Abstract
The prognosis of extranodal nature killer (NK)/T cell lymphoma (ENKL) is dismal because of its aggressive course and multidrug resistance. Currently, for patients with relapsed/refractory ENKL, l-asparaginase-based regimens such as l-asparaginase, ifosfamide, methotrexate, etoposide, and dexamethasone (SMILE) or l-asparaginase, methotrexate, and dexamethasone (AspaMetDex) are recommended. We retrospectively investigated the efficacy and safety of gemcitabine, pegaspargase, cisplatin, and dexamethasone (DDGP) combination chemotherapy in the treatment of 17 relapsed/refractory ENKL patients. Clinical data from these patients were collected and analyzed. The primary end point was overall response rate (ORR). All patients were subjected to 2 to 6 cycles of DDGP chemotherapy, and the median number of cycles of DDGP regimen administrated was four. The ORR was 88.2 % (15/17), with nine patients (52.9 %) achieved complete response (CR) and six patients (35.3 %) achieved partial response (PR). The median follow-up time was 17 months (range 2–28 months). The 1-year overall survival (OS) rate and 1-year progression-free survival (PFS) were 82.4 and 64.7 %, respectively. For those CR responders, the median PFS was 17 months. Grade 3/4 neutropenia occurred in nine patients (52.9 %) and grade 3/4 thrombocytopenia occurred in six patients (35.3 %). DDGP combination chemotherapy produces favorable outcomes in relapsed/refractory ENKL, and more attention should be paid to treatment-related myelosuppression. Further prospective trials are expected to define the efficacy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Extranodal nature killer (NK)/T cell lymphoma (ENKL) is one of the most aggressive lymphoid malignancies with distinct clinical characteristics and poor outcomes. Though it is rare in western countries, populations from Asian and South America are more frequently involved [1]. Most patients with ENKL have stage I/II disease. Primary lesions are mainly present in the upper aerodigestive tract especially nasal cavity, and occasionally in the lung, skin, and testis [2]. As an Epstein-Barr virus (EBV)-associated lymphoma, the quantitation of EBV-DNA in peripheral blood reflects the tumor burden and prognosis of ENKL [3].
Systemic chemotherapy combined with involved field radiotherapy is recommended for localized ENKL [4]. At least 60 ~ 80 % of stage I/II patients can obtain complete response (CR) [5]. With regard to advanced-stage ENKL, conventional anthracycline-containing regimens give unsatisfactory responses, and 1-year overall survival (OS) rate is extremely low. Chemoresistance and early relapse are relatively common due to the overexpression of P-glycoprotein in tumor cells [6, 7]. Although great efforts have been made to explore optimal salvage strategies for relapsed/refractory ENKL, standard therapeutic regimens have not yet been established. In recent years, more effective l-asparaginase-based regimens have been tried for advanced-stage and relapsed/refractory ENKL, and significant responses were observed [8–10]. These regimens generally contain drugs unaffected by mutidrug resistance (MDR) pathway, such as methotrexate, l-asparaginase, and dexamethasone.
Gemcitabine is a deoxycytidine analog widely used against solid tumors. A series of studies have demonstrated the considerable activity of gemcitabine in lymphoma, particularly toward relapsed/refractory patients [11–14]. As a pegylated form of l-asparaginase, pegaspargase has been proved effective in acute lymphoblastic leukemia [15]. However, chemotherapeutic regimens incorporating both gemcitabine and pegaspargase are scarcely reported in curing relapsed/refractory ENKL patients. In this retrospective study, we firstly examined the treatment outcomes of a novel regimen comprising gemcitabine, pegaspargase, cisplatin, and dexamethasone (DDGP) for relapsed/refractory ENKL.
Patients and methods
Patients
Between July 2011 and December 2012, a cohort of 17 patients with relapsed/refractory ENKL was treated with DDGP regimen, at the Department of Medical Oncology, The First Affiliated Hospital of Zhengzhou University. Among them, ten patients were in relapse and seven patients were refractory to first-line therapy. Their pathological data were examined secondly to confirm the diagnosis of NK/T cell lymphoma. Immunohistochemistry analysis showed that all neoplastic cells were typically positive for cytoplasmic CD3ε, CD2, CD56, CD43, granzyme B, TIA-1, and EBER but negative for surface CD3, and CD20.
According to Ann Arbor-cotswolds staging system, pretreatment evaluations included medical history, physical examination, hematological examination, plasmic biochemical analysis for hepatic and renal function, bone marrow biopsy, ultrasonic inspection of superficial lymph nodes as well as computed tomography (CT) scan of nasal cavity, chest, and abdomen. At the end of 2 and 4 cycles and treatment completetion, CT scan and positron emission tomography (PET), if possible, were used to detect residual mass and assess response.
Prior to treatment, patients were fully informed of the adverse reactions and risks and signed an informed consent. Clinical data were collected after approval obtained from local ethics committee.
Treatment protocol
All patients (n = 17) with relapse/refractory ENKL were treated with DDGP regimen. The DDGP protocol was repeated every 3 weeks and administrated as follows: gemcitabine, 800 mg/m2 intravenously, on days 1 and 8; pegaspargase, 2,500 IU/m2 intramuscularly, on day 1; cisplatin, 20 mg/m2 intravenously, on days 1–4; dexamethasone, 15 mg/m2 intravenously, on days 1–5. For patients who were in poor condition or experienced severe toxicities, doses were reduced accordingly. Four patients received additional primary involved field radiotherapy. However, no patients underwent hematopoietic stem cell transplantation (HSCT), and one important reason is negative financial issues. Granulocyte colony-stimulating factor (G-CSF) and granulocyte-macrophage colony stimulating factor (GM-CSF) were given to patients who developed neutropenia and thrombocytopenia as support therapy.
Treatment response and toxicity criteria
Revised Cheson’s standard response criteria were adopted to assess treatment response [16]. CR was defined as no evidence of disease and disease-related symptoms. Partial response (PR) was defined as ≥50 % decrease in sum of the product of the diameters (SPDs) of masses and no new lesions. Stable disease (SD) was defined as a patient who failed to attain CR or PR but did not fulfill those criteria for progressive disease. Progressive disease (PD) was defined as appearance of new sites or ≥50 % increase in SPD of previous lesions from nadir.
Toxicities were graded according to the National Cancer Institute Common Toxicity Criteria, Version 4.0. Physical examination, laboratory tests (routine blood test, plasmic biochemical test, and routine urine test) were used to evaluate adverse reactions and toxicities.
Statistical analysis
The primary end points were overall response rate (ORR) at the end of treatment. The secondary end points were CR, 1-year OS, 1-year progression-free survival (PFS), and toxicity. ORR was defined as the percentage of patients who achieved CR and PR. OS was measured from the date of the treatment started until the date of death or the last follow-up. PFS was measured from the date of the treatment started until the date of disease progression or death from any cause. Graphpad prism v5.0 software was employed to obtain statistical data. The Kaplan-Meier method was used to perform survival analysis for OS and PFS.
Results
Patient characteristics and clinical data
Characteristics of the patients (n = 17) were listed in Table 1. The median age was 42 years (range 13–65 years ). Six patients were male, and eleven patients were female. Eight patients had Ann Arbor stage I/II ENKL, and nine patients had stage III/IV disease. Primary involvement site in the upper aerodigestive tract occurred in 14 patients, the lung in two patients and the skin in one patient. B symptom was observed in 13 patients (76.5 %) and elevated lactate dehydrogenase (LDH) in 10 patients (58.8 %). As to first-line therapy, seven patients (41.2 %) were initially treated with cyclophosphamide, doxorubicin, vincristine, and prednisolone (CHOP) or CHOP-like regimen, four patients (23.5 %) were treated with dexamethasone, methotrexate, ifosfamide, l-asparaginase, and etoposide (SMILE), five patients (29.4 %) were treated with etoposide, ifosfamide, cisplatin, and dexamethasone (VIPD), and one patient (5.9 %) was treated with etoposide, ifosfamide, dexamethasone, and cisplatin (DICE).
Treatment responses and survival
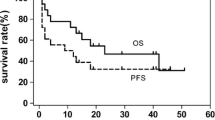
The responses and survival were summarized in Tables 2 and 3. A total of 67 cycles of DDGP regimen were administrated, and the median number of cycles was 4 (range 2–6 cycles). The ORR was 88.2 % (15 of 17 patients). Nine patients (52.9 %) entered CR, and six patients (35.3 %) entered PR. PD was observed in one patient during therapy and SD in one patient. With the median follow-up time of 17 months (range 2–28 months), the 1-year OS rate and 1-year PFS were 82.4 and 64.7 %, respectively (Figs. 1 and 2). Until now, 13 patients are still alive with seven patients in relapse. Three patients died from disease progression and one patient died from infection 3 months after she achieved SD. For those CR patients, the median PFS was 17 months.
Safety
Treatment-related adverse events were shown in Table 4. Grade 3/4 neutropenia and thrombocytopenia occurred in nine patients (52.9 %) and six patients (35.3 %), respectively. With regard to nonhematologic toxicities, all patients experienced various degrees of nausea and vomiting. Six patients (35.3 %) had grade 3/4 infection, and four patients (23.5 %) had liver dysfunction according to elevated alanine aminotransferase (ALT) and aspartate aminotransferase (AST). Allergic reaction was reported in two patients, and no patient developed pancreatitis. Prolonged activated partial thromboplastin time (APTT) was observed in two patients.
Discussion
Owning to MDR, existing salvage regimens for relapsed/refractory ENKL lead to unsatisfactory treatment outcome and efficient protocols are urgently needed. In the present study, we retrospectively evaluated the efficacy and safety of DDGP regimen in the treatment of relapsed/refractory ENKL. The ORR was 88.2 % (15/17) with 52.9 % CR rate. The 1-year OS rate was 82.4 %, and 1-year PFS was 64.7 %. The results we presented here show the excellent antitumor effect of DDGP regimen.
As an agent independent of MDR pathway, l-asparaginase exerts potent effect in inducing apoptosis of tumoral NK cells and acts through depleting asparagines and inhibiting synthesis of proteins, DNA, and RNA in neoplastic cells [17, 18]. Several clinical studies have confirmed the remarkable effectiveness of l-asparaginase in ENKL patients [8, 9, 19, 20]. From a phase II study designed for advanced-stage, relapsed/refractory ENKL, 2 cycles of SMILE regimen were associated with an ORR of 79 % with a CR of 45 % [8]. Pegaspargase, a pegylated form of l-asparaginase, is seldom reported in the treatment of ENKL. Li et al. [21] reported the responses of 12 newly-diagnosed ENKL patients treated with DDGP chemotherapy in our center, and 100 % ORR was achieved. From another report, two patients with relapsed/refractory ENKL received single-agent pegaspargase, and both entered CR [22]. These promising results suggest that pegaspargase should be a potential effective agent employed in the treatment of ENKL.
Frustratingly, part of the patients who have received l-asparaginase-based chemotherapy still experienced failure. At present, there seemed to be no better option to cope with these patients.
Gemcitabine is a pyrimidine antimetabolite with activity against solid tumors. Zinzani et al. [14] reported the role of gemcitabine as a single agent in pretreated T cell lymphoma patients, and the ORR reached 51 %. Combined with other antitumor agents, gemcitabine has also shown substantial clinical activity in aggressive B cell and T cell lymphoma [23, 24]. However, salvage therapeutic strategies containing gemcitabine in the treatment of ENKL were scarcely explored and identified. Recently, a retrospective study of gemcitabine alone or containing chemotherapy for relapsed/refractory ENKL was conducted, and 40 % of patients acquired objective response [25]. Moreover, in vitro and in vivo tumor models have also demonstrated excellent anticancer activity of gemcitabine against NK cell leukemia/lymphoma. Why NK/T tumor cells are especially sensitive to gemcitabine has not been fully elucidated, but increased expression of activating enzymes, such as dCK, ENT1 in tumor cells may be involved in the action mechanism [26].
In this retrospective study, four patients received DDGP regimen as salvage strategy after initial treatment of SMILE regimen. Two patients achieved objective responses and one patient maintained CR status for 28 months. Gemcitabine provides us a better option in managing relapsed/refractory ENKL patients. We have carried out one research aimed to uncover the mechanisms underlying the activity and chemoresistance of gemcitabine. In addition, compared with native asparaginase, pegaspargase has lower incidence of antiasparaginase antibodies and maintains more prolonged asparaginase activity [27].
The estimated 2-year OS and 2-year PFS were 76.5 and 30.9 %, respectively. A great part of the patients who experienced disease-progression acquired favorable treatment outcome after receiving radiotherapy combined with l-asparaginase or etoposide as salvage strategy. However, insufficient follow-up time and sample size restricted the accurate evaluation of 2-year OS and PFS. Further study will enroll more patients with sufficient follow-up to evaluate the 2-year and 5-year OS and PFS.
As to adverse events, grade 3/4 neutropenia and grade 3/4 thrombocytopenia were common. Dose reduction and the application of G-CSF and GM-CSF in time were essential when patients experienced sever cytopenias during and after chemotherapy. Other support treatments such as platelet transfusion and infection prevention were also necessary.
Tumoral NK cells are usually resistant to MDR-dependent drugs, and treatment failure is common. Thus, we assume that combination chemotherapy incorporating MDR-independent drugs such as pegaspargase, gemcitabine, and dexamethasone would be effective in the treatment of ENKL. The results we presented here confirmed our expectation and were consistent to former l-asparaginase-based chemotherapy. Gemcitabine and pegaspargase played a predominant role in improving ORR and prolonging survival. Right now, a multicenter, randomized, controlled trial of DDGP regimen as first-line therapy for newly-diagnosed ENKL has been conducted in our center (Clinical Trials.gov ID, NCT01501136 and NCT01501149), and significant treatment outcomes are expected. Clinical trial (NCT01501136) is a multicenter, randomized, controlled, phase 4 study. There are five treatment arms: sequential DDGP regimen followed by radiotherapy, sequential VIPD regimen followed by radiotherapy, sequential radiotherapy followed by DDGP regimen, sequential radiotherapy followed by VIPD regimen chemotherapy, and suitable type intensity-modulated radiation therapy (IMRT) 50 Gy, respectively. This study is designed to evaluate the efficacy and safety of DDGP regimen for newly diagnosed stage I/II ENKL and explore the optimal treatment strategy for stage I/II ENKL.
In conclusion, DDGP combination chemotherapy showed remarkable effectiveness in pretreated ENKL patients, and toxicity was tolerated. Further prospective trials are expected to validate the efficacy of DDGP regimen in relapsed/refractory ENKL.
References
Au WY, Weisenburger DD, Intragumtornchai T et al (2009) Clinical differences between nasal and extranasal natural killer/T-cell lymphoma: a study of 136 cases from the International Peripheral T-Cell Lymphoma Project. Blood 113:3931–3937
Tse E, Kwong YL (2013) How I treat NK/T-cell lymphomas. Blood 121:4997–5005
Ito Y, Kimura H, Maeda Y et al (2012) Pretreatment EBV-DNA copy number is predictive of response and toxicities to SMILE chemotherapy for extranodal NK/T-cell lymphoma, nasal type. Clin Cancer Res 18:4183–4190
Kwong YL, Anderson BO, Advani R et al (2009) Management of T-cell and natural-killer-cell neoplasms in Asia: consensus statement from the Asian Oncology Summit 2009. Lancet Oncol 10:1093–1101
Kim SJ, Kim K, Kim BS et al (2009) Phase II trial of concurrent radiation and weekly cisplatin followed by VIPD chemotherapy in newly diagnosed, stage IE to IIE, nasal, extranodal NK/T-Cell Lymphoma: consortium for improving survival of lymphoma study. J Clin Oncol 27:6027–6032
Yamaguchi M, Kita K, Miwa H et al (1995) Frequent expression of P-glycoprotein/MDR1 by nasal T-cell lymphoma cells. Cancer 76:2351–2356
Tse E, Kwong YL (2012) Practical management of natural killer/T-cell lymphoma. Curr Opin Oncol 24:480–486
Yamaguchi M, Kwong YL, Kim WS et al (2011) Phase II study of SMILE chemotherapy for newly diagnosed stage IV, relapsed, or refractory extranodal natural killer (NK)/T-cell lymphoma, nasal type: the NK-Cell tumor study group study. J Clin Oncol 29:4410–4416
Jaccard A, Gachard N, Marin B et al (2011) Efficacy of L-asparaginase with methotrexate and dexamethasone (AspaMetDex regimen) in patients with refractory or relapsing extranodal NK/T-cell lymphoma, a phase 2 study. Blood 117:1834–1839
Tsukune Y, Isobe Y, Yasuda H et al (2010) Activity and safety of combination chemotherapy with methotrexate, ifosfamide, l-asparaginase and dexamethasone (MILD) for refractory lymphoid malignancies: a pilot study. Eur J Haematol 84:310–315
Morschhauser F, Depil S, Jourdan E et al (2007) Phase II study of gemcitabine-dexamethasone with or without cisplatin in relapsed or refractory mantle cell lymphoma. Ann Oncol 18:370–375
Dong M, He XH, Liu P et al (2013) Gemcitabine-based combination regimen in patients with peripheral T-cell lymphoma. Med Oncol 30:351
Mahadevan D, Unger JM, Spier CM et al (2013) Phase 2 trial of combined cisplatin, etoposide, gemcitabine, and methylprednisolone (PEGS) in peripheral T-cell non-Hodgkin lymphoma: Southwest Oncology Group Study S0350. Cancer 119:371–379
Zinzani PL, Venturini F, Stefoni V et al (2010) Gemcitabine as single agent in pretreated T-cell lymphoma patients: evaluation of the long-term outcome. Ann Oncol 21:860–863
Escherich G, Zur Stadt U, Zimmermann M et al (2013) Clofarabine in combination with pegylated asparaginase in the frontline treatment of childhood acute lymphoblastic leukaemia: a feasibility report from the CoALL 08-09 trial. Br J Haematol 163:240–247
Cheson BD, Pfistner B, Juweid ME et al (2007) Revised response criteria for malignant lymphoma. J Clin Oncol 25:579–586
Ando M, Sugimoto K, Kitoh T et al (2005) Selective apoptosis of natural killer-cell tumours by L-asparaginase. Br J Haematol 130:860–868
Charamella LJ, Meyer C, Thompson GE et al (1985) Chemotherapeutic agents and modulation of natural killer cell activity in vitro. J Immunopharmacol 7:53–65
Jaccard A, Petit B, Girault S et al (2009) L-asparaginase-based treatment of 15 western patients with extranodal NK/T-cell lymphoma and leukemia and a review of the literature. Ann Oncol 20:110–116
Yong W, Zheng W, Zhu J et al (2009) L-asparaginase in the treatment of refractory and relapsed extranodal NK/T-cell lymphoma, nasal type. Ann Hematol 88:647–652
Li L, Zhang C, Zhang L et al (2014) Efficacy of apegaspargase-based regimen in the treatment of newly-diagnosed extranodal natural killer/T-cell lymphoma. Neoplasma 61:225–232
Reyes VE Jr, Al-Saleem T, Robu VG et al (2010) Extranodal NK/T-cell lymphoma nasal type: efficacy of pegaspargase. Report of two patients from the United Sates and review of literature. Leuk Res 34:e50–e54
Evens AM, Rosen ST, Helenowski I et al (2013) A phase I/II trial of bortezomib combined concurrently with gemcitabine for relapsed or refractory DLBCL and peripheral T-cell lymphomas. Br J Haematol 163:55–61
Mounier N, El Gnaoui T, Tilly H et al (2013) Rituximab plus gemcitabine and oxaliplatin in patients with refractory/relapsed diffuse large B-cell lymphoma who are not candidates for high-dose therapy. A phase II Lymphoma Study Association trial. Haematologica 98:1726–1731
Ahn HK, Kim SJ, Hwang DW et al (2013) Gemcitabine alone and/or containing chemotherapy is efficient in refractory or relapsed NK/T-cell lymphoma. Investig New Drugs 31:469–472
Kim TM, Kim S, Ahn YO et al (2014) Anti-cancer activity of gemcitabine against natural killer cell leukemia/lymphoma. Leuk Lymphoma 55:940–943
Dinndorf PA, Gootenberg J, Cohen MH et al (2007) FDA drug approval summary: pegaspargase (oncaspar) for the first-line treatment of children with acute lymphoblastic leukemia (ALL). Oncologist 12:991–998
Acknowledgments
This work was supported by the National Natural Science Foundation of China [grant number 81172118].
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhou, Z., Li, X., Chen, C. et al. Effectiveness of gemcitabine, pegaspargase, cisplatin, and dexamethasone (DDGP) combination chemotherapy in the treatment of relapsed/refractory extranodal NK/T cell lymphoma: a retrospective study of 17 patients. Ann Hematol 93, 1889–1894 (2014). https://doi.org/10.1007/s00277-014-2136-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-014-2136-7