Abstract
The threshold velocity ≥200 cm/s at transcranial Doppler (TCD) evaluation is a useful cut-off for preventing the stroke (STOP trial) in pediatric patients with sickle cell disease (SCD), term including different types of sickle genotypes. Scanty data are available for adult SCD patients. We compared intracranial blood flow velocities between adult SCD patients and controls using transcranial color Doppler (TCCD), measuring the peak of systolic velocity (PSV) with the insonation angle correction and the pulsatility index (PI), an indicator of endothelial elasticity. Fifty-three adult SCD patients (aged >18 years) were enrolled (15 sickle cell anemia, 26 sickle cell thalassemia, and 12 HbS/HbC). None of the patients presented neurological signs. PSVs in middle cerebral artery (MCA) were higher in SCD patients than in controls (p = 0.001). In sickle cell anemia patients, PSVs were higher when compared to HbS/βThal (p < 0.0060) and HbS/HbC patients (p < 0.0139). PI was within the lower range of normality in SCD patients compared to controls. Moreover, MCA-PSV was higher with lower Hb levels and higher HbS%; PI did not change with variation of Hb levels and HbS%.
PSV and PI in SCD adult patients could be a relevant index to indicate the abnormal cerebral blood flow and to detect the sickle endothelial damage, in order to prevent cerebrovascular accidents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sickle cell disease (SCD) is the term referring to all the clinical syndromes caused by the homozygosis for the βS allele (sickle cell anemia—SCA) or by any other combination of βS allele with β-thalassemic (HbS/β-thalassaemia) or variant alleles (i.e., βS and βC alleles—HbSC disease) [1]. SCD is a multisystem disease, associated with episodes of acute illness and progressive organ damage. Stroke is one of the most severe complications of sickle cell disease (SCD), affecting children as well as adults, as reported by the Collaborative Study of Sickle Cell Disease (CSSCD) [2]. The incidence of stroke is 11% by the age of 16 and 24% by the age of 45 years in patients with SCA, homozygotes for hemoglobin S (HbS), which is ten times higher than in African-Americans without SCD [3].
Transcranial Doppler (TCD) is a practical, inexpensive, and non-invasive method recommended for screening children from 2 to 16 years of age with SCD [4] and in children with SCD at risk for stroke [5, 6].
The threshold velocity ≥200 cm/s measured by time average mean maximum (TAMM) in distal intracranial internal carotid arteries (ICA) and/or proximal middle cerebral arteries (MCA) was the cut-off defined for stroke risk in pediatric SCD patients. SCD children with TAMM ≥200 cm/s had 10% risk per year of a first stroke, which fell to <1% by introducing regular red blood cell transfusions decreasing HbS to <30% [7].
Jones et al. compared TAMM velocities detected by TCD and by transcranial color Doppler (TCCD) and found that TCCD velocities in MCA were lower than TCD velocities from 10 to 15% [8]. Krejza et al. showed that the angle-corrected TCCD velocities are not different from the TCCD velocities in SCD children, but are more precise and more effective in order to study the major intracranial arteries in comparison with TCD [9]. Jones et al. also showed that the derived cut-off points of the peak of systolic velocity (PSV) measured by TCCD [10] had the same predictor value for stroke as those defined by TAMM. PSV cut-off of 200 cm/s measured using TCCD equipment is equivalent to the TCD TAMM threshold velocity of 170 cm/s, which suggests the need of increased surveillance as proposed by the Stroke Prevention Trial in Sickle Cell Anemia (STOP trial) [10,11,12]. Moreover a TAMM velocity of 200 cm/s, which is recommended to start chronic transfusion, is comparable to a TCCD PSV of 250 cm/s [10]. A recent study also showed that PSV can be a better indicator than TAMM because of the highest sensitivity in detecting patients with intracranial vasculopathy [13].
Up to now, most data were based on children assessment [8, 9, 12]. There are few data and no prospective long-term studies on the use of TCCD supported by insonation angle correction, which is a more precise measurement, in adults with SCD [9]. Moreover, few data are available about the evaluation of the endothelial damage of the vessels in adults.
The purpose of this study is to evaluate PSV in intra- and extracranial vessels and the pulsatility index (PI), an indicator of cerebral endothelial elasticity, in a group of adult SCD stroke-free patients with different sickle-genotypes and to compare them with a group of healthy controls matched for age and sex, using angle-corrected TCCD and carotid Doppler sonography (CDS). The results were related to the hemoglobin values.
Materials and methods
Study population
This is a case-control observational study where we evaluated consecutive SCD adult outpatients (aged >18 years) undergoing regular follow-up and treatment at the Rare Diseases Center of Fondazione IRCCS Ca’ Granda, Ospedale Maggiore Policlinico. During a 2.5-year period, we enrolled 53 adult SCD patients (M/F 19/34) and 27 healthy controls, matched for gender, age and, as far as possible, ethnicity; we collected demographic data, hematological tests, and performed clinical neurological assessments. Exclusion criteria were age <18 years, pregnancy, epilepsy, HIV infection and previous bone marrow transplantation.
Laboratory studies
Complete blood cell count (CBC) was performed using Coulter model S.Plus in SCD patients and controls; hemoglobin (Hb) fractions were quantified by high-performance liquid chromatography (HPLC) (Bio Rad Variant Hemoglobin Testing System®). The biochemical tests were measured by standardized methods. Blood tests of SCD patients were evaluated before the transfusion therapy if planned.
TCCD and CDS ultrasound
Both patients and controls were examined with Esaote US system (MyLab Twice, Esaote S.p.A., Genova-Italy) equipped with a linear array transducer probe (LA533, operating bandwidth 3–13 MHz) and a phased array probe (PA240, Esaote; operating bandwidth 1–4 MHz, variable band).
In both patients and controls, all TCCD and CDS exams were performed by the same physician, blind to the pathology of the subjects. During the examination, patients were awake, in order to avoid the increase of intracranial blood flow velocities due to the increase of carbon dioxide (CO2), which occurs during sleep.
Following the STOP protocol, the main intracranial vessels were analyzed: the middle cerebral artery (MCA), the anterior cerebral artery (ACA), the carotid siphon (SIPH), the posterior cerebral artery (PCA), the vertebral arteries (VA V4) (on both right and left side), and the basilar artery (BAS) at a depth of 80–100 mm. The extracranial vessels, the internal carotid artery (ICA), and the vertebral artery in the intertransversal tract (VA V2) were also evaluated. The highest value from the right or left cerebral arteries were assessed.
In this study, PSV were considered, since they are commonly used in vascular ultrasound practice, easier to measure and more reproducible [9, 14]. However, TAMM velocities in the MCA were also measured to compare them with those reported in the literature.
Correction of the insonation angle was carried out in each arterial segment. In the MCA, angle-corrected velocities were measured in at least three points per side, and in particular in the segment with color-aliasing artifice. PSV and TAMM were measured manually to avoid the interference of the signal introduced by electronic wave-follower forms of measurement.
PI was calculated according to the following formula: PI = [(systolic velocity ‐ diastolic velocity)/(mean vel . × 100)].
Blood tests and TCCD/CDS ultrasound were performed on the same occasion.
Magnetic resonance imaging and intracranial magnetic resonance angiography
All SCD patients underwent to 3.0 T device gradient-echo sequence (provide TR/TE/flip angle and spatial resolution) covering the intracranial ICAs, vertebrobasilar arteries, and the circle of Willis in the axial and coronal plane. The cerebral images of the ICAs and branches of the circle of Willis were evaluated for potential intracranial arterial segmental narrowing and flow restriction by a trained operator.
Statistical analysis
The collected data are reported as median and interquartile intervals. Differences between groups were tested using Wilcoxon’s rank test and Kruskal-Wallis test. The significance threshold was set at 0.05 in the case of two comparisons and at 0.017 in the case of three comparisons, in order to avoid an over-estimation of the error of the first type.
Tolerance ellipses of 80% have been calculated using estimates of the slope provided by the linear regression model. They group 80% of the data described function of the two variables considered, while providing a measure of correlation (trend of the main axis), and a measure of dispersion (value of the minor axis).
Finally, using a logistic regression model, a ROC curve was built, allowing us to estimate the threshold variable MCA-PSV in which sensitivity and specificity were highest. All analyses were performed using SAS ver.9.2 package (SAS Institute, Inc. 2008. SAS/STATVR 9.2 user’s guide. Cary, NC: SAS Institute, Inc.)
Results
Patient data
Fifty-three consecutive adult SCD outpatients were recruited (aged >18 years, median age 36 years, range 29–43, M:F 19:34), including 15 patients with SCA (M:F 3:12), 26 with sickle cell β-thalassemia (M:F 11:15) (HbS/βThal of whom 22 HbS/β°Thal; 4 HbS/β+(severe)Thal), and 12 HbS/HbC (M:F 6:7). Patients with SCD were compared to 27 healthy controls (ratio 2:1, median age 31 years, range 25–42, M:F 11:16), matched by gender, age, and ethnicity.
Table 1 shows the hematological data (Hb, Ht, HbS, HbF) in SCD patients and healthy controls. Significant differences were found for Hb values, Ht, RBC, MCV, HbA2% and HbF% and WBC (p < 0.001) when comparing the SCD group with control group. Table 2 shows the hematological and clinical data in the three subgroups of SCD patients.
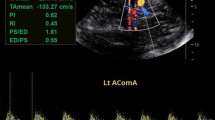
Biochemical tests (iron status, hemolysis indices) were also collected, but not any significant differences were found in the three subgroups of SCD patients. No neurological signs and no cognitive impairment were reported in any SCD patients, despite history of sickle crisis. The values of PSV by angle-corrected TCCD (Fig. 1) in MCA, ACA, SIPH, PCA, and BAS were found to be higher in SCD patients than in controls (median MCA 133.7 vs 112.2 cm/s; p = 0.001); TAMM velocity by TCD in MCA was also higher in SCD patients than in controls (median MCA 96.9 vs 75.9 cm/s p < 0.001) (Table 3), but lower if compared to PSV. No statistical differences in PSVs in extracranial vessels (ICA and VERT) were found.
Both SCD patients and controls have shown a normal range of PI value; however, in MCA, ACA, SIPH, PCA, and VERT, PI was statistically lower in SCD patients (median MCA 0.80 vs 0.89; p = 0.021) than in healthy controls. Any statistic differences in BAS and ICA was found considering.
Considering the three SCD subgroups, higher PSV values were found in SCA patients compared to HbS/βThal and HbS/HbC patients (median MCA 142.5 vs 126.9 cm/s, p < 0.0139 and vs 126.5 cm/s p < 0.006, respectively), corresponding to lower Hb values and a more severe clinical phenotype. TAMM velocity in MCA was also higher in SCA patients than in HbS/βThal and HbS/HbC patients (median MCA 104.2 vs 93.2 cm/s, P < 0.0239 and vs 86.1 cm/s; p < 0.0023, respectively) (Table 4), but lower if compared to PSV.
If considering the three SCD groups altogether, the PI value was not statistically different in the three SCD subgroups.
None of SCD patients showed intracranial arterial narrowing at MRI/MRA, although 20% of cases showed silent cerebral infarcts, similar to which was previously reported in beta thalassemia intermedia patients [15].
Furthermore, MCA-PSV and MCA-IP values were related to Hb levels and HbS%, focusing on the more studied cerebral artery.
Figure 2 shows the relationship between Hb values and MCA-PSV (left) and MCA-PI (right). Eighty percent tolerance ellipses are highlighted for SCD patients and healthy controls. The major axes of the ellipses, even in the presence of a considerable dispersion of the values, show that when the Hb value increases, the MCA-PSV value decreases. MCA-PSV values are lower in healthy controls than in SCD patients; in the latter, the angular coefficient differs in a statistically significant from zero. In healthy controls, MCA-IP values increase when Hb values increase and a substantial stability of MCA-PI was found in SCD patients. The angular coefficient was statistically significant in controls. Even in this case, there is a considerable dispersion of the values in both groups.
Figure 3 shows the relationship between Hb values and MCA-PSV (left) and MCA-IP (right) only for SCD patients, because HbS is absent in healthy controls. Always considering 80% tolerance ellipses, the MCA-PSV value increases together with HbS, even in the presence of a significant dispersion of the data. There is not a significant increase of MCA-IP values with increase of HbS.
Finally, in order to better evaluate the possible use of MCA-PSV as a diagnostic test in differentiating the two groups (SCD patients and controls), the ROC curve was calculated using a logistic regression model. The area under the curve (AUC) was 0.76. The threshold value of PSV of 132.7 provides a sensitivity of 53.8% in identifying a patient SCD and a specificity of 100% in detecting a condition of absence of pathology (Youden’ index = 0.5385) (Fig. 4).
Table 5 shows the distribution of subjects above and below the chosen threshold for the two groups (controls and SCD patients). The highest values of PSV are those that potentially increase the risk of cerebrovascular accident.
Discussion
Ischemic strokes in SCD are usually the result of fibrous proliferative hyperplasia of the miointimal endothelium, leading to intracranial artery stenosis, which can be detected by transcranial Doppler (TCD) [4, 5]. Elevated cerebral blood flow (CBF) velocity (≥200 cm/s) has been widely reported as a risk factor for stroke in children with SCD [6].
Due to improvement in treatment and better life expectancy in SCD patients, cerebrovascular disease in adults with SCD is increasing and receiving greater attention. There are scarce data about the use of TCD in adults with SCD and no prospective long-term studies. Previous studies [16, 17] reported that the pattern of TCD velocities in adults with SCD are lower than those observed in children with SCD, but still higher than those detected in adult control population, confirming that age affects cranial vessel velocities, which are also increased in the presence of anemia [18]. Therefore, the TCD velocity cut-off used in SCD children cannot be used to categorize the risk for stroke in SCD adults. Valadi et al. reported that adults with SCD had a higher TAMM (110.9 ± 25.7 cm/s) compared to healthy controls (71.1 ± 12.0 cm/s) and this difference is apparently proportional to the degree of anemia [19]. In 2004, the American Academy of Neurology also recommended TCD in adults with SCD [20]. Furthermore, recent studies reported the involvement of extracranial internal carotid arteriopathy in the risk of stroke in SCA patients [21, 22].
The use of transcranial color Doppler (TCCD) instead of TCD has the potential to be more accurate, because the former combines color Doppler flow information to B-Mode image and allows determination of the angle between the course of the vessel and the ultrasound beam, leading a more accurate estimation of the velocities. Using the two methods and angle correction, Kreiza et al. [9] found no differences in children. However, no data are available for adults and moreover the transcranial velocities risk threshold for stroke in adults with SCD remains yet to be established.
In this study, a group of stroke-free adult SCD patients was evaluated. We measured PSVs by TCCD using angle correction, because it is a more precise technique to detect CBF velocities, although an overestimation of stroke risk has been reported measuring PSV [23].
Comparing our SCD patients with healthy controls, and with the subgroups of SCA, HbS-βThal and HbS/HbC patients, a direct correlation was found between PSV and Hb levels and an inverse correlation with HbS. Despite the absence of cerebral events, it was observed that adult SCD patients had higher PSVs in any vessels evaluated, compared to healthy controls, showing a more alterated CBF. These findings confirm the important role played by hypoxia in relation to CBF velocities; furthermore, HbS, partially responsible for blood hyperviscosity leading to sickling and consequent vaso-occlusive crisis, is present in different amounts in the three SCD subgroups studied. This could explain the higher PSV values in SCA patients compared to HbS-βThal and HbS/HbC patients and controls. However, in our SCD patients, the highest PSV in MCA, the most representative and best evaluated intracranial vessel, was lower than those reported in the literature in SCD children, and did not relate with overt stroke. Furthermore, no strict relations between CBF velocities and clinical neurological findings were detected. The absence of neurological events is probably due to the absence of hemodynamically relevant stenosis in this group of patients. On the contrary, it was observed that patients with higher PSVs in any evaluated vessels showed a more severe sickle phenotype, in terms of total Hb levels, HbS, blood requirement, and number of sickle crises, even in stroke-free cases. These results suggest that PSV could be an index of severity of disease.
In our series of patients, the ROC curve showed a MCA-PSV values of 134.7 cm/s that can indicate the threshold of abnormal MCA-PSV in SCD patients in comparison with healthy controls. In a previous study, we reported a higher pathological cut-off out of the 95° percentile considering only the group of the SCD patients studied [24].
The pulsatility index (PI) was also considered; PI measurement is considered an indicator of endothelial elasticity. Although it was normal in SCD patients and controls, PI values increase when Hb levels increase with statistical significance only in controls, showing a loss of endothelial elasticity in SCD patients. No relation between PI and HbS was found in SCD patients. The reduced endothelial elasticity in adult SCD patients could justify the higher observed PSV values even though in the absence of stenosis and over stroke events.
In SCD patients, as in the general population, age is a factor responsible for atherosclerosis: atherosclerosis may be a potential physio-pathological mechanism involved in the development of sickle cerebral vasculopathy. Loss of endothelial elasticity in SCD patients is due to inflammation, hypoxia, anemia, hemolysis with subsequent decreased nitric oxide bioavailability, impaired blood rheology and particular local hemodynamic profiles, and genetic factors [25, 26]. The vascular damage caused by sickle cells may be the result of their adherence to the endothelium, leading to secondary degeneration of the arterial wall and compromising the adequate supply of oxygen and other nutrients through the endothelial membrane [27]. In adult SCD patients, PI could be another good indicator of endothelial damage and sclerosis, a potential predictor of acute cerebrovascular events, or an indicator of chronic progression of the disease, and it may be detected with a non-invasive and relatively inexpensive technique. It could be useful to perform this evaluation with PSV to have a complete picture of cerebral vessels, studying at the same time endothelial structure and blood flow velocity, respectively.
A larger prospective study on a larger number of SCD patients could confirm our results.
Conclusions
In conclusion, MCA-PSV measured by angle-corrected TCCD is higher in any cerebral vessels of our group of adult SCD patients compared with healthy age and sex-matched controls and in particular in SCA patients according to the worse hematological phenotype and high HbS levels, despite the absence of acute cerebrovascular events. Furthermore, PI value is within the normal range in both adult SCD patients and healthy controls, but, interestingly, it is in the lower range of normal values in SCD patients.
Considering the life-threatening risk of stroke and the social cost of treatment, it could be helpful to identify adult SCD patients in need of TCCD control in order to indicate abnormal CBF and to detect the severity of the sickle endothelial damage due to the hemodynamic stress. It could be useful to perform a TCCD screening, a non-invasive, convenient, and low-cost procedure, in a large series of adult SCD patients to early identify the patients at risk of stroke and establish appropriate treatment.
References
Rees DC, Williams TN, Gladwin MT (2010) Sickle-cell disease. Lancet 376:2018–2031
Ohene-Frempong K, Weiner SJ, Sleeper LA et al (1998) The cooperative study of sickle cell disease: cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood 91:288–294
Pandey DK, Gorelick PB (2005) Epidemiology of stroke in African Americans and Hispanic Americans. Med Clin North Am 89:739–752
NHLBI Clinical Alert: Periodic transfusions lower stroke risk in children with sickle cell anemia (http://www.nlm.nih.gov/databases/alerts/sickle97.html). Bethesda, MD; National Institutes of Health (1997). Accessed August 6 (2005)
Adams RJ, Nichols FT, Figueroa R et al (1992) Transcranial Doppler correlation with cerebral angiography in sickle cell disease. Stroke 23:1073–1077
Adams RJ, McKie V, Nichols F et al (1992) The use of transcranial ultrasonography to predict stroke in sickle cell disease. N Engl J Med 326:605–610
Adams RJ, McKie VC, Hsu L et al (1998) Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med 339:5–11
Jones AM, Seibert JJ, Nichols FT et al (2001) Comparison of transcranial color Doppler imaging (TCDI) and transcranial Doppler (TCD) in children with sickle cell anemia. Pediatr Radiol 31:461–469
Kreiza J, Rudzinski W, Pawlak MA et al (2007) Angle-corrected imaging transcranial Doppler sonography versus imaging and nonimaging transcranial Doppler sonography in children with sickle cell disease. AJNR Am J Neuroradiol 28:1613–1618
Jones AM, Granger S, Brambilla D et al (2005) Can peak systolic velocities be used for prediction of stroke in sickle cell anemia? Pediatr Radiol 35:66–72
Bulas DI, Jones AM, Siebert JJ et al (2000) Transcranial Doppler (TCD) screening for stroke prevention in sickle cell anemia: pitfalls in technique variation. Pediatr Radiol 3:733–738
Nichols FT, Jones AM, Adams RJ (2001) Stroke prevention in sickle cell disease (STOP) study guidelines for transcranial Doppler testing. J Neuroimaging 11:354–362
Naffaa LN, Tandon YK, Irani N (2015) Transcranial Doppler screening in sickle cell disease: the implications of using peak systolic criteria. World J Radiol 7:52–56
Alexandrov AV, Vital D, Brodie DS et al (1997) Grading carotid stenosis with ultrasound. An interlaboratory comparison. Stroke 28:1208–1210
Musallam KM, Taher AT, Karimi M, Rachmilewitz EA (2012) Cerebral infarction in beta thalassemia intermedia: breaking the silence. Thromb Res 130:695–702
Silva GS, Vicari P, Figueiredo MS et al (2006) Transcranial Doppler in adult patients with sickle cell disease. Cerebrovasc Dis 21:38–41
Silva GS, Vicari P, Figueiredo MS et al (2009) Brain magnetic resonance imaging abnormalities in adult patients with sickle cell disease correlation with transcranial Doppler findings. Stroke 40:2408–2412
Brass LM, Pavlakis SG, De Vivo D et al (1988) Transcranial Doppler measurements of the middle cerebral artery. Effect of hematocrit. Stroke 19:1466–1469
Valadi N, Silva GS, Bowman LS et al (2006) Transcranial Doppler ultrasonography in adults with sickle cell disease. Neurology 67:572–574
Sloan MA, Alexandrov AV, Tegeler CH et al (2004) Therapeutics and technology assessment Subcommittee of the American Academy of neurology: assessment: transcranial Doppler ultrasonography: report of the therapeutics and technology assessment Subcommittee of the American Academy of neurology. Neurology 69:1468–1481
Calviere L, Viguier A, Guidolin B et al (2007) Cervical artery stenoses in sickle cell disease. Eur Neurol 58:120–121
Deane CR, Goss D, Bartram J et al (2010) Extracranial internal carotid arterial disease in children with sickle cell anemia. Haematologica 95:1287–1292
Ishola T, Quinn C (2013) Transcranial doppler peak systolic velocities overestimate the risk of stroke in sickle cell anemia. Abstract, American Society of Hematology. Blood 323:a2240
Graziadei G, Casoni FM, Ridolfi P et al (2013) Transcranial color doppler sonography and magnetic resonance imaging in adult patients with sickle cell disease. Abstract, American Society of Hematology. Blood 122:a2245
Manwani D, Frenette PS (2013) Vaso-occlusion in sickle cell disease: pathophysiology and novel target tharapies. Hematol Am Soc Hematol Educ Program:362–369
Connes P, Verlhac S, Bernaudin F (2013) Advances in understanding the pathogenesis of cerebrovascular vasculopathy in sickle cell anaemia. Br J Haematol 161:484–498
Brandão RA, de Carvalho GT, Reis BL et al (2009) Surgical intracranial aneurysms in sickle cell patients: report of 2 cases and review of the literature. Surg Neurol 72:296–299
Acknowledgments
We thank all the patients for participation in this study. GG and FMC coordinated the study design and contributed to data preparation and analysis; GG and FMC wrote the paper; FA and FMC did transcranial color Doppler; PR and AM recruited the patients; PR, IG, and EDP collected data; IC made statistical analysis; MDC contributed to review and final approval for submission. All authors read and agreed to the final version of the manuscript. This work was supported by Ministry of Health, Italy [RC-2016].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the Ethical Committee of our Institution and the procedures were performed in accordance with the Declaration of Helsinki. All subjects received verbal and written explanation of the aims and procedure of the study and written informed consent was obtained.
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Graziadei, G., Casoni, F.M., Annoni, F. et al. Transcranial color Doppler in stroke-free adult patients with sickle cell disease. Ann Hematol 96, 1547–1555 (2017). https://doi.org/10.1007/s00277-017-3071-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-017-3071-1