Abstract
There is no standard salvage regimen for patients with refractory and relapsed extranodal NK/T-cell lymphoma (NKTCL), nasal type. This study was conduced to evaluate the efficacy of L-asparaginase-based regimen as a salvage regimen, on refractory and relapsed extranodal NKTCL, nasal type. Between March 1996 and March 2008, 45 patients with refractory and relapsed extranodal NKTCL, nasal type, were studied retrospectively. All patients were treated with L-asparaginase-based salvage regimen. Thirty-nine patients also received primary involved-field radiation after L-asparaginase-based chemotherapy. The complete response rate, partial response rate, and overall response rate for the whole group were 55.6%, 26.7%, and 82.2%, respectively. Both of 3-year and 5-year overall survival (OS) rates were 66.9%. The major adverse effects of L-asparaginase were myelosuppression, liver dysfunction, hyperglycemia, and allergic reaction. In general, the side effects could be tolerated. On univariate analysis, age, the stage of disease, and performance status were found to be prognostic factors influencing OS. On multivariate analysis, the stage of disease and age were independent prognostic factors for OS. L-Asparaginase-based regimen was obviously effective for the patients with refractory and relapsed extranodal NKTCL, nasal type.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Extranodal NK/T-cell lymphoma (NKTCL), nasal-type, is a recently recognized distinct entity of non-Hodgkin’s lymphoma (NHL) expressing nature killer cell origin within the new WHO classification of lymphoid tumors [1–3]. This entity is rare, but relatively prevalent in Asia and South America, showing a distinctive geographic distribution. It comprises approximately 6.5%–9% of NHL in Asia [4, 5]. A consistent association with EB virus infection is demonstrated in the lymphoma cells, suggesting a probable pathogenic rule of EBV [1–3, 6–8].
Extranodal NKTCL nasal-type pursues an aggressive clinical course with poor prognosis. Several recent clinical investigations have reported 5-year overall survival (OS) rates ranging from 36% to 49.5% for patients with stage I–IV disease [4, 5, 7–9]. Up to now, optimal treatment strategies have not been fully recognized. Although radiotherapy and chemotherapy are both effective for NKTCL, nasal-type, approximately 50% of the patients still fail in locoregional recurrences or systemic disease progression. Thus, an innovative therapy is urgently needed to improve treatment outcome. Here, the impact of L-asparaginase-based salvage regimen on the treatment outcome of patients with refractory and relapsed extranodal NKTCL, nasal-type, was reported.
Patients and method
Patient eligibility
Between March 1996 and March 2008, 45 Chinese patients with refractory and relapsed extranodal NKTCL, nasal-type, were included. Among them, 41 patients were refractory cases that attained only stabilized disease or disease deterioration after two or more cycles of CHOP chemotherapy, and four patients were recurrent cases after locoregional radiation and CHOP-like chemotherapy.
Histological and immunophenotypic diagnosis
Histological examination was performed on paraffin-embedded tissue sections stained with hematoxilin–eosin. Immunophenotyping was performed using monoclonal antibodies against CD56, CD3 (polyclonal), granzyme B/TIA-1, and CD20. EBER was detected by in situ hybridization technique.
The diagnostic criteria of NKTCL, nasal-type, were as follows: (1) The pathological diagnosis of NKTCL was based on the new WHO classification [1]. (2) In this series, the immunophenotype of tumor cells should express CD56+ and EBER+ or CD56+, granzyme B+/TIA-1+, EBER−. If CD56−, the immunophenotypic expression should demonstrate EBER+, CD3+ (polyclonal), granzyme B+/TIA-1+, and CD20− [1, 10, 11].
Clinical subtypes
Recently, extranodal NKTCL, nasal-type, was divided into two clinical subtypes, namely, upper aerodigestive tract NKTCL (UNKTCL) and extra-upper aerodigestive tract NKTCL (EUNKTCL) according to the primary anatomic site [5, 7, 9]. In this study, UNKTCL referred to tumors primarily occurring in the nasal cavity and the upper aerodigestive tract, and EUNKTCL to those in all sites other than the nasal cavity and the upper aerodigestive tract, e.g., the skin, gastrointestinal tract, salivary glands, testis, and other visceral organs.
Staging
Disease was staged according to Ann Arbor system. The TNMB staging system was used for primary cutaneous NKTCL, nasal type [12]. Staging investigation included a complete history and physical examination; routine blood cell counts; serum biochemistry; a bone marrow aspiration without biopsy; chest X-ray; computed tomography scan of the head, neck, and abdomen; and ultrasound scan of liver, spleen, and lymph nodes.
Prognostic factors
The clinical features evaluated for potential prognostic importance included age, sex, the stage of disease, fever symptom, performance status (PS) (ECOG scale), serum lactate dehydrogenase, and primary anatomic sites of lymphomatous involvement.
Treatment
The patients (n = 41) with primary CHOP resistance were treated with L-asparaginase-based salvage regimen followed by primary involved-field radiation (IF RT). Among them, two patients with intestinal NKTCL, who had received partial enterectomy and CHOP-like chemotherapy, were treated with L-asparaginase-based salvage regimen alone. The relapsed patients (n = 4), who had received locoregional radiation and CHOP-like chemotherapy, were also treated with L-asparaginase-based salvage regimen alone. All the patients received L-asparaginase-based regimen for median three cycles (one to six cycles) with 55.6% of the patients receiving three or more cycles. The L-asparaginase-based regimen consisted of L-asparaginase (preparations prepared from Escherichia coli, Kyowa Hakko Kogyo Tokyo Japan, Chang Zhou Pharmaceutical com. China) 6,000 IU/m2 intravenous drip on days 1 to 7, vincristine 1.4 mg/m2 intravenously on day 1, dexamethasone 10 mg intravenously on days 1 to 7. The cycle was repeated every 28 days. Primary IF RT was delivered using 6-MeV linear accelerator at 2.0 Gy per daily fraction for a total dose of 30–60 Gy (a median of 50 Gy), with 85% of the patients receiving ≥50 Gy, over 3–6 weeks. The institutional review board approved the protocol. Each patient or guardian provided written informed consent.
Statistical analysis
Tumor response was assessed with WHO criteria [13]. The time of OS was measured from the date of diagnosis to the date of death or last follow-up. OS curves were estimated with the Kaplan–Meier method. The log-rank test was used to compare survival curves. Statistical significance was defined as P < 0.05 in the univariate analysis. A backward stepwise Cox regression analysis was performed to define prognostic factors influencing OS. P < 0.05 was considered statistically significant in multivariate analysis.
Results
Clinical and histopathological characteristics
Clinical characteristics of the patients were listed in Table 1. The median age of the patients was 43 years (range, 12–77 years). The male-to-female ratio was 3.1:1. Thirty-three patients (73.3%) had stage I–II disease. Twelve patients (26. 7%) had stage III–IV disease. Among the patients with stage III–IV disease, the most distant spread organs were the skin, intestine, central nervous system, and adrenal gland (Table 2). As to the site distribution of primary involvement, UNKTCL was in 39 patents (86.7%), and EUNKTCL only in six patients (13.3%). Nasal cavity was the most frequently primary organ in 34 patients (75.6%) (Table 2).
Histopathological features showed polymorphic lymphocytic infiltrate and prominent necrosis, and often with angioinvasion. Thirty-seven (82.2%) cases had zonal necrosis. Fifteen cases (33.3%) showed angioinvasion. Forty-four samples (97.8%) were positive for CD56. One sample (2.2%) was negative for CD56 but positive for EBER, granzyme B/TIA-1, and CD3 (Polyclonal). Thirty-six of the 45 samples were detected for EBER; 29 samples (80.6%) were positive.
Treatment results
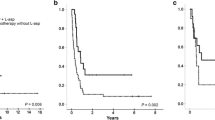
The complete response rate, partial response rate, and overall response rate for the whole group were 55.6% (25/45 cases), 26.7% (12/45 cases), and 82.2% (37/45 cases), respectively. Both the 3-and 5-year OS rates were 66.9% (95% confidence interval 74.0, 107.7) (Fig. 1). The OS curve reached a plateau after 16 months (Fig. 1). The median follow-up period of living patients was 35 months.
Adverse effects of L-Asparaginase
Toxicity was graded according to WHO criteria [13]. The adverse effects of L-Asparaginase were summarized in Table 3. The major side effects were liver dysfunction, myelosuppression, hyperglycemia, hypoalbuminemia, and allergic rash. Pancreatitis developed in one patient. Some patients discontinued or delayed chemotherapy because of adverse events (mainly pancreatitis, allergic reaction, liver dysfunction) or disease deterioration. There was no death related to L-asparaginase.
Causes of death
Fourteen patients died in this series. Among them, five patients died of massive bleeding of tumor tissue, including nasopharyngeal bleeding in two patients and intestinal bleeding in three patients; one patient died of intestinal perforation, one patient died of hemophagocytic syndrome (HPS), and seven patients died of systemic disease progression.
Prognostic factors
The clinical characteristics were tested for prognostic significance on OS (Table 1). On univariate analysis, age, the stage of disease, and PS were found to be prognostic factors influencing OS (Table 1). On multivariate analysis, the stage of disease and age were independent prognostic factors for OS (Table 4).
Discussion
Nasal-type NKTCL is aggressive, often with unsatisfactory response to conventional chemoradiotherapy. Radiation is beneficial to control locoregional disease but may be prone to distant dissemination. Recent clinical studies suggested that a total dose ≥50 Gy resulted in favorable locoregional control [14–18]. Koom et al. reported that the dose–response curve showed the plateau at doses in excess of about 54 Gy [18]. The 5-year OS rate ranged 37.9–66% for stage I–II patients treated with IF RT alone [7–9, 14–16]. The lower 5-year OS was due to local relapse and distant dissemination after radiation. In recent reports, the 5-year OS rates were only 25–38.3% for stage III–IV patients treated with chemoradiotherapy [8, 9, 19, 20]. Conventional chemotherapeutic regimens and radiation are not enough to eradicate local and systemic disease. However, chemotherapy is indicated for both patients with stage III–IV disease and patients with local recurrence or systemic tumor spread after locoregional radiotherapy. The unsatisfactory efficacy of conventional chemotherapy may be due to frequent expression of mdr1 gene in the tumor cells [21, 22]. To improve the conventional chemotherapeutic effect, one of the key issues is to investigate new effective anticancer drugs and chemotherapeutic regimens. It is known that L-asparaginase has a different anticancer mechanism from alkylating agents, plant alkaloids, anticancer antibiotics, and cisplatin. L-Asparaginase hydrolyzes serum asparagine and deprives some cells of the required amino acid to yield anticancer effects in certain tumor cells, especially in lymphoma cells and lymphocytic leukemic cells that lack L-asparagine synthetase [23, 24]. Therefore, we tried to treat the CHOP failures with L-asparaginase-based salvage regimen and obtained obvious effectiveness. The 5-year OS rate was 66.9% in this study. The results showed that L-asparaginase-based regimen was an effective salvage treatment for patients with refractory and relapsed extranodal NKTCL. L-Asparaginase-based regimen could be considered as first-line chemotherapy for patients with stage III or IV disease and should be evaluated in future studies. In general, the side effects of L-asparaginase could be tolerated [20, 23, 24].
Notably, in our series, the lethal complications were massive bleeding of tumor tissue, intestinal perforation, and HPS. The pathologic characteristics of necrotic lesion and progressive angiodestruction may lead to massive bleeding and intestinal perforation. HPS is pathogenically related to an excessive production of cytokines (cytokine storm), in particular, TNFα, from the EBV-infected NK/T lymphoma cells. The cytokine storm can cause histiocytic activation and subsequent hemophagocytic processes [1, 8, 25]. Early and reasonable management of the severe lymphomatous complications would help improve the treatment outcome. In summary, this study suggested that L-asparaginase was an effective option to improve the chemotherapeutic efficacy on NK/T cell lymphoma, nasal type, and is worth further study.
References
Chan JK, Jaffe E, Ralfkiaer E (2001) Extranodal NK/T cell lymphoma, nasal type. In: Jaffe ES, Harris NL, Stein H, Vardiman JW (eds) Tumours of haematopoietic and lymphoid tissues. IARC, Lyon, pp 204–207
Jaffe ES, Chan JKC, Su IJ et al (1996) Report of the workshop on nasal and related extranodal angiocentric T/nature killer cell lymphomas definitions, differential diagnosis, and epidemiology. Am J Surg Pathol 20:103–111
Oshimi K (2003) Leukemia and lymphoma of natural killer lineage cells. Int J Hematol 78:18–23. doi:10.1007/BF02983235
Au WY, Ma SY, Chim CS et al (2005) Clinicopathologic features and treatment outcome of mature T-cell and natural killer-cell lymphomas diagnosed according to the World Health Organization classification scheme: a single center experience of 10 years. Ann Oncol 16:206–214. doi:10.1093/annonc/mdi037
Lee J, Suh C, Park YH et al (2006) Extranodal natural killer T-cell lymphoma, nasal-type: a prognostic model from a retrospective multicenter study. J Clin Oncol 24:612–618. doi:10.1200/JCO.2005.04.1384
Tao Q, Ho FCS, Loke SL et al (1995) Epstein–Barr virus is localized in the tumor cells of nasal lymphoma of NK, T or B cell type. Int J Cancer 60:315–320. doi:10.1002/ijc.2910600306
Kwong YL (2005) Nature killer-cell malignancies: diagnosis and treatment. Leukemia 19:2186–2194. doi:10.1038/sj.leu.2403955
Li CC, Tien HF, Tang JL et al (2004) Treatment outcomes and pattern of failure in 77 patients with sinonasal natural killer /T-cell or T-cell lymphoma. Cancer 100:366–375. doi:10.1002/cncr.11908
Lee J, Park YH, Kim WS et al (2005) Extranodal nasal type NK/T-cell lymphoma: elucidating clinical prognostic factors for risk-based stratification of therapy. Eur J Cancer 41:1402–1408. doi:10.1016/j.ejca.2005.03.010
Chan JK, Tsang WY, Ng CS et al (1995) Discordant CD3 expression in lymphomas when studied on frozen and paraffin sections. Hum Pathol 26:1139–1143. doi:10.1016/0046-8177(95)90277-5
Chan JK, Tsang WY, Ng CS (1996) Clarification of CD3 immunoreactivity in nasal T/natural killer cell lymphomas: the neoplastic cells are often CD3 epsilon+. Blood 87:839–841
Bunn PA Jr, Lamberg SI et al (1979) Report of the committee of staging and classification of cutaneous T-cell lymphomas. Cancer Treat Rep 63:702–728
Miller AB, Hoogstraten B, Staquet M et al (1981) Reporting results of cancer treatment. Cancer 47:207–214. doi:10.1002/1097-0142(19810101)47:1<207::AID-CNCR2820470134>3.0.CO;2-6
Kim GE, Lee SW, Chang SK et al (2001) Combined chemotherapy and radiation versus radiation alone in the management of localized angiocentric lymphoma of the head and neck. Radiother Oncol 61:261–269. doi:10.1016/S0167-8140(01)00428-5
Li JX, Yao B, Jin J et al (2006) Radiotherapy as primary treatment for stage IE and IIE nasal natural killer /T-cell lymphoma. J Clin Oncol 24:181–189. doi:10.1200/JCO.2005.03.2573
Cheung MM, Chan JK, Lau WH et al (2002) Early stage nasal NK/T-cell lymphoma: clinical outcome, prognostic factors, and the effect of treatment modality. Int J Radiat Oncol Biol Phys 54:182–190. doi:10.1016/S0360-3016(02)02916-4
Isobe K, Uno T, Tamaru JI et al (2006) Extranodal natural killer/T-cell lymphoma, nasal type the significance of radiotherapeutic parameters. Cancer 106:609–615. doi:10.1002/cncr.21656
Koom WS, Chung EJ, Yang WI et al (2004) Angiocentric T-cell and NK/T-cell lymphomas: radiotherapeutic viewpoints. Int J Radiat Oncol Biol Phys 59:1127–1137. doi:10.1016/j.ijrobp.2003.12.006
Cheung MMC, Chan JKC, Lau WH et al (1998) Primary non-Hodgkin’s lymphoma of the nose and nasopharynx: clinical features, tumor immunophenotype, and treatment outcome in 113 patients. J Clin Oncol 16:70–77
Yong W, Zheng W, Zhu J et al (2006) Midline NK/T-cell lymphoma nasal-type: treatment outcome, the effect of L-asparaginase based regimen, and prognostic factors. Hematol Oncol 24:28–32. doi:10.1002/hon.765
Chaudhary PM, Mechetner EB, Roninson IB (1992) Expression and activity of the multidrug resistance P-glycoprotein in human peripheral blood lymphocytes. Blood 80:2735–2739
Yamaguchi M, Kita K, Miwa H et al (1995) Frequent expression of P-glycoprotein/MDR1 by nasal T-cell lymphoma cells. Cancer 76:2351–2356. doi:10.1002/1097-0142(19951201)76:11<2351::AID-CNCR2820761125>3.0.CO;2-1
Yong W, Zheng W, Zhu J et al (2003) L-asparaginase-based regimen in the treatment of refractory midline T/NK-cell lymphoma. Int J Hematol 78:163–167. doi:10.1007/BF02983387
Nagafuji K, Fujisaki t, Arima F et al (2001) L-asparaginase induced durable remission of relapsed nasal NK/T-cell lymphoma after autologous peripheral blood stem cell transplantation. Int J Hematol 74:447–450. doi:10.1007/BF02982090
Takahashi N, Miura I, Chubachi A et al (2001) A clinicopathological study of 20 patients with T/natural killer (NK)-cell lymphoma-associated hemophagocytic syndrome with special reference to nasal and nasal-type NK/T-cell lymphoma. Int J Hematol 74:303–308. doi:10.1007/BF02982065
Acknowledgements
This study was supported by Peking University grant 211.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yong, W., Zheng, W., Zhu, J. et al. L-Asparaginase in the treatment of refractory and relapsed extranodal NK/T-cell lymphoma, nasal type. Ann Hematol 88, 647–652 (2009). https://doi.org/10.1007/s00277-008-0669-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-008-0669-3