Abstract
Adenosine is an important molecule that exerts control on the immune system, by signaling through receptors lying on the surface of immune cells. This nucleotide is produced, in part, by the action of the ectoenzymes CD39 and CD73. Interestingly, these proteins are expressed on the cell surface of regulatory T-cells (Tregs) and mesenchymal stromal cells (MSCs)—two cell populations that have emerged as potential therapeutic tools in the field of cell therapy. In fact, the production of adenosine constitutes a mechanism used by both cell types to control the immune response. Recently, great scientific progress was obtained regarding the role of adenosine in the inflammatory environment. In this context, the present review focuses on the advances related to the impact of adenosine production over the immune modulatory activity of Tregs and MSCs, and how this nucleotide controls the biological functions of these cells. Finally, we mention the main challenges and hurdles to bring such molecule to clinical settings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adenosine is a purine nucleoside found in virtually every cell of the human body. It mediates important signaling, which has been involved in several biological events, ranging from cell energy metabolism to complex and multicellular events, such as cardiovascular ischemia-reperfusion response. In fact, adenosine has been used clinically since the 1940s for cardiac protection and vasodilation [1].
Adenosine’s role in the immune response control has been described in the past years, but since then, its fullest potential remains to be fully grasped. Indirectly explored by current drugs, such as methotrexate and caffeine, adenosine is still far from its greatest clinical potential, due to lack of knowledge, as well as to some great challenges that still lie ahead. For instance, the existence of different adenosine receptors present in most tissues at different proportions and associated to different biological effects poses the risk of important side effects, when it comes to systemic therapy using adenosine-based drugs.
In the present review, we go through adenosine’s basic features, such as adenosine formation, clearance, receptors, signaling, and biological effects, giving a major focus on the immunological aspects of such molecule. We then discuss recent observations, which indicate that adenosine signaling, a long known immunomodulation strategy used by cancer and regulatory T-cells (Tregs), is also explored by a new player in the immunological field: the mesenchymal stromal cells (MSCs). Besides reviewing how this novel MSC immunomodulation tool came to light, we quickly review adenosine’s clinical path, as well as the major hurdles still limiting adenosine’s druggability.
Finally, we end our discussion defending a provocative hypothesis, in which we suggest that cell therapy may be adenosine’s major potential to reach the clinic for the treatment of immunological disorders, since such biological entities, which could include Tregs or MSCs, will guarantee local, fine-tuned, and biologically relevant adenosine production, being effective in grasping adenosine’s immunomodulation potential, without provoking the already described and daunting side effects.
Extracellular adenosine production and adenosine signaling
Adenosine is an important molecule for mediating several biologic functions, such as nucleotide biosynthesis and cellular energy metabolism, in addition to acting in the control of immune response. In the extracellular space, adenosine is mainly produced through adenosine 5′-triphosphate (ATP) dephosphorylation by two ectonucleotidases—ectonucleoside triphosphate diphosphohydrolase 1 (CD39) and ecto-5-nucleotidase (CD73). In this process, CD39 hydrolyzes ATP and ADP to AMP, whereas CD73 converts AMP into adenosine. It is worth mentioning that while CD39 activity can be reversed by NDP kinase and adenylate kinase, the activity of CD73 constitutes an irreversible step that culminates in the adenosine generation [2]. Once produced, adenosine exerts its influence on several physiological/pathophysiological processes through signaling of specific receptors, as mentioned below. Adenosine concentration in the extracellular space is maintained at low levels by its degradation, being that the main mechanism of adenosine clearance consists of its deamination to inosine by adenosine deaminase (ADA) [3, 4].
In situations of tissue injury, adenosine production becomes more pronounced due to ATP release from the cells and, once produced, acts as signaling molecules by binding to purinergic transmembrane receptors localized on target cell surfaces, namely adenosine receptors A1 (A1AR), A2A (A2AAR), A2B (A2BAR), and A3 (A3AR) [5]. It is well known that after binding to any of these receptors, adenosine modulation of cell functions will be determined by the inhibition or stimulation of adenylyl cyclase and consequently decrease or increase of intracellular cyclic adenosine monophosphate (cAMP) concentrations [6]. In this sense, there are two different groups of adenosine receptors: A1AR and A3AR, which act by decreasing cAMP levels, and A2AAR and A2BAR, which are able to increase cAMP concentrations.
Therefore, the rationale behind adenosine-mediated immunosuppressive mechanism relies on the fact that ATP has dual roles in cellular physiology, depending on its location. If on one side, intracellular ATP is the main energy unit for cellular energy requiring process, and reaches millimolar concentrations in the cytoplasm, on the other, extracellular ATP is a powerful signaling molecule, performing important signaling function, despite maximal concentrations reaching only low nanomolars [7]. Released by damaged or stressed cells, extracellular ATP acts as a danger signal and induces inflammasome activation, as well as the release of inflammatory cytokines, being strong pro-inflammatory stimuli. Extracellular ATP also promotes phagocyte chemotaxis and leads to damaged cell clearance, as beautifully revised by Corriden and Insel [8]. Accumulated extracellular ATP gradually suffers enzymatic hydrolysis into adenosine, which, in turn, is read as a “reporter of excessive tissue damage,” as shown by Bono et al. [9] As such, adenosine signaling is interpreted as an anti-inflammatory stimulus, generally opposing the effects induced by ATP. Interestingly, ATP may also be released by immune cells upon activation [7] and promotes immunological response fine-tuning.
Adenosine receptors—a brief overview
The expression of adenosine receptors is not homogenous among different tissues, varying according to each cell type. A1AR has high affinity for adenosine and is the most abundant adenosine receptor in the brain, where it modulates several adenosine-induced effects, such as neuronal excitability and synaptic transmission [9, 10]. In addition, pulmonary [11], cardiac [12], hepatic [13], and renal [14] inflammatory models have been explored to demonstrate that A1AR signaling leads to anti-inflammatory effects. According to these studies, besides controlling inflammation, A1AR signaling also regulates polymorphonuclear cell trafficking and constitutes a key process for cytoprotection control. Furthermore, it has been demonstrated that adenosine appears to influence vesicular MHC class I cross-presentation by resting dendritic cells through A1AR signaling [15]. Underscoring A1AR importance, it has been demonstrated recently that the loss of A1AR expression on pancreatic α-cells may be involved on type 1 diabetes pathology [16].
A2AAR can be found in the brain [17], ventricular myocytes [18], endothelial cells [19], carotid body [20], and immune cells [20, 21], among other tissues. With such a wide distribution, A2AAR has been linked to several processes, including protection against ischemia–reperfusion injury [22], coronary vasodilatation [23], sleep regulation [24], control of inflammation [21], citing but a few biological events related to such receptor. Regarding the immune system, A2AAR signaling influences a myriad of events, including dendritic cell maturation [25]; inhibition of neutrophil phagocytosis and adhesion [26, 27]; suppression of proinflammatory response of macrophages [28]; inhibition of inflammatory cytokine production by lymphocytes [29], control of CD8 T-cells cytotoxicity; and modulation of Treg function [30, 31]. Recently, it has also been demonstrated that deletion of A2AAR in mice models causes a decline in the number of naive T-cells in the periphery. Finally, A2AAR signaling appears to prevent IL-7R downregulation after TCR stimulation, increasing naive T-cells survival [32].
In contrast to the activation of A1AR, A2AAR, and A3AR that occurs in physiological conditions with EC50 values between 10 nM and 1 μM, A2BAR activation requires higher adenosine concentrations, exceeding 10 μM, which happens mostly during pathophysiological conditions, such as hypoxia, inflammation, and ischemia [33]. So far, the presence of A2BAR has been detected in lymphocytes [34], neutrophils [35], mast cells [36], dendritic cells [37], endothelial cells [38], fibroblasts [39], and epithelia [40]. Accumulating evidence suggests an important role of A2BAR receptor in the cited cells. For instance, the presence of A2BAR on vascular endothelium appears to be crucial for the maintenance of vascular tonus, since A2BAR activation contributes to the relaxation of aorta through NO production [41]. Moreover, signaling of A2BAR appears to be an important protection mechanism against vascular injuries [42]. It is well known that the inflammatory milieu leads to increased adenosine levels, as well as A2BAR expression. In part, the expression of A2BAR occurs in response to some components present at the inflammatory environment, such as lipopolysaccharide (LPS) and the inflammatory cytokines TNF-α, IL-1β, and IFN-γ [43]. Interestingly, studies conducted in animal models have shown that A2BAR can mediate anti-inflammatory and proinflammatory effects. In mouse models of type 2 diabetes, the increased expression of A2BAR elevates the production of proinflammatory mediators, such as IL-6 and C-reactive protein (CRP) [44]. Likewise, in a study using a mouse model of allergen-induced chronic airway inflammation, the genetic removal of A2BAR inhibited allergen-induced chronic pulmonary inflammation. In contrast, others showed a protective role to A2BAR in inflammatory scenery. A study performed with a mice model of lung injury demonstrated that the use of an A2BAR antagonist enhances pulmonary inflammation, while the use of an A2BAR agonist attenuates the pulmonary inflammation [45]. The conflicting data on A2BAR’s role in inflammatory pulmonary injury allows suggesting that A2BAR function varies according to the phase of disease. Supporting such claim, it was shown that, in a mice model of acute and chronic injury induced by bleomycin, A2BAR exerts an anti-inflammatory role during the acute phase of injury, while inducing fibrosis in the chronic period of this disease [46]. In this line, it has been discussed that in acute injuries, the adenosine response to hypoxic conditions promotes the restoration of normal levels of oxygen and dampens inflammation, promoting tissue adaptation. In contrast, when the elevated levels of adenosine remains beyond the acute phase of the injury, the hypoxic adenosine response changes into tissue injury and fibrosis. Finally, it is important to emphasize that this observation is not limited to the lungs but seems to occur in several other tissues [47].
A3AR can be found, both in humans and rodents, in several tissues, such as the lungs, liver, testis, kidneys, heart, brain, spleen, and placenta. This receptor can also be detected in immune cells, including eosinophils, neutrophils, monocytes, dendritic cells, and lymphocytes. Additionally, A3AR has been described as a cancer marker due to its expression on the colon, breast, lung, pancreatic, and hepatocellular carcinoma. In addition, high levels of this receptor are present in leukemia, lymphoma, and melanoma cells [48, 49]. Although the expression of A3AR is low in the myocardium, this receptor is involved in several effects on this tissue, which may be cytoprotective or cytotoxic, depending on the level of receptor activation. Protective effects include the reduction of infarct size and inhibition of apoptosis and necrosis [50]. A3AR signaling leads to a strong anti-inflammatory effect mediated mainly by the inhibition of proinflammatory cytokines (TNF-α, IL-1, IL-6, and IL-12) and induction of apoptosis, both processes being mediated by the deregulation of the NF-kB signaling pathway [51, 52].
As mentioned above, adenosine receptors are widely distributed in various tissues and have been associated with many pathophysiological alterations. Therefore, the modulation of these receptors constitutes a promising therapeutic strategy in several contexts. Furthermore, the presence of adenosine receptors in, virtually, all immune cells, underscores the importance of this nucleotide in the control of the immune and inflammatory response. Below, we discuss the involvement of the signaling promoted by adenosine on the functions of two cells that have emerged with great potential for cell therapy: Tregs and MSCs.
Regulatory T-cells
Tregs are T lymphocytes with immunomodulatory function, which can be generated during the course of T-cell development in the thymus (thymic Tregs or natural occurring Tregs (nTregs)), or produced in peripheral sites (peripheral Tregs (pTregs)). Alternatively, upon certain conditions, it is possible to induce Treg generation in vitro (iTregs) [53]. nTregs migrate from the thymus to the periphery, where they represent a small population of ∼5–10 % of peripheral CD4+ T-cells [54]. Even though there is no consensus regarding the markers to distinguish nTregs and pTregs, it is believed that at peripheral sites lies a mixture of these two cell populations [55]. The low number of Tregs that can be obtained in the peripheral blood and the great therapeutic potential of these cells led to the search for alternative methods focused on the generation and expansion of Tregs. The first evidence that it is indeed possible to generate Tregs in vitro occurred in 2001, when Yamagiwa et al. [56] showed that TGF-β induces the differentiation of naive T-cells obtained from peripheral blood into iTregs with phenotype and suppressive capacity similar to nTregs. Importantly, iTregs can be expanded in vitro by IL-2, a cytokine that is also paramount to the generation of iTregs from naive T-cells [57]. Another important component in this scenario is the all-trans-retinoic acid (ATRA), which, in the presence of TGF-β, converts naive T-cells into Tregs with a more stable expression of FOXP3 and higher suppressive potential, compared to iTregs generated in the absence of ATRA [58, 59].
Interestingly, during MSC-mediated immunosuppression of T-cells, the former act through cell-cell interaction, producing soluble factors with anti-inflammatory function and generating Tregs [60]. Indeed, MSCs are able to generate Tregs, in part, by the secretion of TGF-β and by promoting Treg expansion [61, 62]. Also, it was reported that rapamycin, an inhibitor of mTOR signaling, promotes the expansion of Tregs obtained from peripheral blood, regardless if used solely or in combination with ATRA [63, 64]. Not only rapamycin was effective in promoting Treg expansion but also in leading to iTreg generation and expansion, when combined with IL-2 [65]. In this sense, recently, it was shown that miR-15b/16 enhances the induction of iTregs by inhibition of mTOR signaling pathway [66]. Similarly, mTOR signaling is also repressed by miR-99a and miR-150, which enhance iTreg differentiation [67].
In recent years, several researchers have sought for additional markers that characterize Tregs. Since FOXP3 is an intracellular marker, the description of cell surface markers has important practical and conceptual implications. For instance, CD39 and CD73 were shown to be effective markers of Foxp3+ Tregs and have been increasingly used to isolate Tregs [68, 69]. Moving beyond Treg identification, the possibility of stratification of different lymphocyte phenotypes capable of suppressing immune response is of interest since it would allow the purification of lymphocytes on the basis of specific surface markers and improve functional assays. In this line, it was demonstrated that CD4+CD25+FOXP3+ T-cells are not a completely homogeneous cell population, being that some subpopulations express T-cell immunoglobulin and mucin domain 3 (TIM-3), while CD4+CD25+FOXP3+TIM-3- subpopulation does not. Functionally, such subpopulations behave differently, being that the former is more efficient at inhibiting pathogenic Th1-cell responses, compared to the latter [70]. Another molecule associated with important biological observation is GITR, since in vitro studies revealed that such cell surface protein could be explored in order to obtain lymphocytes with suppressive function. In fact, both CD4+CD25−GITR+ T-cells and CD4+CD25+GITR+ T-cells are anergic and able to suppress T-cell proliferation [71].
It was also demonstrated that IL-7 receptor (CD127) is downregulated on T-cells and that the majority of these cells express FoxP3 and are immunosuppressive. In addition to the search for alternative markers for identifying lymphocytes with suppressive potential, other lymphocyte populations with different phenotypes compared to the classical Tregs, have been described, including CD8+ regulatory T-cells [72], CD8+CD28− regulatory T-cells [73], CD3+CD4−CD8− regulatory T-cells (double negative) [74] and CD8+CXCR3+ regulatory T-cells [75]. Contributing to this already complex scenario, it was also shown that other immune cells possess capacity to control the immune response, such as regulatory B-cells [76] and myeloid-derived suppressor cells [77]. Recently, the interaction between these regulatory cells with Tregs began to be explored, leading to the observation that both regulatory B-cells and myeloid-derived suppressor cells act converting CD4 T-cells into Tregs [78, 79].
Several studies have been conducted in order to dissect the mechanisms used by Tregs to control the immune response, but such mechanisms still remain to be completely known. Pieces of evidence point to a key role of FOXP3 transcription factor in Treg-mediated immunosuppression, including the fact that CD4+CD25+FOXP3+ T-cells inhibit the development of autoimmune diseases caused by Treg depletion and the knowledge that the forced expression of the Foxp3 gene in naive T-cells converts them into Treg-like cells, able to promote immunosuppressive functions both in vivo and in vitro [80]. It has been shown that FOXP3 binds to ∼700 genes and acts as both transcriptional activator and repressor, a central mechanism for Treg-related processes, such as survival, proliferation, and suppressive potential [81].
It is well established that Tregs act to suppress the immune response by cell-cell contact and by the production of soluble factors, such as IL-10 [82–84]. Interestingly, it was demonstrated that IL-10 potentiates the generation of iTregs and mediates their suppressive function when in presence of TGF-β [85]. In this sense, it is relevant to mention that IL-10 and TGF-β also act cooperatively in other contexts. In fact, it has been described that the production of these factors is controlled by negative feedback regulatory effects that each cytokine has each other [86]. Still with respect to IL-10, another important mechanism involving this interleukin was revealed through the demonstration that Tregs induces long-lasting anergy and production of IL-10 in CD4 T-cells, which then proceeds to suppress the proliferation of syngenic CD4 T-cells via IL-10, independently of direct cell-cell contact, perpetuating the loop that sustains Treg activity [87]. Beyond IL-10, other interleukins appear to play essential roles on Treg-mediated immunossupression. In 2007, IL-35, a member of IL-12 family, was identified in mice as an inhibitory cytokine produced by Tregs which are involved in their suppressive function [88]. Recently, it was shown that Tregs obtained from human peripheral blood produce IL-37 to cause T-cell immunosuppression [89]. Studies performed in mice point to an additional mechanism of immune modulation by Tregs, consisting in the secretion of microvesicles. Considering that Jurkat CD4 T-cells produce exosomes containing molecules with potential immunosuppressive role, it is reasonable to assume that human Tregs may also secrete microvesicles with suppressive function [90].
In contrast to the role of other cytokines, the role of TGF-β as an exclusive Treg mechanism to mediate immunosuppression is still under debate. Available evidence pointing toward an important role of TGF-β in the regulation of Treg function, both in mice and human [91–93]. In stark contrast, others have not found a central role of TGF-β in the suppression promoted by Tregs [94, 95].
Finally, the control of immune response by Tregs may be promoted by cell-cell contact, as demonstrated by perforin-dependent cytotoxicity against activated CD4 and CD8 T-cells, utilizing the adhesive molecule CD18 and causing target-cell death [96].
Overall, it is possible to conclude that in recent years, remarkable progress has been made about the Treg biology. From the several studies presented above, it is clear that human Tregs correspond to a heterogenic population both with regard to their phenotype and to their adopted strategies to control the immune response. Furthermore, despite the fact that Treg immunoregulatory mechanisms are not completely known, several data indicate that FOXP3 acts in a network of binding partners, controlling not only the suppressive potential of Tregs but also other physiological functions of these cells. Concerning clinical use, efforts should be made with the objective of discovering induction methods to generate Tregs able to maintain their suppressive functions at different stages of inflammation. These findings will be useful in order to promote amelioration of various immunological disorders.
Regulatory T-cells and adenosine
As discussed above, the ectonucleotidases CD39 and CD73 are two surface proteins that act by promoting adenosine accumulation outside the cell. The adenosine in the extracellular space can bind to both A2AAR and A2BAR and promote an increase in cAMP concentration, leading to a powerful inhibitory effect over immune response [6]. The expression of CD39 and CD73 has been demonstrated in both mice and human Tregs in different levels [97, 98]. These cells expressed functional ectoenzymes that led to adenosine production [31], as well as inhibition of cytokine production and proliferation of conventional T-cells. Interestingly, it was shown that, in humans, the expression of CD39 is proportional to FOXP3 levels [97, 99, 100]. Since then, as mentioned above, CD39 has been explored as a Tregs marker and a considerable overlap between the expression of this protein and of FOXP3 has been observed [98, 99]. The exploration of this receptor allows the isolation of T-cells with highly suppressive profile. Furthermore, the expression of CD39 efficiently discriminates suppressive T-cells from T-cells with Th17 potential [68, 98].
Moving beyond the CD39/CD73 axis, another protein axis has been identified on the membrane of activated lymphocytes able to generate adenosine. This new adenosinergic axis is composed of the nucleotide-metabolizing ectoenzyme CD38, the ecto-nucleotide pyrophosphatase/phosphodiesterase 1 (NPP1, also known as CD203a) and CD73 [101].
Interestingly, besides having alternative routes, the local production of adenosine may occur as a product of cooperation between different cell types present at any given site, but more specifically, in sites of inflammation. In this sense, it should be noted that endothelial cells express CD39 and CD73 on their surface and can be a source of adenosine [102]. Furthermore, it has recently been demonstrated both in mice and in humans that B-cells express CD39 and CD73, produce adenosine and inhibit T-cell proliferation [103, 104]. Likewise, CD56brightCD16− NK-cells act as regulatory cells, generating adenosine through a CD38-mediated pathway and inhibiting T-cell proliferation [105].
Underscoring adenosine’s omnipresence, CD39 and/or CD73 can be carried to the periphery by microvesicles and support adenosine production far away from their producing cells. Initially, CD39+/CD73+ microvesicles were discovered in different cancer cell types and were actually shown to be able to suppress T-cell function through adenosine production [106]. Contributing to this body of evidence, it was demonstrated that CD4+CD39+Tregs act in cooperation with CD73-expressing-exossomes or with other lymphocytes expressing CD73 to mediate ADO-driven immune suppression [107]. Taken together, these studies clearly build a complex scenario in which adenosine production is the final product of a range of possible dynamic processes that include Tregs, as well as cooperative arrangements between different cell types and membrane-bound vesicles.
As previously commented, adenosine receptors are widely and differentially expressed in immune cells including lymphocytes, macrophages, dendritic cells, and granulocytes; thus, the adenosine produced by Tregs exerts effects on several players of the immune response. In this sense, the suppressive effects of adenosine are largely mediated through A2AAR and A2BAR signaling in immune cells, with a consequent upregulation of cAMP. Indeed, T-cells express high levels of A2AAR, and the use of A2AAR antagonist blocked Treg-mediated immunosuppression [31, 108]. In spite of many papers describing the induction of cAMP due to adenosine-related mechanisms, it is important to note that the accumulation of cAMP on target cells also may also be a consequence of PGE2 production by Tregs [109].
The extension of adenosine action includes NK-cells, which under the influence of adenosine binding to A2AAR, diminish their cytotoxic activity [110]. Also under the influence of adenosine lies dendritic cells, which are recruited in the early phases of immune response, but end up with low expression of costimulatory molecules, due to adenosine-mediated signaling [111]. In this scenario, it was recently demonstrated that adenosine produced by Tregs act as a chemotactic factor and attracts dendritic cells, promoting a cluster among these cells and affecting dendritic cell functions [112].
A previous study showed that adenosine enhances CD73 expression on endothelial cells [113], which can confer to this cells enhanced capacity to generate adenosine. In addition, the use of A2AAR agonist in coculture of allogenic lymphocytes inhibits the activation of cytotoxic effector T-cells and leads to Treg expansion. More importantly, the signaling of A2AAR on Tregs by agonists increases the expression of CTLA-4 and enhances suppressive capacity [114]. Similarly, murine model experiments revealed that A2BAR activation by agonists leads to Treg expansion. Notably, this study showed that A2BAR-deficient mice failed to induce Tregs in the context of inflammation [115]. Overall, these studies clearly show that adenosine acts over several players of the immunological response, promoting direct immune regulation, subject to a feed-forward loop, in addition to the modulation of adenosine production competent cells, which end up reinforcing its suppressive effects.
Mesenchymal stem cells
MSCs were first identified by Friedenstein et al. over 40 years ago in the bone marrow of mice [116]. Described as “spindle-shaped,” “clonogenic” in monolayer cultures and able to generate “colony-forming unit fibroblasts” (CFU-Fs) when plated in specific densities, these bone marrow-derived stromal cells were able to differentiate into adipocytes, chondrocytes, and osteocytes, both in vitro and after transfer in vivo. Succeeding Friedenstein’s seminal work, several research groups continued to investigate such cell population, mainly in the context of regenerative medicine and stem cell biology. In fact, this bone marrow-derived stromal cells was later classified as stem cells and named “mesenchymal stem cells” by Caplan et al. [117], due to their capacity of self-renewal and multipotent differentiation.
The investigation of MSCs by several groups resulted in the identification of similar cell populations in virtually all human tissues, e.g., adipose tissue [118], skin [119], synovia [120], umbilical cord [121], and lungs [122]. As a definition, all MSC populations present the following functional and phenotypic properties: (i) plastic adherence; (ii) specific phenotypic profile consisting of over 95 % of positivity for CD105, CD73, and CD90, as measured by flow cytometry, as well as lack of expression (<2 %) of markers of other cell populations, namely CD45, CD34, CD14 or CD11b, CD79a or CD19, and HLA class II; and (iii) trilineage mesenchymal differentiation capacity as shown by in vitro differentiation into osteoblasts, adipocytes, and chondroblasts under standard conditions. Evidence suggests a perivascular localization of MSCs, justifying their broad distribution throughout the body [123, 124], as well as a function in tissue regeneration and homeostasis, by providing new cells for tissue repair and potentially contributing to immune system regulation.
Currently, over 2000 clinical trials have been performed using bone marrow stem cells to investigate their potential beneficial effects on diseases such as myocardial infarction and other chronic cardiomyopathies, musculoskeletal lesions, stroke, type I diabetes mellitus, autoimmune diseases, metabolic diseases, among others (clinicaltrials.gov). Achieved results in each of such conditions are not the scope of this review and may be revised elsewhere [125–130].
Data from preclinical and clinical trials show that MSCs promote regeneration by inducing angiogenesis, decreasing fibrosis, promoting chemoattraction of specific cell types, and modulating the immune system [131, 132]. Still, clinical data reveals that MSCs are far from reaching their full regenerative potential, since much of the success reported in preclinical studies was not repeated in humans. Recent attempts to boost MSC therapy involve MSC priming prior to injection both as proof of concept (PoC) as well as in clinical trials ([133, 134] NCT00322101 has results but has not been published by the time of this publication).
In contrast to most attempts of applying MSCs to promote tissue regeneration—which have led mostly to inconsistent beneficial results—significant data has been acquired following MSC therapy in immunologic diseases. Surprisingly, the evolution from PoC, obtained from in vitro and in vivo assays, to clinical investigation of immunomodulatory properties of MSCs in humans occurred in a very short time frame. In 2002, Di Nicola et al. [135] observed that MSCs inhibited T-cell activation (measured as proliferation upon stimuli) in vitro, and Bartholomew et al. [136] described that MSCs prolonged skin graft survival in vivo by, among others, affecting T-cell action. In 2004, Le Blanc et al. [137] published data on MSC treatment of a grade IV acute graft-versus-host disease (GVHD) patient, including 1-year follow-up. Le Blanc’s paper is considered a mainstay in the field due to the significance and consistency of their observations [137, 138]. Le Blanc’s work paved the way for the characterization of the broad immunoregulatory properties of MSCs. Apart from a failure in the phase III trial of an industrial application of MSCs in the treatment to GVHD [139, 140], in 2012, the first product based on MSCs in the world aiming to treat GVHD was approved for commercialization [141].
The discovery of MSC immunomodulatory activities was empirical, and the mechanisms by which they unfold are yet to be fully understood. Among the most striking features of MSC-mediated immunomodulation, it is possible to cite the lack of immunogenicity of these cells provided, in part, by a low expression of HLA class I genes, no expression of HLA class II and inducible expression of CD274 (a.k.a. PDL1) [142, 143]. Of note, but still subject for debate, is the capability of MSCs to inhibit T-cell activation [144], proliferation [135], and to affect T-cell differentiation, favoring Th2 and Treg phenotypes by several mechanisms [145–148]. Furthermore, not only MSCs affect dendritic cell recruitment, maturation, and function but also significantly reduce monocyte differentiation into the dendritic cell type [149–153]. Last but not least, MSCs alter the phenotype, proliferation, cytotoxic potential, and cytokine secretion of NK-cells [147, 154, 155] and decrease proliferation, differentiation, chemotactic properties of B-cells [156–159], acting over virtually all of the most important aspects of an immune response.
Gradually, increasing light is being shed on the mechanisms involved in MSC immunomodulation. Among some of the mechanisms already described to be involved in this scenario, there are known regulatory pathways, such as CTLA-4 and PD-1/PD-L1 pathways, the major negative coreceptor expressed on T-cells [160]. While PD-1-related mechanisms act mainly at later stages, CTLA-4 regulates T-cells activity in earlier stages of tumor growth. It is clear that binding to PD-1 receptor on T-cells inhibits their activation and IL-2 production, thus suppressing T-cell attack and inducing immune escape. Also, PD-1 pathway may lead to apoptosis of activated T-cells, facilitate T-cell anergy, decrease their proliferation, while enhancing Treg function [161]. Similar to tumor cells, placenta MSCs express PD-L1 and increase its expression in the presence of IFN-γ [162]. If PD-L1 is blocked, as shown in monoclonal antibody studies, MSC immunosuppression is compromised, underscoring PD-L1 participation in such context [162]. CTLA-4, in contrast to PD-L1, completely blocks costimulation by CD28 through its stronger affinity for B7 molecules. In dendritic cells, interaction between CTLA-4 and B7 molecules led to IDO expression [163].
In addition to membrane-bound molecules, soluble factors have important role in MSC immunomodulation. In antibody-blocking experiments, Di Nicola and coworkers showed that TGF-β and hepatocyte growth factor (HGF) constitute important soluble molecules for T-cell activation, measured as proliferation upon stimuli [135]. Later, TGF-β has also been shown to be important for generation of Tregs [164], as well as in affecting NK-cell proliferation [155], constituting an important player in the MSC immunomodulation scenario. PGE2 is also described as an MSC immunomodulation effector molecule, as revealed in indomethacin inhibition of PGE2 models [165, 166]. It has been shown that PGE2 mediates inhibition of T-cell proliferation, Treg and monocyte differentiation, and NK proliferation/cytotoxic activity [167]. Another interesting mechanism of immunomodulation involves the exhaustion of tryptophan by the enzyme IDO. IDO catalyzes the conversion of tryptophan to kynurenine. In conditions in which substrate is exhausted and product builds up, T-cell proliferation is inhibited. By expressing this enzyme, MSCs promote consumption of tryptophan and compromise T-cell expansion [168, 169]. Another mechanism of MSC immunomodulation is composed of a feed-forward system in which IL-10 and human leukocyte antigen-G5 (HLA-G5) interact. The former induces the expression of the latter in MSCs and as a consequence, and HLA-G5 induces the expression of IL-10. Conflicting data shows that IL-10 may be secreted by MSCs or by T-cells but agrees on the contact dependence of such event [166, 170].
MSCs and adenosine
Recently, an increasing body of evidence has been built leading to the suggestion that another new and exciting mechanism might also be used by MSCs in order to fine tune immune response: adenosine production. Such mechanism, initially suggested by the correlation between adenosine deaminase and immunodeficiency in 1975 [171], was gradually confirmed in the cancer context [9, 172, 173] and also as a strategy used by Tregs [9, 174].
Adding to adenosine’s impressive omnipresence, recent evidence suggesting that MSCs might be using such a strategy to control immune reactions came from the observation made by our group, in which transcriptome analysis of T-cells being downactivated by MSCs revealed an increased expression of adenosine receptors [175]. Later, our group confirmed such observation by conducting functional experiments, in which T-cells were cocultured with MSCs once again. In this paper, we showed that, when in contact with T-cell-conditioned media, MSCs upregulate CD39 and increase adenosine levels. The fact that T-cells also express CD39 may not exclude the possibility of MSCs and T-cell cooperation for the production of adenosine, in which highly CD39 expressing activated T-cells may break ATP into ADP and then AMP, which is used by CD73 expressing MSCs to obtain adenosine. This hypothesis, first discussed by our group, has indeed been confirmed experimentally by Kerkelä et al. [176], in 2016. Adding a parenthesis to this subject, CD39 expression by MSCs has been subject to conflicting observations [177]. In contrast to our observations [175] and others [178, 179], some authors describe a lack or low CD39 expression by MSCs [176, 180]. In our view, this might be due to specific experiment design or to antibody variation. Despite the importance of CD39 in MSC biology and immunosuppression, such conflict is made less important physiologically, the fact being that, no matter if expressed by MSCs, T-cells, endothelial cells, or microvesicles, CD39 is present in the immunosuppressive milieu and contributes to adenosine-dependent MSC-induced immunomodulation.
Recently, MSCs were shown to induce NK-cells to express CD73 [180]. Since NK-cells already express CD39, which is kept in stable levels in the presence of MSCs, CD73 completes the adenosine framework, and in fact, NK-cells themselves end up producing adenosine and inactivating themselves [181]. Such event was also observed in Th-17 cells, which increased their CD39 and CD73 upon coculture with MSCs and had their activation suppressed by adenosine-related mechanisms [179].
Curiously, at the same time that adenosine-related mechanisms for MSCs immunosuppression are being described, the role of adenosine is also being deemed important for MSC biology and differentiation. In fact, MSCs possess all P1 receptors, with A2BAR predominating in undifferentiated cells and during osteoblastogenesis, inducing an osteogenic action and leading to the expression of osteoblast-related genes. Also of interest, adenosine participates in adult neurogenesis. For more information on adenosine importance for MSCs differentiation and neurogenesis, please refer to [182].
Functional role of adenosine in the immunomodulatory arsenal of Tregs and MSCs
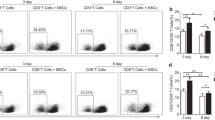
The functional role of adenosine for Treg and MSC immunomodulation is revealed by several investigations performed in vivo. Specifically for Tregs, adenosine role is underscored by several models of CD39 [183, 184] and CD73 knockdown/Ko/blockade [185, 186], which compromise Treg function. Regarding the role of adenosine for MSC function, literature is still restricted. Nevertheless, the abrogation of MSC protective effects by CD73 inhibition [187] and blockage of A2AAR signaling [175] underscores the role of adenosine in MSC immunomodulation. In this sense, Amarnath et al. have also provided important evidence for adenosine role in MSC, by performing interesting experiments involving a GVHD mouse model followed by confirmation at the clinical scenario. In this important paper, Amarnath et al. used clinical grade MSCs and showed that such MSCs were effective in reversing the lethal mouse GVHD model, even though MSCs were only detected in the lungs of treated subjects. The observed immunomodulatory effect was shown to be importantly mediated by adenosine-related mechanisms, as confirmed in a human clinical trial scenario, in which GVHD patients received MSCs. Authors support the adenosine participation in MSC clinical treatment, through the detection of increased circulating CD73+ microvesicles 1 day post-MSC infusion. Notably, the CD73+ microvesicles generated adenosine ex vivo. The data presented in the cited paper has valuable significance for the understanding of adenosine’s action in vivo and promotes a reconciling view between the cell-cell contact dependent adenosine-mediated effects, with the observed paracrine effects of MSCs, which may be, at least in part, mediated by MSC-derived CD73+ microvesicles [188].
It is beyond dispute that Tregs and MSCs may have important immunosuppressive roles in host immune response. As shown, adenosine is a tool used by both cell types to exert their effects over inflammatory cells. Curiously, though, the interaction between Tregs and MSCs has been poorly investigated so far, despite some pieces of evidence pointing at an important interplay between them, which could lead to potent inhibition of immune response. In fact, not only MSC influence Tregs directly, by inducing their formation and expansion, but also indirectly, by modulating antigen-presenting cells and inducing regulatory phenotypes [189].
Interaction of Tregs and MSCs occurs mainly under inflammatory conditions. Traditionally, secretion of IDO, TGF-β, and PGE2 by MSCs promotes Treg induction, expansion, and activation, respectively. In this sense, cell-to-cell contact also seems to be important [189]. In the in vivo scenario, though, adenosine shows up as another important molecule produced by MSCs and Tregs, which influence the function of the latter. In a mice model of kidney ischemia-reperfusion injury (IRI), adenosine activation to A2AAR induced the expression of PD-1 in Tregs and guaranteed their protective effect [190]. A2AAR is also important for dendritic cells, since the strategy of treating such cells with A2AAR agonist successfully resulted in protection of mice from kidney [190, 191].
Recent papers reveal an impressive synergic potential of the association of Tregs and MSCs, being that the coinjection of both cell types in a mice model of GVHD resulted in accelerated proliferation of Th2 and Treg cells, associated with more effective control of Th1 and Th17 cells, compared to infusions of each cell type separately [192]. Interestingly, this effect may be mediated, in part, by a pro-survival effect of Tregs over coinjected MSC, as shown by Zhou et al. [193]. Clinical experience with the combination of Tregs and MSCs is required to obtain further evidence of their synergic potential.
Adenosine application in clinical practice—current trends and present challenges
It is widely recognized that modulating adenosine signaling pathway is an essential mechanism of action of several known drugs with important effects over the immune system, such as methotrexate [194], phosphodiesterase inhibitor pentoxifylline (PTX) [195], sulfasalazine [196], and caffeine [197]. Despite obvious evidence that adenosine receptors are druggable, important hurdles remain to be overcome before new drugs acting over adenosine receptors reach clinical practice. For instance, an important failure in the clinical development of rolofyline, an A1AR antagonist, in acute heart failure, due to lack of efficacy and daunting side effects, including seizures and strokes [198], underscores one of the greatest challenges in using adenosine signaling as therapeutic mechanism: its widespread distribution and the challenge of developing adenosine receptor agonists with tissue specificity. Adding another layer of complexity to the present scenario, the use of caffeine has not been properly controlled in most clinical trial.
In 2013, Chen et al. [1] reviewed several adenosine receptor agonists under clinical trial evaluation. Of those, only two trials were focused on immune-related diseases, namely psoriasis and rheumatoid arthritis. Both trials tested the CF101 compound, which is an A3AR agonist. Due to the fact that A3AR receptor has increased expression restricted to inflammatory cells, it constitutes a very interesting therapeutic target in such context and, in fact, has led to impressive data in a multicenter phase II study aiming at the treatment of rheumatoid arthritis, as reviewed by Fishman et al. [48]. CF101 has also contributed to progressive and linear improvement of psoriasis patients, as presented by Fishman et al. [48]. Such relative success has justified Can-Fite BioPharma Ltd. to plan further clinical trials with CF101 for 2016 (http://ir.canfite.com/press-releases/detail/745/can-fite-announces-2016-clinical-milestones-for-its-pipeline-of-drugs-in-six-indications). Of note, the specificity for A3AR receptor contributes to the decrease of undesired off-target effects, although at a cost of not mobilizing the other adenosine receptors, also important for immune modulation.
Complementing Chen’s review [1], a research on the term “adenosine” at clinicaltrials.gov resulted in 545 trials, 17 of those related to immunological diseases. The present search did not include noninterventional observations, diet modifications/caffeine infusion treatments, asthma, gene therapy, and basic studies, considered beyond the scope of the present review. Finally, terminated and withdrawn studies, involving GVHD studies of CF101, were removed from the search. Drugs under investigation by these studies included pentostatin, etanercept, mycophenolate mofetil, denileukin diftitox, methotrexate, CF101, alemtuzumab, sirolimus, cyclophosphamide, tacrolimus, pentoxifylline, adenosine, sodium prussiate, methylprednisolone, and dipyridamole. Most studies involved GVHD treatment and prevention and also endotoxemia, psoriasis, osteoarthrosis, inflammation, acute pancreatitis, advanced kidney cancer, sickle cell disease and B-thalassemia, rheumatoid arthritis, and arthritis. Therefore, at the moment, CF101 might be the current adenosine-related drug closest to reach the clinic with adenosine signaling as major mechanism of action. The next years will enlighten us, revealing if this silver bullet will be as effective as some expect.
Conclusions
From the context presented, it is possible to grasp the challenges ahead considering pharmacological adenosine receptor modulation. In face of such challenges, adenosine production competent cells—namely, Tregs and MSCs—may arise as the optimal answer to address adenosine-based therapy demands. It is our opinion that Tregs and MSCs may be the most effective and specific strategy to effectively and safely control adenosine signaling pathways, in order to explore this mechanism in its fullest potential. The infusion of self-regulating cells renders possible to specifically produce adenosine at affected sites only, in controlled concentrations and periods, preventing off-target effects and guaranteeing therapeutic efficacy. Taken together, cell therapy for immune-related conditions may be the best token in adenosine’s clinical translation.
References
Chen J-F, Eltzschig HK, Fredholm BB (2013) Adenosine receptors as drug targets—what are the challenges? Nat Rev Drug Discov 12:265–86. doi:10.1038/nrd3955
Allard B, Turcotte M, Stagg J (2012) CD73-generated adenosine: orchestrating the tumor-stroma interplay to promote cancer growth. J Biomed Biotechnol 2012:485156. doi:10.1155/2012/485156
Kaczmarek E, Koziak K, Sévigny J et al (1996) Identification and characterization of CD39/vascular ATP diphosphohydrolase. J Biol Chem 271:33116–33122. doi:10.1074/jbc.271.51.33116
Resta R, Yamashita Y, Thompson LF (1998) Ecto-enzyme and signaling functions of lymphocyte CD73. Immunol Rev 161:95–109. doi:10.1111/j.1600-065X.1998.tb01574.x
Alam M, Costales M, Cavanaugh C, Williams K (2015) Extracellular adenosine generation in the regulation of pro-inflammatory responses and pathogen colonization. Biomolecules 5:775–792. doi:10.3390/biom5020775
Haskó G, Linden J, Cronstein B, Pacher P (2008) Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov 7:759–770. doi:10.1038/nrd2638
Yegutkin GG (2008) Nucleotide- and nucleoside-converting ectoenzymes: important modulators of purinergic signalling cascade. Biochim Biophys Acta Mol Cell Res 1783:673–694. doi:10.1016/j.bbamcr.2008.01.024
Corriden R, Insel PA (2012) New insights regarding the regulation of chemotaxis by nucleotides, adenosine, and their receptors. Purinergic Signal 8:587–598. doi:10.1007/s11302-012-9311-x
Bono MR, Fernández D, Flores-Santibáñez F et al (2015) CD73 and CD39 ectonucleotidases in T cell differentiation: beyond immunosuppression. FEBS Lett 589:3454–3460. doi:10.1016/j.febslet.2015.07.027
Cunha R a (2005) Neuroprotection by adenosine in the brain: from A(1) receptor activation to A (2A) receptor blockade. Purinergic Signal 1:111–34. doi:10.1007/s11302-005-0649-1
Ngamsri K-C, Wagner R, Vollmer I et al (2010) Adenosine receptor A1 regulates polymorphonuclear cell trafficking and microvascular permeability in lipopolysaccharide-induced lung injury. J Immunol 185:4374–84. doi:10.4049/jimmunol.1000433
Liao Y, Takashima S, Asano Y et al (2003) Activation of adenosine A1 receptor attenuates cardiac hypertrophy and prevents heart failure in murine left ventricular pressure-overload model. Circ Res 93:759–766. doi:10.1161/01.RES.0000094744.88220.62
Kim J, Kim M, Song JH, Lee HT (2008) Endogenous A1 adenosine receptors protect against hepatic ischemia reperfusion injury in mice. Liver Transpl 14:845–54. doi:10.1002/lt.21432
Lee HT, Gallos G, Nasr SH, Emala CW (2004) A1 adenosine receptor activation inhibits inflammation, necrosis, and apoptosis after renal ischemia-reperfusion injury in mice. J Am Soc Nephrol 15:102–111
Chen L, Fredholm BB, Jondal M (2008) Adenosine, through the A1 receptor, inhibits vesicular MHC class I cross-presentation by resting DC. Mol Immunol 45:2247–2254. doi:10.1016/j.molimm.2007.11.016
Yip L, Taylor C, Whiting CC, Fathman CG (2013) Diminished adenosine A1 receptor expression in pancreatic α-cells may contribute to the pathology of type 1 diabetes. Diabetes 62:4208–19. doi:10.2337/db13-0614
Rosin DL, Robeva A, Woodard RL et al (1998) Immunohistochemical localization of adenosine A2A receptors in the rat central nervous system. J Comp Neurol 401:163–186
Kilpatrick EL, Narayan P, Mentzer RM, Lasley RD (2002) Cardiac myocyte adenosine A2a receptor activation fails to alter cAMP or contractility: role of receptor localization. Am J Physiol Heart Circ Physiol 282:H1035–40. doi:10.1152/ajpheart.00808.2001
Olanrewaju H a, Mustafa SJ (2000) Adenosine A(2A) and A(2B) receptors mediated nitric oxide production in coronary artery endothelial cells. Gen Pharmacol 35:171–177. doi:10.1016/S0306-3623(01)00107-0
Fagerlund MJ, Kåhlin J, Ebberyd A et al (2010) The human carotid body: expression of oxygen sensing and signaling genes of relevance for anesthesia. Anesthesiology 113:1270–9. doi:10.1097/ALN.0b013e3181fac061
Huang S, Apasov S, Koshiba M, Sitkovsky M (1997) Role of A2a extracellular adenosine receptor-mediated signaling in adenosine-mediated inhibition of T-cell activation and expansion. Blood 90:1600–1610
Milne GR, Palmer TM (2011) Anti-inflammatory and immunosuppressive effects of the A2A adenosine receptor. ScientificWorldJournal 11:320–39. doi:10.1100/tsw.2011.22
Yang Z, Day YJ, Toufektsian MC et al (2005) Infarct-sparing effect of A2A-adenosine receptor activation is due primarily to its action on lymphocytes. Circulation 111:2190–2197. doi:10.1161/01.CIR.0000163586.62253.A5
Shryock JC, Snowdy S, Baraldi PG et al (1998) A2A-adenosine receptor reserve for coronary vasodilation. Circulation 98:711–718. doi:10.1161/01.CIR.98.7.711
Satoh S, Matsumura H, Hayaishi O (1998) Involvement of adenosine A(2A) receptor in sleep promotion. Eur J Pharmacol 351:155–162. doi:10.1016/S0014-2999(98)00302-1
Panther E, Idzko M, Herouy Y et al (2001) Expression and function of adenosine receptors in human dendritic cells. FASEB J 15:1963–70. doi:10.1096/fj.01-0169com
Salmon JE, Cronstein BN (1990) Fc gamma receptor-mediated functions in neutrophils are modulated by adenosine receptor occupancy. A1 receptors are stimulatory and A2 receptors are inhibitory. J Immunol 145:2235–40
Cronstein BN, Levin RI, Philips M et al (1992) Neutrophil adherence to endothelium is enhanced via adenosine A1 receptors and inhibited via adenosine A2 receptors. J Immunol 148:2201–2206
Haskó G, Kuhel DG, Chen JF et al (2000) Adenosine inhibits IL-12 and TNF-[alpha] production via adenosine A2a receptor-dependent and independent mechanisms. FASEB J 14:2065–2074. doi:10.1096/fj.99-0508com
Raskovalova T, Lokshin A, Huang X et al (2007) Inhibition of cytokine production and cytotoxic activity of human antimelanoma specific CD8+ and CD4+ T lymphocytes by adenosine-protein kinase A type I signaling. Cancer Res 67:5949–5956. doi:10.1158/0008-5472.CAN-06-4249
Mandapathil M, Hilldorfer B, Szczepanski MJ et al (2010) Generation and accumulation of immunosuppressive adenosine by human CD4+CD25highFOXP3+ regulatory T Cells. J Biol Chem 285:7176–7186. doi:10.1074/jbc.M109.047423
Cekic C, Sag D, Day Y-J, Linden J (2013) Extracellular adenosine regulates naive T cell development and peripheral maintenance. J Exp Med 210:2693–706. doi:10.1084/jem.20130249
Eltzschig HK (2009) Adenosine: an old drug newly discovered. Anesthesiology 111:904–15. doi:10.1097/ALN.0b013e3181b060f2
Mirabet M, Herrera C, Cordero OJ et al (1999) Expression of A2B adenosine receptors in human lymphocytes: their role in T cell activation. J Cell Sci 112(Pt 4):491–502
Wakai A, Wang JH, Winter DC et al (2001) Adenosine inhibits neutrophil vascular endothelial growth factor release and transendothelial migration via A2B receptor activation. Shock 15:297–301
Marquardt DL, Walker LL, Heinemann S (1994) Cloning of two adenosine receptor subtypes from mouse bone marrow-derived mast cells. J Immunol 152:4508–4515
Addi AB, Lefort A, Hua X et al (2008) Modulation of murine dendritic cell function by adenine nucleotides and adenosine: Involvement of the A2B receptor. Eur J Immunol 38:1610–1620. doi:10.1002/eji.200737781
Feoktistov I, Goldstein AE, Ryzhov S et al (2002) Differential expression of adenosine receptors in human endothelial cells: Role of A2B receptors in angiogenic factor regulation. Circ Res 90:531–538. doi:10.1161/01.RES.0000012203.21416.14
Dubey RK, Gillespie DG, Mi Z, Jackson EK (1997) Exogenous and endogenous adenosine inhibits fetal calf serum-induced growth of rat cardiac fibroblasts: role of A2B receptors. Circulation 96:2656–2666. doi:10.1161/01.CIR.96.8.2656
Strohmeier GR, Reppert SM, Lencer WI, Madara JL (1995) The A2b adenosine receptor mediates cAMP responses to adenosine receptor agonists in human intestinal epithelia. J Biol Chem 270:2387–2394
Ansari HR, Nadeem A, Talukder M a H et al (2007) Evidence for the involvement of nitric oxide in A2B receptor-mediated vasorelaxation of mouse aorta. Am J Physiol Heart Circ Physiol 292:H719–25. doi:10.1152/ajpheart.00593.2006
Yang D, Koupenova M, Mccrann DJ et al (2008) The A2b adenosine receptor protects against vascular injury. Proc Natl Acad Sci 105:792–796. doi:10.1073/pnas.0705563105
Haskó G, Csóka B, Németh ZH et al (2009) A2B adenosine receptors in immunity and inflammation. Trends Immunol 30:263–270. doi:10.1016/j.it.2009.04.001
Figler RA, Wang G, Srinivasan S et al (2011) Links between Insulin resistance, adenosine A2B receptors, and inflammatory markers in mice and humans. Diabetes 60:669–679. doi:10.2337/db10-1070
Eckle T, Grenz A, Laucher S, Eltzschig HK (2008) A2B adenosine receptor signaling attenuates acute lung injury by enhancing alveolar fluid clearance in mice. J Clin Invest 118:3301–3315. doi:10.1172/JCI34203
Zhou Y, Schneider DJ, Morschl E et al (2011) Distinct roles for the A2B adenosine receptor in acute and chronic stages of bleomycin-induced lung injury. J Immunol 186:1097–106. doi:10.4049/jimmunol.1002907
Karmouty-Quintana H, Xia Y, Blackburn MR (2013) Adenosine signaling during acute and chronic disease states. J Mol Med (Berl) 91:173–81. doi:10.1007/s00109-013-0997-1
Fishman P, Bar-Yehuda S, Liang BT, Jacobson K a (2012) Pharmacological and therapeutic effects of A3 adenosine receptor agonists. Drug Discov Today 17:359–366. doi:10.1016/j.drudis.2011.10.007
Borea PA, Varani K, Vincenzi F et al (2015) The A3 adenosine receptor: history and perspectives. Pharmacol Rev 67:74–102. doi:10.1124/pr.113.008540
Hussain A, Gharanei AM, Nagra AS, Maddock HL (2014) Caspase inhibition via A3 adenosine receptors: a new cardioprotective mechanism against myocardial infarction. Cardiovasc Drugs Ther 28:19–32. doi:10.1007/s10557-013-6500-y
Baharav E, Bar-Yehuda S, Madi L et al (2005) Antiinflammatory effect of A3 adenosine receptor agonists in murine autoimmune arthritis models. J Rheumatol 32:469–76
Fishman P, Bar-Yehuda S, Madi L et al (2006) The PI3K-NF-kappaB signal transduction pathway is involved in mediating the anti-inflammatory effect of IB-MECA in adjuvant-induced arthritis. Arthritis Res Ther 8:R33. doi:10.1186/ar1887
Shevach EM, Thornton AM (2014) tTregs, pTregs, and iTregs: similarities and differences. Immunol Rev 259:88–102. doi:10.1111/imr.12160
Sakaguchi S (2004) Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol 22:531–562. doi:10.1146/annurev.immunol.21.120601.141122
Horwitz D a, Zheng SG, Gray JD (2008) Natural and TGF-beta-induced Foxp3+CD4+ CD25+ regulatory T cells are not mirror images of each other. Trends Immunol 29:429–435. doi:10.1016/j.it.2008.06.005
Yamagiwa S, Gray JD, Hashimoto S, Horwitz DA (2001) A role for TGF-beta in the generation and expansion of CD4+CD25+ regulatory T cells from human peripheral blood. J Immunol 166:7282–7289
Zheng SG, Wang J, Wang P et al (2007) IL-2 is essential for TGF-β to convert naive CD4+CD25- cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J Immunol 178:2018–2027. doi:10.4049/jimmunol.178.4.2018
Lu L, Zhou X, Wang J et al (2010) Characterization of protective human CD4+CD25+ FOXP3+ regulatory T cells generated with IL-2, TGF-β and retinoic acid. PLoS One 5:1–12. doi:10.1371/journal.pone.0015150
Wang J, Huizinga TWJ, Toes REM (2009) De novo generation and enhanced suppression of human CD4+CD25+ regulatory T cells by retinoic acid. J Immunol 183:4119–26. doi:10.4049/jimmunol.0901065
Haddad R, Saldanha-Araujo F (2014) Mechanisms of T-cell immunosuppression by mesenchymal stromal cells: what do we know so far? Biomed Res Int 2014:216806. doi:10.1155/2014/216806
Melief SM, Schrama E, Brugman MH et al (2013) Multipotent stromal cells induce human regulatory T cells through a novel pathway involving skewing of monocytes toward anti-inflammatory macrophages. Stem Cells 31:1980–91. doi:10.1002/stem.1432
Cahill EF, Tobin LM, Carty F et al (2015) Jagged-1 is required for the expansion of CD4(+) CD25(+) FoxP3(+) regulatory T cells and tolerogenic dendritic cells by murine mesenchymal stromal cells. Stem Cell Res Ther 6:19. doi:10.1186/s13287-015-0021-5
Battaglia M, Stabilini A, Migliavacca B et al (2006) Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J Immunol 177:8338–47. doi:10.4049/jimmunol.177.12.8338
Scottà C, Esposito M, Fazekasova H et al (2013) Differential effects of rapamycin and retinoic acid on expansion, stability and suppressive qualities of human CD4+CD25+FOXP3+ T regulatory cell subpopulations. Haematologica 98:1291–1299. doi:10.3324/haematol.2012.074088
Long S, Buckner JH (2008) Combination of rapamycin and IL-2 increases de novo induction of human CD4(+)CD25(+)FOXP3(+) T cells. J Autoimmun 30:293–302
Singh Y, Garden OA, Lang F, Cobb BS (2015) MicroRNA-15b/16 enhances the induction of regulatory T cells by regulating the expression of Rictor and mTOR. J Immunol 195:5667–5677. doi:10.4049/jimmunol.1401875
Warth SC, Hoefig KP, Hiekel A et al (2015) Induced miR-99a expression represses Mtor cooperatively with miR-150 to promote regulatory T-cell differentiation. EMBO J 34:1195–213, doi:10.15252/embj.201489589
Mandapathil M, Lang S, Gorelik E, Whiteside TL (2009) Isolation of functional human regulatory T cells (Treg) from the peripheral blood based on the CD39 expression. J Immunol Methods 346:55–63. doi:10.1016/j.jim.2009.05.004
Schuler PJ, Harasymczuk M, Schilling B et al (2011) Separation of human CD4+CD39+ T cells by magnetic beads reveals two phenotypically and functionally different subsets. J Immunol Methods 369:59–68. doi:10.1016/j.jim.2011.04.004
Gautron A-S, Dominguez-Villar M, de Marcken M, Hafler D a (2014) Enhanced suppressor function of TIM-3(+) FoxP3(+) regulatory T cells. Eur J Immunol 44(9):2703–11. doi:10.1002/eji.201344392
Uraushihara K, Kanai T, Ko K et al (2003) Regulation of murine inflammatory bowel disease by CD25+ and CD25–CD4+ glucocorticoid-induced TNF receptor family-related gene+ regulatory T cells. J Immunol 171:708–716. doi:10.4049/jimmunol.171.2.708
Mahic M, Henjum K, Yaqub S et al (2008) Generation of highly suppressive adaptive CD8+CD25+FOXP3+ regulatory T cells by continuous antigen stimulation. Eur J Immunol 38:640–646. doi:10.1002/eji.200737529
Chang CC, Ciubotariu R, Manavalan JS et al (2002) Tolerization of dendritic cells by T(S) cells: the crucial role of inhibitory receptors ILT3 and ILT4. Nat Immunol 3:237–243. doi:10.1038/ni760
Fischer K, Voelkl S, Heymann J et al (2005) Isolation and characterization of human antigen-specific TCR alpha beta+ CD4(−)CD8- double-negative regulatory T cells. Blood 105:2828–2835. doi:10.1182/blood-2004-07-2583
Shi Z, Okuno Y, Rifa’i M et al (2009) Human CD8+CXCR3+ T cells have the same function as murine CD8+CD122+ Treg. Eur J Immunol 39:2106–2119. doi:10.1002/eji.200939314
Brisslert M, Bokarewa M, Larsson P et al (2006) Phenotypic and functional characterization of human CD25+ B cells. Immunology 117:548–557. doi:10.1111/j.1365-2567.2006.02331.x
Nagaraj S, Gabrilovich DI (2007) Myeloid-derived suppressor cells. Adv Exp Med Biol 601:213–223
Hoechst B, Gamrekelashvili J, Manns MP et al (2011) Plasticity of human Th17 cells and iTregs is orchestrated by different subsets of myeloid cells. Blood 117:6532–6541. doi:10.1182/blood-2010-11-317321
Flores-Borja F, Bosma A, Ng D et al (2013) CD19+CD24hiCD38hi B cells maintain regulatory T cells while limiting TH1 and TH17 differentiation. Sci Transl Med 5:173ra23. doi:10.1126/scitranslmed.3005407
Sakaguchi S, Wing K, Onishi Y et al (2009) Regulatory T cells: how do they suppress immune responses? Int Immunol 21:1105–1111. doi:10.1093/intimm/dxp095
Zheng Y, Josefowicz SZ, Kas A et al (2007) Genome-wide analysis of Foxp3 target genes in developing and mature regulatory T cells. Nature 445:936–940. doi:10.1038/nature05563
Thornton AM, Shevach EM (1998) CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med 188:287–296. doi:10.1084/jem.188.2.287
Boussiotis VA, Tsai EY, Yunis EJ et al (2000) IL-10-producing T cells suppress immune responses in anergic tuberculosis patients. J Clin Invest 105:1317–1325. doi:10.1172/JCI9918
Dieckmann D, Plottner H, Berchtold S et al (2001) Ex vivo isolation and characterization of CD4(+)CD25(+) T cells with regulatory properties from human blood. J Exp Med 193:1303–10. doi:10.1084/jem.193.11.1303
Hsu P, Santner-Nanan B, Hu M et al (2015) IL-10 potentiates differentiation of human induced regulatory T cells via STAT3 and Foxo1. J Immunol 195:3665–3674. doi:10.4049/jimmunol.1402898
Horwitz DA, Zheng SG, Gray JD (2003) The role of the combination of IL-2 and TGF-β or IL-10 in the generation and function of CD4+ CD25+ and CD8+regulatory T cell subsets. J Leukoc Biol 74:471–478. doi:10.1189/jlb.0503228
Dieckmann D, Bruett CH, Ploettner H et al (2002) Human CD4(+)CD25(+) regulatory, contact-dependent T cells induce interleukin 10-producing, contact-independent type 1-like regulatory T cells [corrected]. J Exp Med 196:247–253. doi:10.1084/jem.20020642
Collison LW, Workman CJ, Kuo TT et al (2007) The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature 450:566–569. doi:10.1038/nature06306
Shuai X, Wei-Min L, Tong Y et al (2015) Expression of IL-37 contributes to the immunosuppressive property of human CD4+CD25+ regulatory T cells. Sci Rep 5:14478. doi:10.1038/srep14478
Agarwal A, Fanelli G, Letizia M et al (2014) Regulatory T cell-derived exosomes: possible therapeutic and diagnostic tools in transplantation. Front Immunol 5:555. doi:10.3389/fimmu.2014.00555
Levings MK, Sangregorio R, Sartirana C et al (2002) Human CD25+CD4+ T suppressor cell clones produce transforming growth factor, but not interleukin 10, and are distinct from type 1 T regulatory cells. J Exp Med 196:1335–1346. doi:10.1084/jem.20021139
Nakamura K, Kitani A, Strober W (2001) Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med 194:629–44. doi:10.1084/jem.194.5.629
Annunziato F, Cosmi L, Liotta F et al (2002) Phenotype, localization, and mechanism of suppression of CD4(+)CD25(+) human thymocytes. J Exp Med 196:379–387. doi:10.1084/jem.20020110
Piccirillo CA, Letterio JJ, Thornton AM et al (2002) CD4(+)CD25(+) regulatory T cells can mediate suppressor function in the absence of transforming growth factor beta1 production and responsiveness. J Exp Med 196:237–246
Kullberg MC, Hay V, Cheever AW et al (2005) TGF-beta1 production by CD4+ CD25+ regulatory T cells is not essential for suppression of intestinal inflammation. Eur J Immunol 35:2886–95. doi:10.1002/eji.200526106
Grossman WJ, Verbsky JW, Barchet W et al (2004) Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity 21:589–601. doi:10.1016/j.immuni.2004.09.002
Deaglio S, Dwyer KM, Gao W et al (2007) Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med 204:1257–65. doi:10.1084/jem.20062512
Dwyer KM, Hanidziar D, Putheti P et al (2010) Expression of CD39 by human peripheral blood CD4+CD25 + T cells denotes a regulatory memory phenotype. Am J Transplant 10:2410–2420. doi:10.1111/j.1600-6143.2010.03291.x
Borsellino G, Kleinewietfeld M, Di Mitri D et al (2007) Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: Hydrolysis of extracellular ATP and immune suppression. Blood 110:1225–1232. doi:10.1182/blood-2006-12-064527
Kobie JJ, Shah PR, Yang L et al (2006) T regulatory and primed uncommitted CD4 T cells express CD73, which suppresses effector CD4 T cells by converting 5’-adenosine monophosphate to adenosine. J Immunol 177:6780–6786. doi:10.4049/jimmunol.177.10.6780
Horenstein AL, Chillemi A, Zaccarello G et al (2013) A CD38/CD203a/CD73 ectoenzymatic pathway independent of CD39 drives a novel adenosinergic loop in human T lymphocytes. Oncoimmunology 2, e26246. doi:10.4161/onci.26246
Haskó G, Cronstein BN (2004) Adenosine: an endogenous regulator of innate immunity. Trends Immunol 25:33–39. doi:10.1016/j.it.2003.11.003
Kaku H, Cheng KF, Al-Abed Y, Rothstein TL (2014) A novel mechanism of B cell-mediated immune suppression through CD73 expression and adenosine production. J Immunol 193:5904–13. doi:10.4049/jimmunol.1400336
Saze Z, Schuler PJ, Hong C-S et al (2013) Adenosine production by human B cells and B cell-mediated suppression of activated T cells. Blood 122:9–18. doi:10.1182/blood-2013-02-482406
Morandi F, Horenstein AL, Chillemi A et al (2015) CD56brightCD16- NK cells produce adenosine through a CD38-mediated pathway and act as regulatory cells inhibiting autologous CD4+ T cell proliferation. J Immunol 195:965–72. doi:10.4049/jimmunol.1500591
Clayton A, Al-Taei S, Webber J et al (2011) Cancer exosomes express CD39 and CD73, which suppress T cells through adenosine production. J Immunol 187:676–683. doi:10.4049/jimmunol.1003884
Schuler PJ, Saze Z, Hong CS et al (2014) Human CD4+CD39+ regulatory T cells produce adenosine upon co-expression of surface CD73 or contact with CD73+ exosomes or CD73+ cells. Clin Exp Immunol 177:531–543. doi:10.1111/cei.12354
Whiteside TL, Mandapathil M, Schuler P (2011) The role of the adenosinergic pathway in immunosuppression mediated by human regulatory T cells (Treg). Curr Med Chem 18:5217–5223. doi:10.2174/092986711798184334
Mandapathil M, Szczepanski MJ, Szajnik M et al (2010) Adenosine and prostaglandin e2 cooperate in the suppression of immune responses mediated by adaptive regulatory T cells. J Biol Chem 285:27571–27580. doi:10.1074/jbc.M110.127100
Hoskin DW, Mader JS, Furlong SJ et al (2008) Inhibition of T cell and natural killer cell function by adenosine and its contribution to immune evasion by tumor cells (review). Int J Oncol 32:527–535
Dwyer KM, Deaglio S, Gao W et al (2007) CD39 and control of cellular immune responses. Purinergic Signal 3:171–180. doi:10.1007/s11302-006-9050-y
Ring S, Pushkarevskaya A, Schild H et al (2015) Regulatory T cell-derived adenosine induces dendritic cell migration through the Epac-Rap1 pathway. J Immunol 194:3735–44. doi:10.4049/jimmunol.1401434
Narravula S, Lennon PF, Mueller BU, Colgan SP (2000) Regulation of endothelial CD73 by adenosine: paracrine pathway for enhanced endothelial barrier function. J Immunol 165:5262–5268. doi:10.4049/jimmunol.165.9.5262
Ohta A, Kini R, Ohta A et al (2012) The development and immunosuppressive functions of CD4+ CD25+ FoxP3+ regulatory T cells are under influence of the adenosine-A2A adenosine receptor pathway. Front Immunol 3:190. doi:10.3389/fimmu.2012.00190
Ehrentraut H, Westrich JA, Eltzschig HK, Clambey ET (2012) Adora2b adenosine receptor engagement enhances regulatory T cell abundance during endotoxin-induced pulmonary inflammation. PLoS One 7(2):e32416. doi:10.1371/journal.pone.0032416
Friedenstein AJ et al (1974) Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation 17:331–340. doi:10.1097/00007890-197404000-00001
Caplan A (1991) Mesenchymal stem cells. J Orthop Res 9:641–50. doi:10.1002/jor.1100090504
Zuk P a, Zhu M, Mizuno H et al (2001) Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7:211–28. doi:10.1089/107632701300062859
Toma JG, Akhavan M, Fernandes KJ et al (2001) Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol 3:778–84. doi:10.1038/ncb0901-778
De Bari C, Dell’accio F, Tylzanowski P, Luyten FP (2001) Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum 44:1928–1942. doi:10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P
Romanov YA, Svintsitskaya VA, Smirnov VN (2003) Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC-like cells from umbilical cord. Stem Cells 22:105–110. doi:10.1634/stemcells.21-1-111
Sabatini F, Petecchia L, Tavian M et al (2005) Human bronchial fibroblasts exhibit a mesenchymal stem cell phenotype and multilineage differentiating potentialities. Lab Investig 85:962–971. doi:10.1038/labinvest.3700300
da Silva ML, Caplan AI, Nardi NB et al (2008) In search of the in vivo identity of mesenchymal stem cells. Stem Cells 26:2287–99. doi:10.1634/stemcells.2007-1122
Covas DT, Panepucci R a, Fontes AM et al (2008) Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene-expression profile with CD146 + perivascular cells and fibroblasts. Exp Hematol 36:642–654. doi:10.1016/j.exphem.2007.12.015
Steinert AF, Rackwitz L, Gilbert F et al (2012) Concise review: the clinical application of mesenchymal stem cells for musculoskeletal regeneration: current status and perspectives. Stem Cells Transl Med 1:237–47. doi:10.5966/sctm.2011-0036
Behfar A, Crespo-Diaz R, Terzic A, Gersh BJ (2014) Cell therapy for cardiac repair—lessons from clinical trials. Nat Rev Cardiol 11:232–46. doi:10.1038/nrcardio.2014.9
Rosado-De-Castro PH, Pimentel-Coelho PM, da Fonseca LMB et al (2013) The rise of cell therapy trials for stroke: review of published and registered studies. Stem Cells Dev 22:2095–111. doi:10.1089/scd.2013.0089
Domínguez-Bendala J, Lanzoni G, Inverardi L, Ricordi C (2012) Concise review: mesenchymal stem cells for diabetes. Stem Cells Transl Med 1:59–63. doi:10.5966/sctm.2011-0017
Sokal EM (2014) Treating inborn errors of liver metabolism with stem cells: current clinical development. J Inherit Metab Dis 37:535–539. doi:10.1007/s10545-014-9691-x
Munir H, Mcgettrick HM (2015) Mesenchymal stem cells therapy for autoimmune disease: risks and rewards. Stem Cells Dev 24:2091–2100. doi:10.1089/scd.2015.0008
Hahn JY, Cho HJ, Kang HJ et al (2008) Pre-treatment of mesenchymal atem cells with a combination of growth factors enhances gap junction formation, cytoprotective effect on cardiomyocytes, and therapeutic efficacy for myocardial infarction. J Am Coll Cardiol 51:933–943. doi:10.1016/j.jacc.2007.11.040
Khubutiya MS, Vagabov AV, Temnov AA, Sklifas AN (2014) Paracrine mechanisms of proliferative, anti-apoptotic and anti-inflammatory effects of mesenchymal stromal cells in models ofacute organ injury. Cytotherapy 16:579–585. doi:10.1016/j.jcyt.2013.07.017
Carvalho JL, Braga VBA, Melo MB et al (2013) Priming mesenchymal stem cells boosts stem cell therapy to treat myocardial infarction. J Cell Mol Med 17:617–625. doi:10.1111/jcmm.12036
Hu X, Yu SP, Fraser JL et al (2008) Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J Thorac Cardiovasc Surg 135:799–808. doi:10.1016/j.jtcvs.2007.07.071
Di Nicola M, Carlo-Stella C, Magni M et al (2002) Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 99:3838–3843. doi:10.1182/blood.V99.10.3838
Bartholomew A, Sturgeon C, Siatskas M et al (2002) Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol 30:42–8. doi:10.1016/S0301-472X(01)00769-X
Le Blanc K, Rasmusson I, Sundberg B et al (2004) Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 363:1439–1441. doi:10.1016/S0140-6736(04)16104-7
Frassoni F, Labopin M, Bacigalupo A et al (2002) Expanded mesenchymal stem cells (MSC), co-infused with HLA identical hematopoietic stem cell transplants, reduce acute and chronic graft-versus-host disease: a matched pair analysis. Bone Marrow Transplant 29:s2, abstract
Martin P, Uberti J, Soiffer R et al (2010) Prochymal improves response rates in patients with steroid-refractory acute graft versus host disease (SR-GVHD) involving the liver and gut: results of a randomized, placebo-controlled, multicenter phase III trial in GVHD. Biol Blood Marrow Trasnplant 16:S169–S170
Cyranoski D (2012) Canada approves stem cell product. Nat Biotechnol 30:571
Vaes B, Van’t Hof W, Deans R, Pinxteren J (2012) Application of Multistem® allogeneic cells for immunomodulatory therapy: clinical progress and pre-clinical challenges in prophylaxis for graft versus host disease. Front Immunol 3:345. doi:10.3389/fimmu.2012.00345
Klyushnenkova E, Mosca JD, Zernetkina V et al (2005) T cell responses to allogeneic human mesenchymal stem cells: immunogenicity, tolerance, and suppression. J Biomed Sci 12:47–57. doi:10.1007/s11373-004-8183-7
Najar M, Raicevic G, Kazan HF et al (2012) Immune-related antigens, surface molecules and regulatory factors in human-derived mesenchymal stromal cells: the expression and impact of inflammatory priming. Stem Cell Rev Rep 8:1188–1198. doi:10.1007/s12015-012-9408-1
Le Blanc K, Tammik L, Sundberg B et al (2003) Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol 57:11–20. doi:10.1046/j.1365-3083.2003.01176.x
Ghannam S, Pène J, Torcy-Moquet G et al (2010) Mesenchymal stem cells inhibit human Th17 cell differentiation and function and induce a T regulatory cell phenotype. J Immunol 185:302–312. doi:10.4049/jimmunol.0902007
Luz-Crawford P, Kurte M, Bravo-Alegría J et al (2013) Mesenchymal stem cells generate a CD4+CD25+Foxp3+ regulatory T cell population during the differentiation process of Th1 and Th17 cells. Stem Cell Res Ther 4:65. doi:10.1186/scrt216
Aggarwal S, Pittenger MF (2005) Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105:1815–1822. doi:10.1182/blood-2004-04-1559
Saldanha-Araujo F, Haddad R, Malmegrim De Farias KCR et al (2012) Mesenchymal stem cells promote the sustained expression of CD69 on activated T lymphocytes: Roles of canonical and non-canonical NF-kB signalling. J Cell Mol Med 16:1232–1244. doi:10.1111/j.1582-4934.2011.01391.x
Jiang X-X, Zhang Y, Liu B et al (2005) Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood 105:4120–4126. doi:10.1182/blood-2004-02-0586.Supported
Maccario R, Podestà M, Moretta A et al (2005) Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4 + T-cell subsets expressing a regulatory/suppressive phenotype. Haematologica 90:516–525
Nauta AJ, Kruisselbrink AB, Lurvink E et al (2006) Mesenchymal stem cells inhibit generation and function of both CD34+−derived and monocyte-derived dendritic cells. J Immunol 177:2080–2087
Spaggiari GM, Abdelrazik H, Becchetti F, Moretta L (2009) MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: central role of MSC-derived prostaglandin E2. Blood 113:6576–6583. doi:10.1182/blood-2009-02-203943
Zhang W, Ge W, Li C et al (2004) Effects of mesenchymal stem cells on differentiation, maturation, and function of human monocyte-derived dendritic cells. Stem Cells Dev 13:263–71. doi:10.1089/154732804323099190
Krampera M, Cosmi L, Angeli R et al (2006) Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells 24:386–98. doi:10.1634/stemcells.2005-0008
Sotiropoulou P a, Perez S a, Gritzapis AD et al (2006) Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells 24:74–85. doi:10.1634/stemcells.2004-0359
Corcione A, Benvenuto F, Ferretti E et al (2006) Human mesenchymal stem cells modulate B-cell functions. Blood 107:367–372. doi:10.1182/blood-2005-07-2657
Tabera S, Pérez-Simón JA, Díez-Campelo M et al (2008) The effect of mesenchymal stem cells on the viability, proliferation and differentiation of B-lymphocytes. Haematologica 93:1301–1309. doi:10.3324/haematol.12857
Comoli P, Ginevri F, Maccario R et al (2008) Human mesenchymal stem cells inhibit antibody production induced in vitro by allostimulation. Nephrol Dial Transplant 23:1196–1202. doi:10.1093/ndt/gfm740
Rosado MM, Bernardo ME, Scarsella M et al (2015) Inhibition of B-cell proliferation and antibody production by mesenchymal stromal cells is mediated by T cells. Stem Cells Dev 24:93–103. doi:10.1089/scd.2014.0155
Mu CY, Huang JA, Chen Y et al (2011) High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med Oncol 28:682–688. doi:10.1007/s12032-010-9515-2
He J, Hu Y, Hu M, Li B (2015) Development of PD-1/PD-L1 pathway in tumor immune microenvironment and treatment for non-small cell lung cancer. Sci Rep 5:13110. doi:10.1038/srep13110
Chang C-J, Yen M-L, Chen Y-C et al (2006) Placenta-derived multipotent cells exhibit immunosuppressive properties that are enhanced in the presence of interferon-gamma. Stem Cells 24:2466–2477. doi:10.1634/stemcells.2006-0071
Munn DH, Mellor AL (2013) Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol 34:137–143. doi:10.1016/j.it.2012.10.001
English K, Ryan JM, Tobin L et al (2009) Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25(High) forkhead box P3+ regulatory T cells. Clin Exp Immunol 156:149–60. doi:10.1111/j.1365-2249.2009.03874.x
Baratelli F, Lin Y, Zhu L et al (2005) Prostaglandin E2 induces FOXP3 gene expression and T regulatory cell function in human CD4+ T cells. J Immunol 175:1483–1490. doi:10.4049/jimmunol.175.3.1483
Ryan JM, Barry F, Murphy JM, Mahon BP (2007) Interferon-γ does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin Exp Immunol 149:353–363. doi:10.1111/j.1365-2249.2007.03422.x
Maria Spaggiari G, Moretta L, Spaggiari GM, Moretta L (2013) Cellular and molecular interactions of mesenchymal stem cells in innate immunity. Immunol Cell Biol 91:27–31. doi:10.1038/icb.2012.62
Meisel R, Zibert A, Laryea M et al (2004) Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood 103:4619–4621. doi:10.1182/blood-2003-11-3909
Chinnadurai R, Copland IB, Patel SR, Galipeau J (2014) IDO-independent suppression of T cell effector function by IFN-γ-licensed human mesenchymal stromal cells. J Immunol 192:1491–501. doi:10.4049/jimmunol.1301828
Tomchuck SL, Zwezdaryk KJ, Coffelt SB et al (2008) Toll-like receptors on human mesenchymal stem cells drive their migration and immunomodulating responses. Stem Cells 26:99–107. doi:10.1634/stemcells.2007-0563
Meuwissen H, Pollara B, Pickering R (1975) Combined immunodeficiency disease associated with adenosine deaminase deficiency. Report on a workshop held in Albany, New York, October 1, 1973. J Pediatr 86:169–81
Kumar V (2013) Adenosine as an endogenous immunoregulator in cancer pathogenesis: where to go? Purinergic Signal 9:145–165. doi:10.1007/s11302-012-9349-9
Blay J, White TD, Hoskin DW (1997) The extracellular fluid of solid carcinomas contains immunosuppressive concentrations of adenosine. Cancer Res 57:2602–2605
Ohta A, Sitkovsky M (2014) Extracellular adenosine-mediated modulation of regulatory T cells. Front Immunol 5:304. doi:10.3389/fimmu.2014.00304
Saldanha-Araujo F, Ferreira FIS, Palma PV et al (2011) Mesenchymal stromal cells up-regulate CD39 and increase adenosine production to suppress activated T-lymphocytes. Stem Cell Res 7:66–74. doi:10.1016/j.scr.2011.04.001
Kerkelä E, Laitinen A, Räbinä J et al (2016) Adenosinergic immunosuppression by human mesenchymal stromal cells requires co-operation with T cells. Stem Cells 34:781–90
Saldanha-Araujo F, Panepucci R a (2011) CD39 expression in mesenchymal stromal cells. J Immunother 34:568. doi:10.1097/CJI.0b013e3182298c8f
Sattler C, Steinsdoerfer M, Offers M et al (2010) Inhibition of T cell proliferation by murine multipotent mesenchymal stromal cells is mediated by CD39/CD73 coexpression and adenosine generation. Cell Transplant 20:1221–1230. doi:10.3727/096368910X546553\rct0203sattler
Lee JJ, Jeong HJ, Kim MK et al (2013) CD39-mediated effect of human bone marrow-derived mesenchymal stem cells on the human Th17 cell function. Purinergic Signal 10(2):357–365. doi:10.1007/s11302-013-9385-0
Sundin M, D’Arcy P, Johansson CC et al (2011) Multipotent mesenchymal stromal cells express FoxP3: a marker for the immunosuppressive capacity? J Immunother 34:336–42. doi:10.1097/CJI.0b013e318217007c
Chatterjee D, Tufa D, Baehre H et al (2014) Natural killer cells acquire CD73 expression upon exposure to mesenchymal stem cells. Blood 123:594–5
Cavaliere F, Donno C, D’Ambrosi N (2015) Purinergic signaling: a common pathway for neural and mesenchymal stem cell maintenance and differentiation. Front Cell Neurosci 9:211. doi:10.3389/fncel.2015.00211
Li P, Gao Y, Cao J et al (2015) CD39(+) regulatory T cells attenuate allergic airway inflammation. Clin Exp Allergy 45:1126–37. doi:10.1111/cea.12521
Yoshida O, Dou L, Kimura S et al (2015) CD39 deficiency in murine liver allografts promotes inflammatory injury and immune-mediated rejection. Transpl Immunol 32:76–83. doi:10.1016/j.trim.2015.01.003
Ehrentraut H, Clambey ET, Mcnamee EN et al (2013) CD73+ regulatory T cells contribute to adenosine-mediated resolution of acute lung injury. FASEB J 27:2207–19. doi:10.1096/fj.12-225201
Wang L, Fan J, Chen S et al (2013) Graft-versus-host disease is enhanced by selective CD73 blockade in mice. PLoS One 8(3), e58397. doi:10.1371/journal.pone.0058397
Chen X, Shao H, Zhi Y et al (2016) CD73 pathway contributes to the immunosuppressive ability of mesenchymal stem cells (MSCs) in intraocular autoimmune responses. Stem Cells Dev 25:337–46
Amarnath S, Foley JE, Farthing DE et al (2015) Bone marrow-derived mesenchymal stromal cells harness purinergenic signaling to tolerize human th1 cells in vivo. Stem Cells 33:1200–1212. doi:10.1002/stem.1934
Burr SP, Dazzi F, Garden O a (2013) Mesenchymal stromal cells and regulatory T cells: the Yin and Yang of peripheral tolerance? Immunol Cell Biol 91:12–8. doi:10.1038/icb.2012.60
Kinsey GR, Huang L, Jaworska K et al (2012) Autocrine adenosine signaling promotes regulatory T cell-mediated renal protection. J Am Soc Nephrol 23:1528–37. doi:10.1681/ASN.2012010070
Kinsey GR, Sharma R, Okusa MD (2013) Regulatory T cells in AKI. J Am Soc Nephrol 24:1720–6. doi:10.1681/ASN.2013050502
Lim J-Y, Park M-J, Im K-I et al (2013) Combination cell therapy using mesenchymal stem cells and regulatory T cells provides a synergistic immunomodulatory effect associated with reciprocal regulation of Th1/Th2 and Th17/Treg cells in a murine acute graft-versus-host disease model. Cell Transplant 23:703–714. doi:10.3727/096368913X664577
Zhou Y, Singh AK, Hoyt RF et al (2014) Regulatory T cells enhance mesenchymal stem cell survival and proliferation following autologous cotransplantation in ischemic myocardium. J Thorac Cardiovasc Surg 148:1131–7. doi:10.1016/j.jtcvs.2014.06.029
Cronstein B (2010) How does methotrexate suppress inflammation? Clin Exp Rheumatol 28(5 Suppl 61):S21–S23
Kreth S, Ledderose C, Luchting B et al (2010) Immunomodulatory properties of pentoxifylline are mediated via adenosine-dependent pathways. Shock 34:10–6. doi:10.1097/SHK.0b013e3181cdc3e2
Cronstein BN, Montesinos MC, Weissmann G (1999) Salicylates and sulfasalazine, but not glucocorticoids, inhibit leukocyte accumulation by an adenosine-dependent mechanism that is independent of inhibition of prostaglandin synthesis and p105 of NFkappaB. Proc Natl Acad Sci U S A 96:6377–81. doi:10.1073/pnas.96.11.6377
Horrigan LA, Kelly JP, Connor TJ (2006) Immunomodulatory effects of caffeine: friend or foe? Pharmacol Ther 111:877–892. doi:10.1016/j.pharmthera.2006.02.002
Massie BM, O’Connor CM, Metra M et al (2010) Rolofylline, an adenosine A1-receptor antagonist, in acute heart failure. N Engl J Med 363:1419–1428. doi:10.1056/NEJMoa0912613
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
de Oliveira Bravo, M., Carvalho, J.L. & Saldanha-Araujo, F. Adenosine production: a common path for mesenchymal stem-cell and regulatory T-cell-mediated immunosuppression. Purinergic Signalling 12, 595–609 (2016). https://doi.org/10.1007/s11302-016-9529-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-016-9529-0