress. J Cancer Surviv. https://doi.org/10.1007/s11764-023-01458-2
Bennink MC et al (2022) Impact of comorbidities on Health-related quality of life in Nontransplant Eligible patients with newly diagnosed multiple myeloma. Hemasphere 6(7):e744
Yang J et al (2016) Discover the network underlying the connections between aging and age-related diseases. Sci Rep 6:32566
Cummings A et al (2018) Comorbidities are associated with poorer quality of life and functioning and worse symptoms in the 5 years following colorectal cancer surgery: results from the ColoREctal Well-being (CREW) cohort study. Psychooncology 27(10):2427–2435
Götze H et al (2018) Comorbid conditions and health-related quality of life in long-term cancer survivors-associations with demographic and medical characteristics. J Cancer Surviv 12(5):712–720
Efficace F et al (2019) Health-related quality of life, symptom burden, and comorbidity in long-term survivors of acute promyelocytic leukemia. Leukemia 33(7):1598–1607
Vissers PA et al (2013) The impact of comorbidity on Health-Related Quality of Life among cancer survivors: analyses of data from the PROFILES registry. J Cancer Surviv 7(4):602–613
Charlson ME et al (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40(5):373–383
Oken MM et al (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5(6):649–655
Palumbo A et al (2015) Geriatric assessment predicts survival and toxicities in elderly myeloma patients: an International Myeloma Working Group report. Blood 125(13):2068–2074
Gay F et al (2018) From transplant to novel cellular therapies in multiple myeloma: European Myeloma Network guidelines and future perspectives. Haematologica 103(2):197–211
Unger JM et al (2019) Association of Patient Comorbid conditions with Cancer Clinical Trial Participation. JAMA Oncol 5(3):326–333
Bellera C et al (2013) Barriers to inclusion of older adults in randomised controlled clinical trials on Non-hodgkin’s lymphoma: a systematic review. Cancer Treat Rev 39(7):812–817
Arcari A et al (2023) New treatment options in elderly patients with diffuse large B-cell lymphoma. Front Oncol 13:1214026
Terpos E et al (2021) Management of patients with multiple myeloma beyond the clinical-trial setting: understanding the balance between efficacy, safety and tolerability, and quality of life. Blood Cancer J 11(2):40
Davies A et al (2020) Health-related quality of life in the phase III GALLIUM study of obinutuzumab- or rituximab-based chemotherapy in patients with previously untreated advanced follicular lymphoma. Ann Hematol
Cheson BD et al (2017) Health-related quality of life and symptoms in patients with rituximab-refractory indolent non-hodgkin lymphoma treated in the phase III GADOLIN study with obinutuzumab plus bendamustine versus bendamustine alone. Ann Hematol 96(2):253–259
Burke JM et al (2016) Differences in quality of life between bendamustine-rituximab and R-CHOP/R-CVP in patients with previously untreated Advanced Indolent Non-hodgkin Lymphoma or Mantle Cell Lymphoma. Clin Lymphoma Myeloma Leuk 16(4):182–190e1
Paunescu AC et al (2022) Correction to: quality of life of survivors 1 year after the diagnosis of diffuse large B-cell lymphoma: a LYSA study. Ann Hematol 101(2):333
Ekels A et al (2022) Persistent symptoms of fatigue, neuropathy and role-functioning impairment among indolent non-hodgkin lymphoma survivors: a longitudinal PROFILES registry study. Br J Haematol 197(5):590–601
Oerlemans S et al (2011) The impact of treatment, socio-demographic and clinical characteristics on health-related quality of life among Hodgkin’s and Non-hodgkin’s lymphoma survivors: a systematic review. Ann Hematol 90(9):993–1004
Hurria A et al (2014) Designing therapeutic clinical trials for older and frail adults with cancer: U13 conference recommendations. J Clin Oncol 32(24):2587–2594
Basch E et al (2021) Composite grading algorithm for the National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). Clin Trials 18(1):104–114
van de Poll-Franse LV et al (2011) The patient reported outcomes following initial treatment and long term evaluation of survivorship registry: scope, rationale and design of an infrastructure for the study of physical and psychosocial outcomes in cancer survivorship cohorts. 47(14): pp. 2188–2194
Jack A et al (2000) International classification of diseases for oncology: ICD-O. World Health Organization
Sangha O et al (2003) The self-administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Care Research: Official J Am Coll Rheumatol 49(2):156–163
Aaronson NK et al (1993) The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. JNCI: J Natl Cancer Inst 85(5):365–376
Oerlemans S et al (2023) International validation of a health-related quality-of-life questionnaire for Hodgkin lymphoma: the EORTC QLQ-HL27. Blood Adv 7(22):7045–7055
Oerlemans S et al (2023) International validation of two EORTC questionnaires for assessment of health-related quality of life for patients with high-grade non-hodgkin lymphoma (QLQ-NHL-HG29) and low-grade non-hodgkin lymphoma (QLQ-NHL-LG20). Cancer 129(17):2727–2740
Cocks K et al (2011) Evidence-based guidelines for determination of sample size and interpretation of the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30. J Clin Oncol 29(1):89–96
Cocks K et al (2012) Evidence-based guidelines for interpreting change scores for the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30. Eur J Cancer 48(11):1713–1721
Osoba D et al (2023) Interpreting the significance of changes in Health-Related Quality-of-life scores. J Clin Oncol 41(35):5345–5350
Osoba D et al (1998) Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol 16(1):139–144
van de Poll-Franse LV et al (2011) Normative data for the EORTC QLQ-C30 and EORTC-sexuality items in the general Dutch population. Eur J Cancer. 47(5):667–675. https://doi.org/10.1016/j.ejca.2010.11.004
Basch E et al (2016) Symptom Monitoring with patient-reported outcomes during Routine Cancer treatment: a Randomized Controlled Trial. J Clin Oncol 34(6):557–565
van den Hurk CJG et al (2022) A narrative review on the Collection and Use of Electronic patient-reported outcomes in Cancer Survivorship Care with emphasis on Symptom Monitoring. Curr Oncol 29(6):4370–4385
Georges GE et al (2020) Survivorship after autologous hematopoietic cell transplantation for Lymphoma and multiple myeloma: late effects and Quality of Life. Biol Blood Marrow Transpl 26(2):407–412
D’Souza A et al (2023) Trajectories of quality of life recovery and symptom burden after autologous hematopoietic cell transplantation in multiple myeloma. Am J Hematol 98(1):140–147
Schwartz CE et al (2006) The clinical significance of adaptation to changing health: a meta-analysis of response shift. Qual Life Res 15(9):1533–1550
Mols F et al (2012) Health-related quality of life and disease-specific complaints among multiple myeloma patients up to 10 year after diagnosis: results from a population-based study using the PROFILES registry. Eur J Haematol 89(4):311–319
van Yperen NC et al (2023) Selection bias in follow-up studies of stem cell transplantation survivors: an experience within the Maastricht Observational study of late effects after stem cell trAnsplantation (MOSA). Ann Hematol 102(3):641–649
Ramsey I et al (2019) Cancer survivors who fully participate in the PROFILES registry have better health-related quality of life than those who drop out. J Cancer Surviv 13(6):829–839
Acknowledgements
This study was financially supported by the Jonker-Driessen Foundation. The PROFILES registry was funded by an investment grant (#480-08-009) of the Netherlands Organization for Scientific Research (The Hague, The Netherlands). Both funding agencies had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of this paper; and in the decision to submit the paper for publication.
Author information
Authors and Affiliations
Contributions
Conception and design: A.E, L.vd.P and S.O. Inclusion of patients: D.I, M.H, M.N, A.K, C.d.J, A.A, N.T, L.T, L.t.B, L.B, N.T, G.V, F.d.B, M.K and E.P. Data analysis and interpretation: A.E, L.vd.P and S.O. Manuscript writing and revision: A.E, L.P and S.O. Final approval of manuscript: all authors.
Corresponding authors
Ethics declarations
Ethical approval
Ethical approval for the study was obtained from a certified Medical Ethics Committee (of the Maxima Medical Centre in Veldhoven, the Netherlands; NL20.011 and NL78561.015.21).
Consent to participate
Informed consent for collecting data was obtained from all patients participating in this study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ekels, A., van de Poll-Franse, L.V., Issa, D.E. et al. Impact of comorbidity on health-related quality of life in newly diagnosed patients with lymphoma or multiple myeloma: results from the PROFILES-registry. Ann Hematol (2024). https://doi.org/10.1007/s00277-024-06006-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00277-024-06006-1