Abstract
Purpose
The main objective of the present study is to compare the safety, technical success and diagnostic yield of endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) versus ultrasound-guided percutaneous core-needle biopsy (US-CNB) in patients with solid pancreatic lesions.
Methods
This is a retrospective study that involved all patients with a solid pancreatic lesion who underwent EUS-FNA or US-CNB between November 2019 and February 2021. Of all patients, 69 (84.1%) had inoperable malignancy, whereas 13 (15.9%) had chronic pancreatitis. Resectability status was ascertained by computed tomography. All core needle biopsies were performed by the same interventional radiologist via ultrasound guidance with an 18-gauge semi-automatic tru-cut needle. All EUS-FNA procedures were performed by the same gastroenterologist with a 27-gauge EUS-FNA needle. Technical success is defined as if the region of interest is reached and specimen taken from the pancreatic lesion. Diagnostic yield is defined as the procurement of sufficient tissue for pathological examination.
Results
Overall, 52 patients (mean age 58.5 ± 9.8 years) who underwent EUS-FNA and 30 patients (60.1 ± 12.1 years) who underwent US-CNB were included. Solid lesions were most commonly (61.5% in EUS-FNA and 50.0% in US-CNB groups) located in pancreatic head in both groups. Mean size of the lesions was comparable in both groups as well. The technical success was 100% in both groups. In 12 (14.6%) patients, pathology results revealed inadequate sampling (11 × in the EUS-FNA and 1 × in the US-CNB group). The diagnostic yield was significantly higher in US-CNB group than in EUS-FNA group (96.7% vs. 78.8%, respectively, p = 0.048). Of 11 patients in the EUS-FNA with inadequate sampling, pancreatic lesions were located in the pancreatic head in 7 (63.6%). No major complications were observed in neither of the groups. As a minor complication, one case of slight abdominal pain was detected in the EUS-FNA group.
Conclusion
Based on the results of the present study, both US-CNB and EUS-FNA appeared safe; however, diagnostic yield in the US-CNB group was significantly higher.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pancreatic cancer ranks as the 4th most fatal cancer in both males and females in the general population [1].
Since solid pancreatic lesions have a heterogenous etiology, tissue diagnosis is required in most cases [2]. Pancreatic adenocarcinoma constitutes the great majority (80–90%) of neoplastic solid pancreatic lesions, followed by neuroendocrine tumors and metastatic lesions. Chronic pancreatitis should also be considered in the differential diagnosis. When chronic inflammation occurs focally, it may present as a solid lesion, i.e., pseudotumor [3].
Multidetector computed tomography (CT) is recommended as the initial imaging technique in the diagnosis and staging of pancreatic adenocarcinoma [1]. Endoscopic ultrasonography (EUS) is used as an adjunct imaging modality in most cases, particularly in assessing the presence of lymph node metastasis and vascular invasion. European Society for Medical Oncology (ESMO) guidelines recommend using EUS when the pancreatic adenocarcinoma is deemed resectable and no metastases are detected [1]. EUS is also recommended when the cancer is locally advanced to obtain a biopsy for definitive diagnosis.
Ultrasound guided percutaneous core needle biopsy (US-CNB) of solid pancreatic can be used as an alternative diagnostic modality to EUS-guided fine needle aspiration (EUS-FNA) [4,5,6]. Some studies [7, 8] reported higher diagnostic sensitivity rates with CNB compared to EUS-FNA owing to the larger volume of extracted biopsy samples. Moreover, CNB under ultrasound guidance may be faster and does not require radiation exposure. On the other hand, US-CNB is theoretically riskier for seeding of the primary pancreatic tumor due to a longer trajectory and larger bore of the biopsy needles. However, there is no direct evidence of this risk in the literature. Nevertheless, the National Comprehensive Cancer Network (NCCN) guideline recommends performance US-FNA over US-CNB based on the belief that the latter is associated with less major complications and seeding in patients with resectable pancreatic lesions [5, 9, 10]. It can be considered that the potential risk of seeding is less of a problem in locally advanced and already metastatic disease.
Several studies have evaluated diagnostic yield and complication rates of percutaneous CNB in solid pancreatic lesions [5, 11, 12]. However, data comparing diagnostic utility and adverse events of US-CNB with that of EUS-FNA in solid pancreatic lesions are scarce [7, 13]. Therefore, in the present study, we aimed to compare technical success rate, diagnostic yield as well as procedure-related adverse events between US-CNB and EUS-FNA in patients with solid pancreatic lesions.
Materials and Methods
Study Design, Subjects, and Setting
This is a retrospective study that involved all patients with a solid pancreatic lesion who underwent EUS-FNA or US-CNB at our institution. The institutional review board approved the study protocol.
Solid pancreatic lesions had been detected by abdominal CT and/or magnetic resonance imaging ordered by the primary physicians of the patients, mainly gastroenterologists. Unresectable pancreatic cancer was defined as International Union Against Cancer clinical stage III (locally advanced disease: T4N0-1 and M0) or IV (metastatic disease: T1-4N0-1 and M1). We included all patients who underwent US-guided core-needle biopsy or EUS- FNA between November 2019 and February 2021 at the National Cancer Center of Azerbaijan for suspected pancreatic carcinoma.
Patients who had a contraindication for EUS (structural abnormalities such as duodenal or esophageal stenosis) and US-FNA (poor performance status and cardiopulmonary reserve, inadequate visualization, and untreated bleeding tendency) were excluded from the study. Patients who lacked biopsy results and other study data were excluded, either. Resectability status was ascertained based on the resectability criteria of NCCN guidelines [14]. “In this study EUS-guided FNA was the initial biopsy modality. However, if the lesion deemed to be unsuitable for EUS-guided FNA, or if pancreatitis was suspected, the clinician would consider US-guided CNB as the first biopsy modality.
All inoperable patients were administered systemic chemotherapy. The clinical course and outcome will not be followed of the patients after pancreatic biopsy procedures.
US-CNB and EUS-FNA Procedures
All core-needle biopsies were performed by the same interventional radiologist after overnight fasting. While patients were lying supine, a 5-MHz ultrasound probe (SonoAce X6 scanner, Samsung Medison, Seoul, Korea) was used to visualize the solid pancreatic lesion. The needle insertion point was planned and trajectory to obtain the shortest possible distance to the lesion without causing any injury to adjacent organs. Local anesthesia was performed with lidocaine at the needle insertion site without systemic sedation. An 18-gauge, 15 cm semi-automatic tru-cut needle (Geotek Semi-Automatic Biopsy Needle, Ankara, Turkey) was inserted under the continuous guidance of ultrasound. At least two biopsy cores were collected, which were fixated in 10% formalin for further pathological examination at the pathology department. After the second extraction of the needle, local pressure was applied for 5 min to avoid local bleeding and hematoma formation (Figs. 1, 2).
A In a 50-year-old female patient, a hyperdense nodular lesion in the pancreas is seen on contrast-enhanced CT (white arrow). B Hypoechoic nodule in the pancreas on transabdominal ultrasonography (white arrow). C Core biopsy was performed from the hypoechogenic nodular lesion in the pancreas via percutaneous trans-abdominal ultrasonography guidance (black arrow)
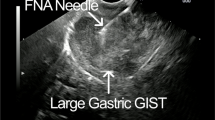
A In a 60-year-old male patient, a hypointense lesion (white arrow) in the pancreas is seen on T1 weighted MR imaging. B Hypointense lesion (black arrow) in the pancreas is seen on T2 weighted MR imaging. C Endoscopic ultrasonography showed a hypoechoic nodular lesion (white arrow) in the pancreas. D Endoscopic ultrasonographic fine-needle aspiration biopsy was performed from a hypoechoic nodular lesion (white arrow)
All EUS-FNA procedures were performed by the same gastroenterologist after midazolam sedation and pharyngeal lidocaine application. Solid pancreatic lesions were visualized using endoscopic ultrasound via linear echoendoscopes (EU-ME2 Premier Plus Olympus). 27-gauge EUS-FNA needles (Acquire endoscopic ultrasound fine needle biopsy device, Boston Scientific Co., Natick, MA, USA) were used to biopsy the pancreatic lesions. Minor and major complications were defined according to the guidelines for percutaneous needle biopsy of the Society of Interventional Radiology [15].
Definitions
Technical success was defined as if the region of interest was reached and specimen was taken from the pancreatic lesion Diagnostic yield was accepted as the procurement of sufficient tissue to make a diagnosis via pathological examination.
Statistical Analysis
The Kolmogorov–Smirnov test was used to check whether the distribution of the variables was normal. Age was given as mean ± standard deviation since it was normally distributed. Lesion diameter was presented as median (interquartile range) because it was not normally distributed. Categorical variables were presented as numbers and percentages. The non-parametric Mann–Whitney U test was used to compare the lesion diameter of the EUS-FNA and US-CNB groups. To compare age between the groups, the independent samples t-test was used. The Chi-Square test and Fisher's exact test were used to compare categorical variables between the groups.
A probability value of p < 0.05 was considered statistically significant, and two-tailed p values were used for all statistics. Statistical analysis was performed using the SPSS 25.0 statistical software package (SPSS Inc. Chicago, IL, USA).
Results
Overall, 52 patients (mean age 58.5 ± 9.8 years) who underwent EUS-FNA and 30 patients (mean age 60.1 ± 12.1 years) who underwent US-CNB were available for the analysis. There was no difference in terms of sex distribution between the groups. In both groups, more than two-thirds of the patients were male. Of all patients, 69 (84.1%) patients were referred for biopsy with a clinical and radiologic diagnosis of malignancy. In addition, 13 (15.9%) patients with a solid pancreatic lesion had a history of chronic pancreatitis.
In patients who underwent EUS-FNA, solid lesions were most commonly located in the pancreatic head (61.5%), followed by the neck and body. This was also the case in the US-CNB group (50.0%). There was no statistically significant difference in the location of the pancreatic lesions in both groups. The mean size of the lesions was comparable in both groups. The technical failure rate was zero in both groups since, in all patients, the pancreatic lesion could be reached either by US-CNB or EUS-FNA. The number of patients with a diagnostic yield was 41 (78.8%) in the EUS-FNA group, and 29 (96.7%) among the patients who underwent the US-CNB procedure. In 12 (14.6%) patients (11 in US-FNA and 1 in US-CNB group), pathology results revealed inadequate sampling. The diagnostic yield was significantly higher in the US-CNB group than in the EUS-FNA group (p = 0.048) (Fig. 3). Of 12 patients whose biopsy result was reported as inadequate sampling, pancreatic lesions were located in the pancreatic head in 7 patients (63.6%), whereas 4 lesions were located in the body and tail of the pancreas. The sole inadequately sampled lesion in the US-CNB group was located in the head of the pancreas. All biopsy pathology results were compatible with presumed pre-biopsy diagnosis in patients who were deemed to have unresectable malignancy. Biopsy results in both groups were compatible with chronic pancreatitis in patients who had a history and diagnostic imaging clues for chronic pancreatitis. Pancreatic ductal adenocarcinoma was the most commonly diagnosed cancer in both groups (Fig. 4). Table 1 summarizes demographic features and pancreatic lesion characteristics including the biopsy results. No major complications were observed in neither of the groups. As a minor complication, only one patient complained of slight abdominal pain in the EUS-FNA group.
Discussion
The salient findings of the present study were as follows: First, the technical was 100% in both groups. However, the diagnostic yield was significantly higher in the US-CNB group compared with the EUS-FNA group. Second, both biopsy procedures were safe, and no major complications were observed.
Currently, major oncology guidelines recommend tissue diagnosis in patients with a solid pancreatic lesion if the patient was considered to have an unresectable solid mass before administration of therapy. Besides, they suggest EUS-FNA as the primary means of tissue sampling from the pancreas and US-CNB as only an adjunct modality in case of technical failure or inadequate tissue sampling. The primary concern of guideline bodies is the risk of seeding of the pancreatic tumor by US-CNB [1, 14]. Huang et al., in their systematic review of studies of US-CNB reported that seeding was not reported in the studies they examined [5]. However, Bhatti and colleagues retrospectively evaluated 153 CT-guided percutaneous pancreas biopsies in patients in palliative care. The authors, though did not provide actual numbers, concluded that there was no evidence of seeding in our cohort [5, 6]. All of the patients in our study had a pre-diagnosis of pancreatic malignancy based on clinical findings, imaging studies, and tumor markers. Patients with chronic pancreatitis also had a predisposing factor for chronic pancreatitis along with imaging features compatible with chronic background pancreatitis. Hence, we did not perform US-CNB in patients with a potentially resectable pancreatic tumor with concerns of seeding.
In a meta-analysis Huang et al. [5] and reported the sensitivity and specificity of percutaneous US-CNB as 94.4% and 97.9%, respectively. The negative predictive value of the procedure was 76.3%. The procedure-related complication rate was 2.08%. However, data with respect to the direct comparison of percutaneous CNB and EUS-FNA for the diagnosis of solid pancreatic lesions are much scarcer. Sur and colleagues compared the diagnostic yield of percutaneous US-CNB and EUS-FNA in patients with solid pancreatic lesions [7]. The accuracy, technical failure rate, sensitivity, and specificity were found to be similar for both techniques. The sensitivity and specificity were 87.1% and 100%, respectively, for the US-CNB. The similar technical failure rate coupled with less diagnostic yield reflects most likely the fact that larger tissue samples could be obtained by CNB compared with EUS-FNA. The results of the present study actually confirmed the results of the study by Sur et al. in terms of diagnostic yield. Only 3.3% of the biopsy attempts resulted in inadequate sampling in patients who underwent US-CNB.
In some patients with a solid pancreatic lesion, it might be difficult to distinguish inflammatory reaction from true neoplasm with fine-needle aspiration. Moreover, diagnosis of neuroendocrine tumors, which usually necessitates the use of immunohistochemical examination, might be difficult solely based on FNA cytology [16, 17]. The presence of an on-site cytopathologist was supposed as a requirement for better results with EUS-FNA [18, 19]; however, a recent prospective study and a study with a modified biopsy technique reported successful results even in the absence of a readily available cytopathologist during the performance of the EUS-FNA [20, 21]. In our study, we did not have an on-site cytopathologist readily available during biopsy procedure. Nevertheless, the diagnostic yield was close to 80% for patients who underwent EUS-FNA.
One of the potential limitations of the EUS-FNA is the relative difficulty of biopsying the solid pancreatic lesions located in the pancreatic body, uncinate process, and tail [2]. However, in this study, among cases with inadequate sampling who underwent EUS-FNA, most of the lesions were localized in the head of the pancreas (63.6%). The technical failure rate both for percutaneous US-CNB and EUS-FNA was zero.
Percutaneous conventional fine needle biopsy provides a small amount of target organ tissue, causing a low negative predictive value [22]. Accuracy has been reported to be much higher in pancreas tru-cut biopsies compared to fine needle biopsies [23]. Another important advantage of the ultrasound-guided techniques is the capability of showing vascular structures in real-time [22].
Biopsy of pancreatic lesions via EUS-FNA is not without adverse events. Particularly, it is known that EUS-FNA of pancreatic lesions is associated with higher complication rates compared to EUS-FNA of other organs [24]. In a systematic review including 51 studies and more than 10 thousand patients who underwent EUS-FNA for a solid pancreatic lesion, 0.98% of all patients developed procedure-related pancreatitis, abdominal/chest pain, bleeding, or infection. The mortality rate attributable to EUS-FNA was 0.02%. Most of the studies reported in this analysis used 22G biopsy needles. Nevertheless, the median major complication rate was found to be 2.08% among 13 studies that used US-CNB to biopsy solid pancreatic lesions [5]. In the present study, none of the patients in either group experienced a major complication.
Several limitations of the present study are worthy of mention. First, this was a retrospective study with a relatively small sample size. Secondly, it was not possible to obtain tissue diagnosis from another route, such as a biopsy of a metastatic lesion. Thus, accuracy, sensitivity, or specificity for neither of the biopsy procedures could not be reported. However, it was made a head-to-head comparison of EUS-FNA and US-CNB in solid pancreatic lesions. The mean size of the lesions and frequency of location in a different part of the pancreas were similar in both groups, which made the comparison more straightforward. In a similar way, the rates of pancreatic malignancy and chronic pancreatitis were also comparable in both groups. In the present study, patient follow-up was not included. Therefore, the histopathological diagnoses was not clinically confirmed.
In conclusion, it was demonstrated that both techniques were safe and technically successful; however, US-CNB had a higher diagnostic yield.
Data Availability
We added data of the present study with a link*. *https://figshare.com/projects/Diagnostic_Evaluation_of_Solid_Pancreatic_Lesions_Endoscopic_Ultrasound-Guided_Fine_Needle_Aspiration_versus_Ultrasound-Guided_Core_Needle_Biopsy/128942.
References
Ducreux M, Cuhna AS, Caramella C, Hollebecque A, Burtin P, Goere D, et al. Cancer of the pancreas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v56-68.
Tokar JL, Walia R. Diagnostic evaluation of solid pancreatic masses. Curr Gastroenterol Rep. 2013;15(10):347.
Guarneri G, Gasparini G, Crippa S, Andreasi V, Falconi M. Diagnostic strategy with a solid pancreatic mass. Presse Med. 2019;48(3 Pt 2):e125–45.
Chen PT, Liu KL, Cheng TY, Chang CC, Chang YC. Indirect percutaneous core needle biopsy of solid pancreatic or peripancreatic lesions. Abdom Radiol (NY). 2019;44(1):292–303.
Huang Y, Shi J, Chen YY, Li K. Ultrasound-guided percutaneous core needle biopsy for the diagnosis of pancreatic disease. Ultrasound Med Biol. 2018;44(6):1145–54.
Bhatti I, Ojo D, Dennison AR, Rees Y, Elabassy M, Garcea G. Percutaneous pancreatic biopsies-still an effective method for histologic confirmation of malignancy. Surg Laparosc Endosc Percutan Tech. 2016;26(4):334–7.
Sur YK, Kim YC, Kim JK, Lee JH, Yoo BM, Kim YB. Comparison of ultrasound-guided core needle biopsy and endoscopic ultrasound-guided fine-needle aspiration for solid pancreatic lesions. J Ultrasound Med. 2015;34(12):2163–9.
Schoellnast H, Komatz G, Bisail H, Talakic E, Fauster M, Ehammer T, et al. CT-guided biopsy of lesions of the lung, liver, pancreas or of enlarged lymph nodes: value of additional fine needle aspiration (FNA) to core needle biopsy (CNB) in an offsite pathologist setting. Acad Radiol. 2010;17(10):1275–81.
Micames C, Jowell PS, White R, Paulson E, Nelson R, Morse M, et al. Lower frequency of peritoneal carcinomatosis in patients with pancreatic cancer diagnosed by EUS-guided FNA vs. percutaneous FNA. Gastrointest Endosc. 2003;58(5):690–5.
Tempero MA, Malafa MP, Al-Hawary M, Behrman SW, Benson AB, Cardin DB, et al. Pancreatic adenocarcinoma, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2021;19(4):439–57.
Kahriman G, Ozcan N, Dogan S, Ozmen S, Deniz K. Percutaneous ultrasound-guided core needle biopsy of solid pancreatic masses: Results in 250 patients. J Clin Ultrasound. 2016;44(8):470–3.
Yang RY, Ng D, Jaskolka JD, Rogalla P, Sreeharsha B. Evaluation of percutaneous ultrasound-guided biopsies of solid mass lesions of the pancreas: a center’s 10-year experience. Clin Imaging. 2015;39(1):62–5.
Mallery JS, Centeno BA, Hahn PF, Chang Y, Warshaw AL, Brugge WR. Pancreatic tissue sampling guided by EUS, CT/US, and surgery: a comparison of sensitivity and specificity. Gastrointest Endosc. 2002;56(2):218–24.
Tempero MA, Malafa MP, Al-Hawary M, Asbun H, Bain A, Behrman SW, et al. Pancreatic adenocarcinoma, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2017;15(8):1028–61.
Gupta S, Wallace MJ, Cardella JF, Kundu S, Miller DL, Rose SC, et al. Quality improvement guidelines for percutaneous needle biopsy. J Vasc Interv Radiol. 2010;21(7):969–75.
Kanno A, Ishida K, Hamada S, Fujishima F, Unno J, Kume K, et al. Diagnosis of autoimmune pancreatitis by EUS-FNA by using a 22-gauge needle based on the International Consensus Diagnostic Criteria. Gastrointest Endosc. 2012;76(3):594–602.
Bhatia V, Varadarajulu S. Endoscopic ultrasonography-guided tissue acquisition: how to achieve excellence. Dig Endosc. 2017;29(4):417–30.
Ecka RS, Sharma M. Rapid on-site evaluation of EUS-FNA by cytopathologist: an experience of a tertiary hospital. Diagn Cytopathol. 2013;41(12):1075–80.
Roy A, Kim M, Hawes R, Varadarajulu S. Changing trends in tissue acquisition in malignant pancreatic neoplasms. J Gastroenterol Hepatol. 2016;31(2):501–5.
Kanata R, Sasaki T, Matsuyama M, Ishigaki K, Yamada I, Ozaka M, et al. Prospective study of EUS-guided tissue acquisition with a 20G core biopsy needle with a forward bevel for solid pancreatic mass. Medicine (Baltimore). 2021;100(2):e24193.
Chen YI, Chatterjee A, Berger R, Kanber Y, Wyse JM, Lam E, et al. EUS-guided fine needle biopsy alone vs. EUS-guided fine needle aspiration with rapid on-site evaluation of cytopathology in pancreatic lesions: a multicenter randomized trial. Endoscopy. 2021;54:4–12.
Xin Y, Yang Y, Chen Y, Wang Y, Cao XJ, Zhou X. Safety and efficacy of ultrasound-guided percutaneous coaxial core biopsy of pancreatic lesions: a retrospective study. J Ultrasound. 2021;24(3):269–77.
Stella SF, Van Borsel M, Markose G, Nair SB. Image-guided percutaneous biopsy for pancreatic lesions: 10-year experience in a tertiary cancer center. Can Assoc Radiol J. 2019;70(2):199–203.
Wang KX, Ben QW, Jin ZD, Du YQ, Zou DW, Liao Z, et al. Assessment of morbidity and mortality associated with EUS-guided FNA: a systematic review. Gastrointest Endosc. 2011;73(2):283–90.
Funding
O.A. was partially supported by the NIH/NCI Cancer Center Support Grant P30 CA008748.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Caymaz, I., Afandiyeva, N. Diagnostic Evaluation of Solid Pancreatic Lesions: Endoscopic Ultrasound-Guided Fine Needle Aspiration Versus Percutaneous Ultrasound-Guided Core Needle Biopsy. Cardiovasc Intervent Radiol 46, 1596–1602 (2023). https://doi.org/10.1007/s00270-023-03494-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-023-03494-y