Abstract
Primary immune thrombocytopenia is an autoimmune disease, characterized with decreased platelet and increased risk of bleeding. Recent studies have shown the reduction and dysfunction of regulatory T (Treg) cells in ITP patients. CD39 is highly expressed on the surface of Treg cells. It degrades ATP to AMP and CD73 dephosphorylates AMP into adenosine. Then adenosine binds with adenosine receptor and suppresses immune response by activating Treg cells and inhibiting the release of inflammatory cytokines from effector T (Teff) cells. Adenosine receptor has several subtypes and adenosine A2A receptor (A2AR) plays a crucial role especially within lymphocytes. The CD39+ Treg cells and the expression of A2AR showed abnormality in some autoimmune disease. But knowledge of CD39+ Treg cells and A2AR which are crucial in the adenosine immunosuppressive pathway is still limited in ITP. Thirty-one adult patients with newly diagnosed ITP were enrolled in this study. CD39 and A2AR expression was measured by flow cytometry and RT-PCR. The function of CD39 was reflected by the change of ATP concentration detected by CellTiter-Glo Luminescent Cell Viability Assay. CD39 expression within CD4+CD25+ Treg cells in ITP patients was decreased compared to normal controls. After high-dose dexamethasone therapy, response (R) group showed increased CD39 expression within Treg cells while non-response (NR) group did not show any difference in contrast to those before treatment. The expression of A2AR in CD4+CD25− Teff and CD4+CD25+ Treg cells was both lower in ITP patients than that of normal controls. After therapy, CD4+CD25− Teff cells had higher A2AR expression while CD4+CD25+ Treg cells did not show any difference in comparison to that before treatment. The enzymatic activity of CD39 was damaged in ITP patients and improved after high-dose dexamethasone therapy. In ITP, there was not only numerical decrease but also impaired enzymatic activity in CD39+ Treg cells. After high-dose dexamethasone treatment, these two defects could be reversed. Our results also suggested that ITP patients had reduced A2AR expression in both CD4+CD25+ Treg cells and CD4+CD25− Teff cells. CD4+CD25− Teff cells had increased A2AR expression after treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Primary immune thrombocytopenia is an autoimmune disease, characterized with decreased platelet and increased risk of bleeding [1, 2]. Besides platelet-specific antibodies produced by auto-reactive B cells [3] and imbalance of Th1/Th2 cell [4,5,6], study of regulatory T (Treg) cell abnormalities is getting more and more attention. Lots of studies have revealed both the numerical and functional defect of Treg cells in ITP [7,8,9,10,11,12,13,14,15,16,17]. Effective treatment can significantly increase Treg cell amount and restore their function [7,8,9,10,11, 14,15,16,17]. Usually, Treg cells were measured by the expression of Foxp3 in CD4+CD25+ cells; Foxp3 transcription factor can be used as a specific molecular marker for Treg cells. However, its location inside the cell limits its application in some Treg cell studies. Researches have shown that CD39 is highly expressed on the surface of Treg cells and has been increasingly used as a marker of Treg cells [18, 19]. In mouse, CD39 is expressed in almost all Foxp3+ regulatory T cells. In human, CD39 is mainly expressed in regulatory T cells with memory activation. CD39 could be a marker which is independent of Foxp3, and CD39 subgroup could also be considered a subtype of Treg cells [18, 19]. CD39 is an integral membrane protein and a member of ecto-nucleoside triphosphate diphosphohydrolase family (E-NTPDase) [20]. Of this family, CD39 is the dominant ectoenzyme in the immune system [21, 22]. CD39 degrades extracellular adenosine triphosphate (ATP) to adenosine monophosphate (AMP), and CD73 (ecto-5′-nucleotidase, Ecto5’NTase) dephosphorylates AMP into adenosine [23, 24]. This conversion is considered one of the mechanisms by which Treg cells reduce inflammation. Then adenosine binds with adenosine receptor on the membrane and suppresses immune response by activating Treg cells and inhibiting the release of inflammatory cytokines from Teff cells [25]. Treg cells separated from CD39 knockout mice lost their function to suppress the proliferation of Teff cells [18]. It shows that CD39 plays a crucial part in immunoregulation. Adenosine receptor has several subtypes: A1R/A2AR/A2BR/A3R and A2AR play a crucial role especially within lymphocytes [26,27,28,29]. Treg and Teff cells both express A2AR on their cellular membrane. Therefore, the expression of CD39 and the enzymatic activity of CD39 determine the production of adenosine. And the expression of adenosine A2A receptor determines the effect of adenosine immunosuppressive pathway.
Defective numbers of CD39+ Treg cells have been reported in multiple sclerosis, where these cells were also impaired in their ability to suppress IL-17 production [30,31,32]. The abnormal amount and dysfunction of CD39+ Treg cells have been found in other autoimmune diseases such as autoimmune hepatitis, rheumatoid arthritis, inflammatory bowel disease, and systematic lupus erythematosus [33,34,35,36,37]. The expression of adenosine A2A receptor also showed abnormality in myasthenia gravis and type 2 diabetes mellitus [38, 39] which were considered to be immune-involved diseases. Given the key role of CD39 and A2AR in the adenosine immunosuppressive pathway, our current study aimed to explore CD39+ Treg cell amount, the expression of A2AR in both Treg and Teff cells, and the enzymatic activity of CD39 within Treg cells.
Subjects and methods
Patients and controls
Thirty-one adult patients with newly diagnosed ITP according to an international working group [1, 40, 41] were enrolled in the study (Table 1). Their blood platelet count was less than 30 × 109/L and therefore they required medical intervention. Pregnant females, secondary ITP, and those who could not undergo corticosteroid treatment due to certain reasons like contraindications were excluded. All patients received HD-DXM regimen (40 mg of oral dexamethasone daily for 4 consecutive days). Before and one month after treatment, 10 ml venous blood samples of patients and normal controls was collected. Patients whose platelet counts were remarkably increased and efficacy was validated in accordance with the Vicenza Consensus Conference were described as the response (R) group [1, 40, 41]. Patients whose platelet counts were not remarkably increased were described as the non-response (NR) group [1, 40, 41]. Patients were paired with their own before (Pre-T group) and after treatment (Post-T group). Healthy volunteers were taken as normal controls (NC group).
The study was approved by local Medical Ethics Committees of Zhongshan Hospital, Fudan University. Written informed consent was obtained from each patient enrolled in the study.
Sample preparation
Venous blood samples were collected in ethylenediaminetetraacetic acid–treated tubes and diluted 1:2 with Hanks balanced salt solution (HBSS) before Ficoll-Hypaque gradient centrifugation (2500 rpm at room temperature for 15 min). Washed and resuspended, isolated peripheral blood mononuclear cells (PBMCs) were cryopreserved in fetal bovine serum containing 10% dimethyl sulfoxide (DMSO), and stored in liquid nitrogen for future flow cytometric analysis (FCM) and real-time polymerase chain reaction (RT-PCR).
Cell culture
Cryopreserved PBMCs were thawed at 37 °C, washed twice with HBSS, and stained with trypan blue to testify cell viability. PBMCs were seeded at 5 × 105/mL in 24-well plates in RPMI1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 200 U/mL penicillin, and 100 μg/mL streptomycin for 24 h.
Flow cytometric analysis
Cultured cells were stained with FITC-conjugated anti-CD4/APC-conjugated anti-CD25/PE-conjugated anti-CD39 antibodies at 4 °C for 20 min, washed twice, and resuspended in staining buffer for analysis according to the manufacturer’s protocol (ebioscience, USA). For adenosine A2A receptor intracellular-staining, the cells were incubated with FITC-conjugated anti-CD4/APC-conjugated anti-CD25 monoclonal antibodies and then fixed with fixative buffer for 20 min. Afterward, the cells were permeabilized with permeabilization buffer and stained with PE-conjugated anti-A2A monoclonal antibodies (Santa Cruz Biotechnology, Inc.) for 30 min in the dark. Stained cells were tested on a FACS Aria II flow cytometer (BD, USA) and then analyzed using Flowjo software version 7.6.
Real-time polymerase chain reaction
Total RNA was isolated from PBMCs by Trizol reagent (Invitrogen, USA) and converted into cDNA using a Prime-Script RT reagent kit (Takara, Japan) according to the manufacturer’s instructions. CD39 and A2AR gene expression was quantified using the SYBR Premix Ex Taq (Takara, Japan) on a MasterCycler Realplex4 system (Eppendorf, German), with GAPDH expression as a control. Amplification was performed in a total volume of 20 μL for 40 cycles of 5 s at 95 °C and 30 s at 60 °C after initial denaturation (95 °C, 30 s). The primer sequences were as follows: GAPDH-sense: 5′-CATCAGCAATGCCTCCTGCAC-3′; GAPDH-antisense: 5′-TGAGTCCTTCCACGATACCAAAGTT-3′; CD39-sense: 5′-CCATCCTTGGCTTCTCCTCTAT-3′; CD39-antisense: 5′-CCACGCCTGTGTCATTCTCCT-3′; A2AR-sense: 5′-CACGAGGGCTAAGGGCATCATT-3′; A2AR-antisense: 5′-GCAGTCGGGGCAGAAGAAAGT-3′. Samples were analyzed in triplicate. 2-ΔΔCt was used to calculate fold change of mRNA expression.
ATP hydrolysis assay
PBMCs of the same density were incubated in medium containing 10 μM ATP for 30 min. ATP concentration in the supernatant was determined using the CellTiter-Glo Luminescent Cell Viability Assay (Promega cat. no. G7570). ATP standard luminescence curve was drawn using the ATP disodium salt (Sigma cat. no. A7699).
Statistical analysis
All analyses were performed with SPSS 19.0 software. Continuous variables data were expressed as mean ± SD. Normality was assessed by Shapiro-Wilk test and homogeneity of variances was evaluated by Levene’s test. One-way ANOVA and Kruskal-Wallis test were used for data that fulfilled normal distribution and for those that did not, respectively. Paired student t test was used to evaluate the differences between patients before and after therapy. A value of p < 0.05 was considered statistically significant.
Results
The percentage of CD4+CD25+ Treg cells was decreased in ITP patients and could be corrected by effective corticosteroid therapy
Groups were age and sex matched. The result showed a notable decrease of the percentage of CD4+CD25+ Treg cells in the Pre-T group compared with the NC group (NC vs. Pre-T—(10.22 ± 7.77)% vs. (3.80 ± 3.54)%, p < 0.001, Fig. 1b). Among the response group (R), the Post-T group possessed significantly higher CD4+CD25+ Treg cells percentage than the Pre-T group (Pre-T vs. Post-T—(4.59 ± 3.99)% vs. (8.52 ± 5.02)%, p = 0.011, Fig. 1c) and no difference between the NC group and the Post-T group (p = 0.975, Fig. 1b). Among the non-response group (R), there is no difference between the Pre-T group and the Post-T group (Pre-T vs. Post-T—(1.07 ± 0.53)% vs. (1.59 ± 0.87)%, p = 0.644, Fig. 1c). The representative dot plots are shown in Fig. 1a.
Frequency of CD4+CD25+ cells was decreased in ITP patients and increased after treatment in the response group. a The representative dot plots of CD4+CD25+ cells within lymphocytes in different groups. b, c The mean ± SD of percentage of CD4+CD25+ cells within lymphocytes in different groups. p value is shown in the figure. NC, normal control (n = 20). Pre-T, ITP patients before treatment (n = 23). Post-T, ITP patients after treatment (n = 23). R, response group (n = 16). NR, non-response group (n = 7)
The expression of CD39 in CD4+CD25+ Treg cells was decreased in ITP patients and could be corrected by high-dose dexamethasone therapy
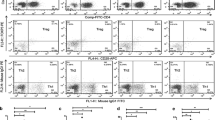
Groups were age and sex matched. A population of CD25+CD39+ cells within CD4+ lymphocytes and CD39+ cells within CD4+CD25+ lymphocytes were detected, which were both remarkably lower in the Pre-T group than in the NC group (p < 0.001, p < 0.001, respectively; Fig. 2b, Fig. 3b). The CD39 mRNA of PBMCs was also decreased in the Pre-T group compared with the NC group (NC vs. Pre-T—1.38 ± 1.64 vs. 0.59 ± 0.37, p = 0.002, Fig. 3d). Among the response group (R), both the population of CD25+CD39+ cells within CD4+ lymphocytes and CD39+ cells within CD4+CD25+ lymphocytes were increased compared with the Pre-T group (p < 0.001, p < 0.001, respectively; Fig. 2c, Fig. 3c). On the other hand, among the non-response group (NR), there was no difference between the Pre-T and Post-T groups (p = 0.154, p = 0.399, respectively, Fig. 2c, Fig. 3c). The representative dot plots are shown in Fig. 2a and Fig. 3a.
CD25+CD39+ cells within CD4+ lymphocytes was reduced in ITP patients and elevated after treatment in the response group. a The representative dot plots of CD25+CD39+ cells within CD4+ lymphocytes in different groups. b, c The mean ± SD of percentage of CD25+CD39+ cells within CD4+ lymphocytes in different groups. p value is shown in the figure. NC, normal control (n = 20). Pre-T, ITP patients before treatment (n = 23). Post-T, ITP patients after treatment (n = 23). R, response group (n = 16). NR, non-response group (n = 7)
Expression of CD39 in Treg cells was decreased in ITP patients and increased after treatment in the response group. a The representative dot plots of CD39+ cells within Treg cells in different groups. b, c The mean ± SD of percentage of CD39+ cells within Treg cells in different groups. p value is shown in the figure. d The mean ± SD of CD39 mRNA within lymphocytes in different groups. e The mean ± SD of CD39 mRNA within lymphocytes in ITP patients before and after treatment. p value is shown in the figure. NC, normal control (n = 20). Pre-T, ITP patients before treatment (n = 23). Post-T, ITP patients after treatment (n = 23). R, response group (n = 16). NR, non-response group (n = 7)
In accordance with CD39 staining, the expression of CD39 mRNA in PBMCs was also elevated after therapy at the R group (Pre-T vs Post-T—0.63 ± 0.38 vs. 1.03 ± 0.36, p = 0.003, Fig. 3e) and showed no difference at the NR group (Pre-T vs Post-T—0.51 ± 0.36 vs. 0.50 ± 0.49, p = 0.735, Fig. 3e).
The expression of adenosine A2A receptor in CD4+ lymphocytes was decreased in ITP patients
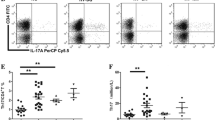
Groups were age and sex matched. The expression of A2A receptor in both CD4+CD25− Teff cells and CD4+CD25+ Treg cells has been assessed. The percentage of A2A+ CD25− cells within CD4+ lymphocytes in both Pre-T group and Post-T groups was decreased compared with that in the NC group (p < 0.001, p = 0.001, respectively, Fig. 4a). The same result went for A2A+ CD25+ cells within CD4+ lymphocytes (p = 0.003, p = 0.006, respectively, Fig. 4b). The frequency of A2A+ CD25− Teff cells was elevated in the Post-T group (Pre-T vs Post-T—(2.47 ± 1.02)% vs. (4.31 ± 1.86)%, p = 0.012, Fig. 4a), whereas no significant difference was observed between the Pre-T group and the Post-T group in A2A+ CD25+ cells (Pre-T vs Post-T—(0.36 ± 0.24)% vs. (0.53 ± 0.55)%, p = 0.93, Fig. 4b). The A2AR mRNA of PBMCs was also decreased in both Pre-T group and Post-T groups compared with that in the NC group (p < 0.001 (Pre-T), p < 0.001 (Post-T R), p = 0.008 (Post-T NR), respectively, Fig. 4c). A2AR mRNA in PBMCs was elevated after therapy at the R group (p = 0.024, Fig. 4s) and showed no difference at the NR group (p = 0.856, Fig. 4D).
Expression of adenosine A2A receptor and enzymatic activity of CD39 was decreased in ITP patients. a The mean ± SD of percentage of A2AR+ CD25− cells within CD4+ lymphocytes in different groups. b The mean ± SD of percentage of A2AR+ CD25+ cells within CD4+ lymphocytes in different groups. p value is shown in the figure. NC, normal control (n = 10). Pre-T, ITP patients before treatment (n = 8). Post-T, ITP patients after treatment (n = 8). c The mean ± SD of A2AR mRNA within lymphocytes in different groups. d The mean ± SD of A2AR mRNA within lymphocytes in ITP patients before and after treatment. p value is shown in the figure. A2AR, adenosine A2A receptor. NC, normal control (n = 20). Pre-T, ITP patients before treatment (n = 23). Post-T, ITP patients after treatment (n = 23). R, response group (n = 16). NR, non-response group (n = 7). e The mean ± SD of ATP consumption in different groups. p value is shown in the figure. NC, normal control (n = 10). Pre-T, ITP patients before treatment (n = 8). Post-T, ITP patients after treatment (n = 8)
The enzymatic activity of CD39 was damaged in ITP patients and could be improved after therapy
Groups were age and sex matched. To test for the functional activity of CD39, the consumption of exogenous ATP by PBMCs was assessed. Ectonucleotidase activity was lower in the Pre-T group than in the NC group (NC vs. Pre-T—87.87 ± 8.70 vs. 54.09 ± 21.51, p = 0.001, Fig. 4e). But it is increased after therapy (Pre-T vs. Post-T—54.09 ± 21.51 vs. 78.42 ± 26.05, p = 0.024, Fig. 4e).
Discussion
Previous investigations have found CD39 defects of CD4+CD25+ (we called Treg cells in this study) cells in several immune-related diseases, such as autoimmune hepatitis and multiple sclerosis [30,31,32, 34]. Inconsistent with these studies, we found that the expression of CD39 in CD4+CD25+Treg cells was decreased at both protein and mRNA levels in ITP patients compared with normal controls. Apart from that, our research also revealed that CD39+ Treg cells were impaired in the ability to hydrolyze ATP and ADP in ITP patients in comparison to normal controls. Those two defects led to a reduced production of immunosuppressive adenosine and persistently high levels of pro-inflammatory ATP in ITP patients which lead to activation and perpetuation of inflammation. After high-dose dexamethasone treatment, the amount and the hydrolytic activity of CD39+ Treg cells were both restored. However, among patients who had no response to corticosteroids, CD39 expression remained impaired in CD4+CD25+ Treg cells. These results suggested that expression and function of CD39 in CD4+CD25+ Treg cells may be correlated with the state of disease and may contribute to the initiation and perpetuation of ITP. CD39 may also be involved in therapeutic effect and low expression can possibly result in lack of response to treatment. Improvement of CD39 expression and function may be involved in the remission and recovery after effective treatment. Furthermore, CD39 may be used to assess the therapeutic effect of ITP and potential therapy for boosting CD39 expression in ITP should be explored in the future. Although CD25 is widely used to identify Treg cells, its specificity is limited because its expression is also induced upon activity of conventional T cells. CD39 could help in the characterization of Treg cells and is likely to be used as a novel functional marker. Expression of CD39 is regulated mainly by two transcriptional factors: sp1 and stat3 which can be triggered by hypoxia, ischemia, and oxidative stress [42, 43]. Moreover, low expression of CD39 in inflammatory bowel disease was reported to be associated with CD39 polymorphism, suggesting a genetically encoded defect of immunoregulation in this condition [33]. Exploration of upstream signals such as sp1 and factors such as CD39 polymorphism which may account for the defect of CD39 in patients with ITP deserves extended studies.
Among CD4+ T lymphocytes, the activation of adenosine A2A receptor can suppress the production of IL-2, IL-4, and IFN-γ; upregulate the expression of CTLA-4 and PD-1; and downregulate the expression of CD40L [44,45,46,47]. A2AR stimulation in Treg cells can also increase the expression of Foxp3 known as a transcription factor and a maker of Treg cells [48, 49]. Researches have found the abnormality of adenosine A2A receptor expression in some immune-related diseases, such as myasthenia gravis and type 2 diabetes mellitus [38, 39]. In our study, we demonstrated that the expression of A2A adenosine receptor in both Treg cells and Teff cells was diminished in ITP patients in contrast to normal controls. After effective corticosteroid treatment, Teff cells expressed more A2AR but no changes were observed in Treg cells. These findings indicated that the low expression of A2AR in CD4+ T lymphocytes could play a crucial role in the immune disorder in ITP. In experimental autoimmune myasthenia gravis (EMAG), Na Li et al. revealed the administration of A2AR agonist CGS21680 not only led to a decrease in anti-AChR IgG levels but also partially restored the imbalance between Th1/Th2/Th17/Treg cell subset. Furthermore, preventive treatment of EAMG with CGS21680 was effective in down-modulating disease manifestations and therapeutic treatment partly attenuated the severity of established EAMG [38]. Among other autoimmune diseases such as inflammatory bowel disease and experimental autoimmune encephalomyelitis, A2A receptor agonists also showed effective in animal models [50,51,52]. Moreover, A2A receptor agonists, through activation of Tregs and immune tolerance, have proven to be effective in reducing and preventing graft versus host disease (GVHD) development in murine models of the disease [53, 54]. On the opposite side, improving anti-tumor T cell activity by A2AR antagonists suggested promise for disengaging the adenosine-mediated immunosuppression in the tumor microenvironment like non-small-cell lung cancer [55, 56]. Therefore, ongoing development of therapeutic strategies targeting the A2AR may have beneficial therapeutic applications in ameliorating severity of disease and offer promise for the management of some autoimmune diseases such as ITP.
In conclusion, this study has shown that, in ITP, there was a numerical decrease in CD39 expression of CD4+CD25+ cells. These CD39+ Treg cells were impaired in their enzymatic and suppressive abilities. After effective corticosteroid treatment, these two defects could be reversed. Our results also demonstrated that ITP patients had reduced A2AR expression in both CD4+CD25+ Treg cells and CD4+CD25− Teff cells. Therefore, reduced production of adenosine and decreased expression of adenosine A2A receptor in CD4+ T lymphocytes may be both involved in the development of ITP. Therapies targeting adenosine production and A2AR such as A2AR agonists may have a promising future.
References
Rodeghiero F, Stasi R, Gernsheimer T, Michel M, Provan D, Arnold DM, Bussel JB, Cines DB, Chong BH, Cooper N, Godeau B, Lechner K, Mazzucconi MG, McMillan R, Sanz MA, Imbach P, Blanchette V, Kuhne T, Ruggeri M, George JN (2009) Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood 113(11):2386–2393. https://doi.org/10.1182/blood-2008-07-162503
Swinkels M, Rijkers M, Voorberg J, Vidarsson G, Leebeek FWG, Jansen AJG (2018) Emerging concepts in immune thrombocytopenia. Front Immunol 9:880. https://doi.org/10.3389/fimmu.2018.00880
McMillan R (2000) Autoantibodies and autoantigens in chronic immune thrombocytopenic purpura. Semin Hematol 37(3):239–248
Ogawara H, Handa H, Morita K, Hayakawa M, Kojima J, Amagai H, Tsumita Y, Kaneko Y, Tsukamoto N, Nojima Y, Murakami H (2003) High Th1/Th2 ratio in patients with chronic idiopathic thrombocytopenic purpura. Eur J Haematol 71(4):283–288
Takahashi N, Saitoh T, Gotoh N, Nitta Y, Alkebsi L, Kasamatsu T, Minato Y, Yokohama A, Tsukamoto N, Handa H, Murakami H (2017) The cytokine polymorphisms affecting Th1/Th2 increase the susceptibility to, and severity of, chronic ITP. BMC Immunol 18(1):26. https://doi.org/10.1186/s12865-017-0210-3
Gu D, Chen Z, Zhao H, Du W, Xue F, Ge J, Sui T, Wu H, Liu B, Lu S, Zhang L, Yang R (2010) Th1 (CXCL10) and Th2 (CCL2) chemokine expression in patients with immune thrombocytopenia. Hum Immunol 71(6):586–591. https://doi.org/10.1016/j.humimm.2010.02.010
Ling Y, Cao X, Yu Z, Ruan C (2007) Circulating dendritic cells subsets and CD4+Foxp3+ regulatory T cells in adult patients with chronic ITP before and after treatment with high-dose dexamethasome. Eur J Haematol 79(4):310–316. https://doi.org/10.1111/j.1600-0609.2007.00917.x
Liu B, Zhao H, Poon MC, Han Z, Gu D, Xu M, Jia H, Yang R, Han ZC (2007) Abnormality of CD4(+)CD25(+) regulatory T cells in idiopathic thrombocytopenic purpura. Eur J Haematol 78(2):139–143. https://doi.org/10.1111/j.1600-0609.2006.00780.x
Bao W, Bussel JB, Heck S, He W, Karpoff M, Boulad N, Yazdanbakhsh K (2010) Improved regulatory T-cell activity in patients with chronic immune thrombocytopenia treated with thrombopoietic agents. Blood 116(22):4639–4645. https://doi.org/10.1182/blood-2010-04-281717
Nishimoto T, Satoh T, Takeuchi T, Ikeda Y, Kuwana M (2012) Critical role of CD4(+)CD25(+) regulatory T cells in preventing murine autoantibody-mediated thrombocytopenia. Exp Hematol 40(4):279–289. https://doi.org/10.1016/j.exphem.2012.01.001
Stasi R, Cooper N, Del Poeta G, Stipa E, Laura Evangelista M, Abruzzese E, Amadori S (2008) Analysis of regulatory T-cell changes in patients with idiopathic thrombocytopenic purpura receiving B cell-depleting therapy with rituximab. Blood 112(4):1147–1150. https://doi.org/10.1182/blood-2007-12-129262
Aslam R, Hu Y, Gebremeskel S, Segel GB, Speck ER, Guo L, Kim M, Ni H, Freedman J, Semple JW (2012) Thymic retention of CD4+CD25+FoxP3+ T regulatory cells is associated with their peripheral deficiency and thrombocytopenia in a murine model of immune thrombocytopenia. Blood 120(10):2127–2132. https://doi.org/10.1182/blood-2012-02-413526
Zhang J, Zhang Q, Li Y, Tao L, Wu F, Shen Y, Tao Q, Xu X, Wu C, Ruan Y, Wang J, Wang J, Wang Y, Zhai Z (2018) Immune dysregulation in primary immune thrombocytopenia patients. Hematology 23:1–7. https://doi.org/10.1080/10245332.2018.1435021
Li J, Wang Z, Hu S, Zhao X, Cao L (2013) Correction of abnormal T cell subsets by high-dose dexamethasone in patients with chronic idiopathic thrombocytopenic purpura. Immunol Lett 154(1–2):42–48. https://doi.org/10.1016/j.imlet.2013.08.006
Li F, Ji L, Wang W, Hua F, Zhan Y, Zou S, Yuan L, Ke Y, Min Z, Song D, Sun L, Chen H, Cheng Y (2015) Insufficient secretion of IL-10 by Tregs compromised its control on over-activated CD4+ T effector cells in newly diagnosed adult immune thrombocytopenia patients. Immunol Res 61(3):269–280. https://doi.org/10.1007/s12026-014-8620-2
Nishimoto T, Kuwana M (2013) CD4+CD25+Foxp3+ regulatory T cells in the pathophysiology of immune thrombocytopenia. Semin Hematol 50(Suppl 1):S43–S49. https://doi.org/10.1053/j.seminhematol.2013.03.018
Ji L, Zhan Y, Hua F, Li F, Zou S, Wang W, Song D, Min Z, Chen H, Cheng Y (2012) The ratio of Treg/Th17 cells correlates with the disease activity of primary immune thrombocytopenia. PLoS One 7(12):e50909. https://doi.org/10.1371/journal.pone.0050909
Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC (2007) Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med 204(6):1257–1265. https://doi.org/10.1084/jem.20062512
Dwyer KM, Hanidziar D, Putheti P, Hill PA, Pommey S, McRae JL, Winterhalter A, Doherty G, Deaglio S, Koulmanda M, Gao W, Robson SC, Strom TB (2010) Expression of CD39 by human peripheral blood CD4+ CD25+ T cells denotes a regulatory memory phenotype. Am J Transplant Off J Am Soc Transplant Am Soc Transplant Surg 10(11):2410–2420. https://doi.org/10.1111/j.1600-6143.2010.03291.x
Heine P, Braun N, Sevigny J, Robson SC, Servos J, Zimmermann H (2001) The C-terminal cysteine-rich region dictates specific catalytic properties in chimeras of the ectonucleotidases NTPDase1 and NTPDase2. European J Biochem FEBS 268(2):364–373
Mizumoto N, Kumamoto T, Robson SC, Sevigny J, Matsue H, Enjyoji K, Takashima A (2002) CD39 is the dominant Langerhans cell-associated ecto-NTPDase: modulatory roles in inflammation and immune responsiveness. Nat Med 8(4):358–365. https://doi.org/10.1038/nm0402-358
Robson SC, Sevigny J, Zimmermann H (2006) The E-NTPDase family of ectonucleotidases: structure function relationships and pathophysiological significance. Purinergic Signal 2(2):409–430. https://doi.org/10.1007/s11302-006-9003-5
Kobie JJ, Shah PR, Yang L, Rebhahn JA, Fowell DJ, Mosmann TR (2006) T regulatory and primed uncommitted CD4 T cells express CD73, which suppresses effector CD4 T cells by converting 5′-adenosine monophosphate to adenosine. J Immunol 177(10):6780–6786
Bastid J, Cottalorda-Regairaz A, Alberici G, Bonnefoy N, Eliaou JF, Bensussan A (2013) ENTPD1/CD39 is a promising therapeutic target in oncology. Oncogene 32(14):1743–1751. https://doi.org/10.1038/onc.2012.269
de Oliveira Bravo M, Carvalho JL, Saldanha-Araujo F (2016) Adenosine production: a common path for mesenchymal stem-cell and regulatory T-cell-mediated immunosuppression. Purinergic Signal 12(4):595–609. https://doi.org/10.1007/s11302-016-9529-0
Fredholm BB (2007) Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ 14(7):1315–1323. https://doi.org/10.1038/sj.cdd.4402132
Fredholm BB, Irenius E, Kull B, Schulte G (2001) Comparison of the potency of adenosine as an agonist at human adenosine receptors expressed in Chinese hamster ovary cells. Biochem Pharmacol 61(4):443–448
Alam MS, Costales MG, Cavanaugh C, Williams K (2015) Extracellular adenosine generation in the regulation of pro-inflammatory responses and pathogen colonization. Biomolecules 5(2):775–792. https://doi.org/10.3390/biom5020775
Mandapathil M, Hilldorfer B, Szczepanski MJ, Czystowska M, Szajnik M, Ren J, Lang S, Jackson EK, Gorelik E, Whiteside TL (2010) Generation and accumulation of immunosuppressive adenosine by human CD4+CD25highFOXP3+ regulatory T cells. J Biol Chem 285(10):7176–7186. https://doi.org/10.1074/jbc.M109.047423
Peelen E, Damoiseaux J, Smolders J, Knippenberg S, Menheere P, Tervaert JW, Hupperts R, Thewissen M (2011) Th17 expansion in MS patients is counterbalanced by an expanded CD39+ regulatory T cell population during remission but not during relapse. J Neuroimmunol 240-241:97–103. https://doi.org/10.1016/j.jneuroim.2011.09.013
Muls NG, Dang HA, Sindic CJ, van Pesch V (2015) Regulation of Treg-associated CD39 in multiple sclerosis and effects of corticotherapy during relapse. Mult Scler 21(12):1533–1545. https://doi.org/10.1177/1352458514567215
Fletcher JM, Lonergan R, Costelloe L, Kinsella K, Moran B, O'Farrelly C, Tubridy N, Mills KH (2009) CD39+Foxp3+ regulatory T cells suppress pathogenic Th17 cells and are impaired in multiple sclerosis. J Immunol 183(11):7602–7610. https://doi.org/10.4049/jimmunol.0901881
Friedman DJ, Kunzli BM, Yi AR, Sevigny J, Berberat PO, Enjyoji K, Csizmadia E, Friess H, Robson SC (2009) From the cover: CD39 deletion exacerbates experimental murine colitis and human polymorphisms increase susceptibility to inflammatory bowel disease. Proc Natl Acad Sci U S A 106(39):16788–16793. https://doi.org/10.1073/pnas.0902869106
Grant CR, Liberal R, Holder BS, Cardone J, Ma Y, Robson SC, Mieli-Vergani G, Vergani D, Longhi MS (2014) Dysfunctional CD39(POS) regulatory T cells and aberrant control of T-helper type 17 cells in autoimmune hepatitis. Hepatology 59(3):1007–1015. https://doi.org/10.1002/hep.26583
Loza MJ, Anderson AS, O'Rourke KS, Wood J, Khan IU (2011) T-cell specific defect in expression of the NTPDase CD39 as a biomarker for lupus. Cell Immunol 271(1):110–117. https://doi.org/10.1016/j.cellimm.2011.06.010
Peres RS, Donate PB, Talbot J, Cecilio NT, Lobo PR, Machado CC, Lima KWA, Oliveira RD, Carregaro V, Nakaya HI, Cunha TM, Alves-Filho JC, Liew FY, Louzada-Junior P, Cunha FQ (2018) TGF-beta signalling defect is linked to low CD39 expression on regulatory T cells and methotrexate resistance in rheumatoid arthritis. J Autoimmun 90:49–58. https://doi.org/10.1016/j.jaut.2018.01.004
Gibson DJ, Elliott L, McDermott E, Tosetto M, Keegan D, Byrne K, Martin ST, Rispens T, Cullen G, Mulcahy HE, Cheifetz AS, Moss AC, Robson SC, Doherty GA, Ryan EJ (2015) Heightened expression of CD39 by regulatory T lymphocytes is associated with therapeutic remission in inflammatory bowel disease. Inflamm Bowel Dis 21(12):2806–2814. https://doi.org/10.1097/MIB.0000000000000566
Li N, Mu L, Wang J, Zhang J, Xie X, Kong Q, Tang W, Yao X, Liu Y, Wang L, Wang G, Wang D, Jin L, Sun B, Li H (2012) Activation of the adenosine A2A receptor attenuates experimental autoimmune myasthenia gravis severity. Eur J Immunol 42(5):1140–1151. https://doi.org/10.1002/eji.201142088
Guzman-Flores JM, Cortez-Espinosa N, Cortes-Garcia JD, Vargas-Morales JM, Catano-Canizalez YG, Rodriguez-Rivera JG, Portales-Perez DP (2015) Expression of CD73 and A2A receptors in cells from subjects with obesity and type 2 diabetes mellitus. Immunobiology 220(8):976–984. https://doi.org/10.1016/j.imbio.2015.02.007
Provan D, Stasi R, Newland AC, Blanchette VS, Bolton-Maggs P, Bussel JB, Chong BH, Cines DB, Gernsheimer TB, Godeau B, Grainger J, Greer I, Hunt BJ, Imbach PA, Lyons G, McMillan R, Rodeghiero F, Sanz MA, Tarantino M, Watson S, Young J, Kuter DJ (2010) International consensus report on the investigation and management of primary immune thrombocytopenia. Blood 115(2):168–186. https://doi.org/10.1182/blood-2009-06-225565
Neunert C, Lim W, Crowther M, Cohen A, Solberg L Jr, Crowther MA, American Society of H (2011) The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood 117(16):4190–4207. https://doi.org/10.1182/blood-2010-08-302984
Eltzschig HK, Kohler D, Eckle T, Kong T, Robson SC, Colgan SP (2009) Central role of Sp1-regulated CD39 in hypoxia/ischemia protection. Blood 113(1):224–232. https://doi.org/10.1182/blood-2008-06-165746
Deaglio S, Robson SC (2011) Ectonucleotidases as regulators of purinergic signaling in thrombosis, inflammation, and immunity. Adv Pharmacol 61:301–332. https://doi.org/10.1016/B978-0-12-385526-8.00010-2
Naganuma M, Wiznerowicz EB, Lappas CM, Linden J, Worthington MT, Ernst PB (2006) Cutting edge: critical role for A2A adenosine receptors in the T cell-mediated regulation of colitis. J Immunol 177(5):2765–2769
Sevigny CP, Li L, Awad AS, Huang L, McDuffie M, Linden J, Lobo PI, Okusa MD (2007) Activation of adenosine 2A receptors attenuates allograft rejection and alloantigen recognition. J Immunol 178(7):4240–4249
Lappas CM, Rieger JM, Linden J (2005) A2A adenosine receptor induction inhibits IFN-gamma production in murine CD4+ T cells. J Immunol 174(2):1073–1080
Csoka B, Himer L, Selmeczy Z, Vizi ES, Pacher P, Ledent C, Deitch EA, Spolarics Z, Nemeth ZH, Hasko G (2008) Adenosine A2A receptor activation inhibits T helper 1 and T helper 2 cell development and effector function. FASEB J : official publication of the Federation of American Societies for Experimental Biology 22(10):3491–3499. https://doi.org/10.1096/fj.08-107458
Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, Hopner S, Centonze D, Bernardi G, Dell’Acqua ML, Rossini PM, Battistini L, Rotzschke O, Falk K (2007) Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood 110(4):1225–1232. https://doi.org/10.1182/blood-2006-12-064527
Zarek PE, Huang CT, Lutz ER, Kowalski J, Horton MR, Linden J, Drake CG, Powell JD (2008) A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood 111(1):251–259. https://doi.org/10.1182/blood-2007-03-081646
Odashima M, Bamias G, Rivera-Nieves J, Linden J, Nast CC, Moskaluk CA, Marini M, Sugawara K, Kozaiwa K, Otaka M, Watanabe S, Cominelli F (2005) Activation of A2A adenosine receptor attenuates intestinal inflammation in animal models of inflammatory bowel disease. Gastroenterology 129(1):26–33
Liu Y, Alahiri M, Ulloa B, Xie B, Sadiq SA (2018) Adenosine A2A receptor agonist ameliorates EAE and correlates with Th1 cytokine-induced blood brain barrier dysfunction via suppression of MLCK signaling pathway. Immun Inflammation Dis 6(1):72–80. https://doi.org/10.1002/iid3.187
Liu Y, Zou H, Zhao P, Sun B, Wang J, Kong Q, Mu L, Zhao S, Wang G, Wang D, Zhang Y, Zhao J, Yin P, Liu L, Zhao X, Li H (2016) Activation of the adenosine A2A receptor attenuates experimental autoimmune encephalomyelitis and is associated with increased intracellular calcium levels. Neuroscience 330:150–161. https://doi.org/10.1016/j.neuroscience.2016.05.028
Han KL, Thomas SV, Koontz SM, Changpriroa CM, Ha SK, Malech HL, Kang EM (2013) Adenosine A(2) A receptor agonist-mediated increase in donor-derived regulatory T cells suppresses development of graft-versus-host disease. J Immunol 190(1):458–468. https://doi.org/10.4049/jimmunol.1201325
Lappas CM, Liu PC, Linden J, Kang EM, Malech HL (2010) Adenosine A2A receptor activation limits graft-versus-host disease after allogenic hematopoietic stem cell transplantation. J Leukoc Biol 87(2):345–354. https://doi.org/10.1189/jlb.0609388
Mediavilla-Varela M, Castro J, Chiappori A, Noyes D, Hernandez DC, Allard B, Stagg J, Antonia SJ (2017) A novel antagonist of the immune checkpoint protein adenosine A2a receptor restores tumor-infiltrating lymphocyte activity in the context of the tumor microenvironment. Neoplasia 19(7):530–536. https://doi.org/10.1016/j.neo.2017.02.004
Gessi S, Bencivenni S, Battistello E, Vincenzi F, Colotta V, Catarzi D, Varano F, Merighi S, Borea PA, Varani K (2017) Inhibition of A2A adenosine receptor signaling in cancer cells proliferation by the novel antagonist TP455. Front Pharmacol 8:888. https://doi.org/10.3389/fphar.2017.00888
Funding
This study was supported by grants from the National Natural Science Foundation of China(81870098, 81600090, 81470282,81500090), the Science and Technology Commission of Shanghai Municipality (18ZR1407200), and the Shanghai Municipal Health Bureau (20134Y117, 20144Y0194), and grant from Zhongshan Hospital (2015ZSYXGG09).
Author information
Authors and Affiliations
Contributions
YL and YC conceived the study; YL, LC, FL, LJ, and YC performed the literature review, and drafted and revised the manuscript; YL and YC contributed to the critical revision of the manuscript; YL, YZ, LC, XS, BW, CL, ZM, YK, LS, and HC performed the experiments and analyzed the data. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
The study was approved by local Medical Ethics Committees of Zhongshan Hospital, Fudan University. Written informed consent was obtained from each patient enrolled in the study.
Competing interests
The authors declare that they have no conflict of interest.
Data sharing statement
Data sharing: no additional data available
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lu, Y., Cheng, L., Li, F. et al. The abnormal function of CD39+ regulatory T cells could be corrected by high-dose dexamethasone in patients with primary immune thrombocytopenia. Ann Hematol 98, 1845–1854 (2019). https://doi.org/10.1007/s00277-019-03716-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-019-03716-9