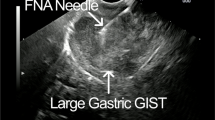
Abstract
Objectives
To evaluate the diagnostic accuracy and complication rate of percutaneous ultrasound-guided fine-needle aspiration (US-FNA) of solid pancreatic neoplasms through the analysis of 10-year experiences of two centres.
Methods
Clinical, radiological and pathologic data of 2,024 patients with solid pancreatic masses who underwent US-FNAs were retrospectively evaluated. Indications for aspiration were: unresectable lesions before neo-adjuvant therapy; doubtful imaging findings; and suspicion of uncommon neoplasms with prognostic or therapeutic implications such as metastases or lymphoma. US-FNAs were performed using aspiration needles with a cytopathologist present in centre 1. In centre 2, cytologic samples were collected with Chiba needles and separately evaluated by a cytopathologist.
Results
US-FNA had a diagnostic sample rate of 92.2 % (centre 1: 95.9 %; centre 2: 87.2 %). US-FNA repetition after non-diagnostic samples provided a diagnosis in 86.3 % of cases. Sensitivity, specificity, positive and negative predictive values, and accuracy were 98.7 %, 100 %, 100 %, 75.5 %, and 98.7 %, respectively. The complication rate was 0.8 %.
Conclusions
Percutaneous US-FNA is a sensitive, accurate and safe method for the invasive diagnosis of solid pancreatic neoplasms. The use of aspiration needles and the on-site presence of a cytopathologist may lead to a high rate of diagnostic samples, thus reducing the need for US-FNA repetition.
Key Points
• Percutaneous ultrasound-guided fine-needle aspiration of pancreatic neoplasms is sensitive and accurate.
• The short-term complication rate of percutaneous ultrasound-guided fine-needle aspiration is low.
• Technical aspects may influence the rate of diagnostic samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fine-needle aspiration (FNA) is widely used as an invasive diagnostic technique for solid pancreatic masses. Radiological guidance is required in most cases. Many imaging modalities can be chosen: ultrasound, either trans-abdominal (US) or endoscopic (EUS), computed tomography (CT), or magnetic resonance imaging (MRI). Different factors may influence this choice: availability, safety, and, last but not least, local expertise. Ultrasound provides real-time guidance, and is widely available and biologically safe. Previously published papers have reported high sensitivity and accuracy values for percutaneous US-FNA, with low complication rates [1, 2]. Percutaneous FNA can be also performed under CT guidance, but this technique has some drawbacks, such as the lack of real-time visualization and the radiation dose. EUS guidance can be also used for FNA; however, it is not available in all institutions, is more expensive than percutaneous FNA [2], and must be performed at least under deep sedation. No significant differences in terms of diagnostic accuracy have been reported between the endoscopic and percutaneous approaches for the diagnosis of solid pancreatic malignant masses [3]. Percutaneous FNA and EUS-FNA have similar relatively low complication rates, ranging between 0 % and 5 % [1–8].
The aim of this paper is to evaluate the diagnostic value and rate of complications for percutaneous US-FNA of solid pancreatic neoplasms over a 10-year experience at two high-volume centres for pancreatic diseases, in which US-FNA was used as the first invasive diagnostic tool.
Materials and methods
Patient population
Institutional review board approval was not required for this retrospective study.
Clinical, radiological and pathological data for all patients who underwent percutaneous US-FNA of solid pancreatic masses over a 10-year period in two centres were retrospectively evaluated. Inclusion periods were January 2004 through September 2014 for centre 1 and January 2002 through December 2012 for centre 2.
For each patient, the following features were reviewed and analysed: lesion size and location; number of needle passes; number of smeared slides; cytologic report; histological diagnosis after surgical resection or clinical/radiological follow-up findings; and short-term complications after FNA (≤7 days after the procedure, or until discharge if this occurred before day 7).
According to the literature [9, 10], indications for FNA included a non-resectable (metastatic or locally advanced) solid pancreatic neoplasm before neo-adjuvant therapies; inconclusive or controversial findings at previous imaging examinations; and suspicion of an uncommon neoplasm that could influence the prognosis (for example, metastases) or that could be better managed with medical therapy than with surgical resection (for example, lymphoma).
All lesions had been evaluated with contrast-enhanced CT or MRI and contrast-enhanced US before FNA. Non-steroidal anti-inflammatory drugs and/or antiaggregants were discontinued 1 week before FNA; patients treated with antiaggregant therapy were treated with low-molecular weight heparin, which was discontinued 12 hours before FNA and resumed 12 hours after the procedure. Exclusion criteria for US-FNA were a platelet count <50,000/mL or other coagulation disorders (APTT or PT ratio values >1.5). All patients provided written informed consent before the procedure.
Sampling technique and specimen preparation
Previous imaging examinations were thoroughly reviewed before FNA in order to identify the best site for sampling, avoiding necrotic areas; for the same reason, all lesions were evaluated with B-mode US immediately before the procedure. Peripancreatic vessels were examined with colour Doppler US in order to choose the safest percutaneous approach.
Prior to the sample, routine sterile preparation of the abdominal wall was performed, and 10 ml of local anaesthetic (lidocaine 2 %) was injected at the chosen entry point.
In centre 1, US-FNAs were performed by a radiologist (MD or EM, with 10 and 12 years of experience in interventional US-guided procedures, respectively) using a Sequoia 512 system (Acuson/Siemens Healthcare, Mountain View, CA, USA). Convex multi-frequency probes with a lateral guidance kit and 20G or 21G modified Menghini-type aspiration needles were used. An experienced cytopathologist (FB or EM, with 30 and 15 years of experience in pancreatic diseases, respectively) attended each procedure, and immediately smeared the cytologic sample on a glass slide, fixed it with 95 % alcohol and stained the sample using the modified Papanicolaou method. Cytologic samples were then immediately evaluated by the same cytopathologist, providing an assessment of sample adequacy and a same-session cytologic diagnosis.
All procedures in centre 2 were performed by a radiologist (EB, with 30 years of experience) using an Aplio system (Aplio 80 or Aplio XG; Toshiba Medical Systems, Tokyo, Japan) and 20G Chiba needles. The samples were immediately smeared on glass slides and fixed with 95 % alcohol by the radiologist. Cytologic samples were then stained and separately evaluated by a cytopathologist (SG, with 10 years of experience), who did not attend the procedures.
Evaluation of FNA/follow-up findings and data analysis
Mass size was measured as the largest of all reported measurements, and is presented in millimetres (mm). The number of needle passes for each procedure was evaluated and recorded.
Samples were considered “diagnostic” when a formal pathological report was made; they were considered “non-diagnostic” if the collected sample was deemed inadequate for a proper cytologic analysis.
FNA diagnosis was considered true positive if malignant features or those strongly suggestive of malignancy (“atypia” or “suspicious for malignancy”) were present: these were pooled and considered as positive, given their high associated risk of malignancy (79–96 %), similar to procedures described by Layfield et al. [11].
FNA was considered false positive if positive for malignancy but clinical setting or imaging findings suggested the benignity of the lesion.
Since false-negative FNA results have been previously reported [1–7], all patients with negative samples were followed up with clinical and radiological (CT and/or MRI) evaluations. FNA diagnosis was then considered true negative if no malignant features were found at cytologic analysis, without clinical or radiological evidences of malignancy at follow-up. FNA samples were considered false negative if the cytologic analysis was negative but clinical/imaging follow-up findings suggested malignancy.
Sensitivity, specificity, positive and negative predictive values, and accuracy of US-FNA were evaluated overall and separately for the centres.
Statistical analysis was performed using SPSS Statistics, version 21 software (IBM Corp, Armonk, NY, USA).
Results
Two thousand and twenty-four patients (1,106 men; 918 women) were included in this study: 1,170 (57.8 %) from centre 1 and 854 (42.2 %) from centre 2.
The mean lesion size was 42 mm (range 10–150 mm); lesions were located primarily in the head or uncinate process of the pancreas (64.9 %).
The mean number of needle passes was 1.1 (range, 1–4). A mean of 8 slides per patient were obtained (range, 1–23).
Details on patient population are reported in Table 1.
Sample analysis
FNA diagnoses are reported in Table 2. Overall, US-FNA provided diagnostic samples in most cases (1867/2024, 92.2 %); centre 1 had a higher rate of diagnostic samples (95.9 %) than centre 2 (87.2 %).
Forty-two patients were lost to follow-up after a non-diagnostic sample was obtained. A final diagnosis was obtained in most (88.7 %) of the remaining patients (115) with previously non-diagnostic samples. There were differences between centres regarding the modality of re-evaluation of patients with non-diagnostic samples: US-FNA was repeated in a second session in two cases in centre 1 and in 93 cases in centre 2, and provided a final diagnosis in 86.3 % of cases, while 13 samples were still non-diagnostic.
Among patients with diagnostic samples, most had a diagnosis of malignancy (1767/1867). In most cases the diagnosis was of ductal adenocarcinoma (1584/1767, 89.6 %), while other pancreatic neoplasms, such as neuroendocrine tumours and rare neoplasms (i.e., metastases, lymphoma, solid pseudopapillary tumors) were less common (4.7 % and 3.6 %, respectively). Thirty-six patients (2.1 %) had a diagnosis of “atypia” or “suspicious for malignancy.” As previously stated, these diagnoses were pooled and considered positive.
False/true positive and negative results of US-FNA are presented in Table 3.
Overall, 100 negative samples (47 in centre 1 and 53 in centre 2) were found. Two patients with negative samples were lost to follow-up at centre 1, and were therefore excluded from this analysis. In centre 1, absence of malignancy was confirmed at surgery in 4/40 cases (focal areas of fibrosis in a setting of chronic pancreatitis); the remaining 36 patients had clinical and radiological follow-up findings (median follow-up period 15 months) consistent with non-malignant lesions. Five samples in centre 1 were found to be US-FNA false-negative: in four cases, the final diagnosis was of ductal adenocarcinoma, established by disease progression at follow-up (three cases) or with surgical biopsy (one case). One patient had a diagnosis of neuroendocrine tumour at surgery.
Thirty-four of 53 negative samples in centre 2 were confirmed as true negative at follow-up (median length 17 months), while 19/53 were found to be false negative, since patients presented disease progression at follow-up.
There were no false-positive FNA results.
Diagnostic value analysis
Considering all non-diagnostic procedures (157) as false negative, percutaneous US-FNA demonstrated overall sensitivity and accuracy of 90.7 % [95 % confidence interval (CI), 89.3–91.9 %] and 91 % (95 % CI, 89.6–91.9 %), respectively. Excluding non-diagnostic samples, percutaneous US-FNA showed 98.7 % sensitivity (95 % CI, 97.9–99.1 %), 100 % specificity and positive predictive value (95 % CI, 93.8–100 % and 99.7–100 %), 75.5 % negative predictive value (95 % CI, 65.5–83.3 %), and 98.7 % accuracy (95 % CI, 97.9–99.1 %). Details are reported in Table 4.
Evaluation of complications
In 2007 of the 2024 cases (99.2 %), the procedure was uneventful. Imaging examinations (US or CT) were performed in all patients with suspicion of post-procedural complications. No major complications occurred. In five patients, abdominal effusion was observed immediately after the procedure, but follow-up evaluation excluded severe complications. Twelve patients experienced post-procedural pain after US-FNA that required administration of analgesic drugs, without relevant findings at subsequent imaging.
Discussion
Data derived from the largest published series reveal that percutaneous FNA of pancreatic masses reveal diagnostic accuracy of between 75 and 99.4 % [1, 2, 4–7] (Table 5). Considering US guidance alone, accuracy of between 91 and 99.4 % has been reported, while sensitivity ranges between 81 and 99.4 % [1, 2, 6, 7]. The results of the present study therefore compare favourably with literature data in terms of sensitivity and diagnostic accuracy (98.7 %), but are based on a larger number of cases.
The many published papers dealing with pancreatic FNA have shown a variable rate of inadequate samples [1, 2, 12, 13], which surpassed 15 % for the percutaneous approach [2, 4–7]. In the present series, differences in the diagnostic sample rates were found between the centres involved in this study , with rates higher in centre 1 than in centre 2 (95.9 % versus 87.2 %, respectively).
Although this paper does not specifically aim to compare different sampling techniques, the higher diagnostic sample rate and the larger number of smeared slides reported in centre 1 could be explained by differences in the collection and evaluation of cytologic samples: the on-site presence of a cytopathologist in centre 1 allowed an immediate evaluation of the cytologic sample, thus enabling a re-sample during the same session. Moreover, Menghini-modified needles work with a suction/aspiration modality, while Chiba needles collect cells through capillarity. Previous studies have reported the superiority of aspiration needles, especially for lesions with low cellular density [14, 15]. With regard to pancreatic masses specifically, Hopper et al. [16] reported a clearly poorer performance of the capillary method sampling with regard to the amount of cellular material obtained, degree of cellular trauma, and overall diagnostic value.
There were differences between the centres involved in this study with regard to the modality of re-evaluation in patients with non-diagnostic samples: excluding 42 patients who were lost to follow-up after non-diagnostic samples were obtained (26 in centre 1 and 16 in centre 2), most patients in centre 1 were referred to surgery (12/22) or EUS-FNA (5/22), while in centre 2 US-FNA was repeated in all cases. Among a total of 95 patients between the two centres who underwent repeated US-FNA, a final diagnosis was obtained in most cases (82/95, 86.3 %). Even after repeated US-FNAs, 13 patients had non-adequate samples, which may have been attributable to the presence of extremely desmoplastic and paucicellular lesions [17].
False-negative results of percutaneous FNA have been reported in the literature [1, 2, 4–7]. In the present series, the overall rate of negative samples was 5.4 %. Excluding two negative samples of patients who were lost to follow-up after a negative FNA, five false-negative results were found in centre 1: malignancy was found at surgery in two cases, and during follow-up in the remaining three. False-negative results were demonstrated in 19 cases in centre 2: malignancy was established by the onset of disease progression at clinical/radiological follow-up. Negative results of pancreatic US-FNA, therefore, should be viewed with caution, and radiological and clinical findings should be always taken into account during cytologic examination. In this regard, a study by Spier et al. [18] suggested that in patients with a definite solid lesion with imaging features suspicious for malignancy such as invasion of the adjacent vessels or the presence of peripancreatic lymph nodes—despite negative FNA—the FNA result should be considered as false negative FNA, and the patients followed closely, with an early repeat attempt at tissue acquisition and repeat imaging, or strongly considered for direct referral for an intra-operative sample. Moreover, these authors reported that in the majority of patients with a false-negative FNA who were diagnosed with cancer, the diagnosis was made within 90 days after FNA. Therefore, patients who have cancer (the false negatives) will almost always be diagnosed with that malignancy within 90 days of the FNA, and will tend to have imaging characteristics of a malignancy present despite the negative FNA. Beyond 3 months post-FNA, if a patient has not been diagnosed with cancer despite clinical suspicion or imaging findings, it is unlikely that the patient has an underlying malignancy that was missed at the time of FNA. Thus, after a negative FNA in patients who do not have suspicious imaging findings, it is reasonable to perform one or two follow-up CT/MRI examinations in the first 3–6 months after a negative FNA, but longer-term surveillance is rarely needed [18].
Short-term complications following percutaneous US-FNA have been reported at rates of 0 % to 4.9 % [2, 6, 7]; our findings are in line with these data (overall rate of complications of 0.8 %). Long-term complications were not evaluated in this study, largely because most patients were not available for long-term follow-up, having returned to other institutions for further, often palliative, treatment. Tumour seeding has been described as a possible long-term complication of percutaneous FNA [19, 20]. It must be noted, however, that Johnson et al. [21] found no difference in median survival between patients with pancreatic cancer who underwent percutaneous FNA and those who did not; Hernandez et al. strengthened these findings in their 1,406-patient review [22]. Moreover, the small number of cases of peritoneal seeding after FNA published thus far suggests that this complication is more common after EUS-FNA, especially when the sample is performed on cystic lesions [23–25]. It must be also noted that a reliable distinction between peritoneal nodules secondary to disease progression and those induced by peritoneal seeding following FNA can be hard to achieve, even at laparotomy [26].
Pancreatic FNA can be also performed endoscopically. The largest published meta-analyses of EUS-FNA in solid pancreatic lesions showed sensitivity and specificity values of 83–92 % and 94–100 %, respectively [10, 12, 27, 28], although the false-negative rate of EUS-FNA can reach as high as 12 % [28–33]. Moreover, a randomized controlled trial by Horwhat et al. [3] comparing EUS-FNA to CT-/US-FNA revealed no significant differences between the endoscopic and percutaneous approaches for the diagnosis of malignant pancreatic lesions. Given these results in terms of diagnostic accuracy, comparable low complication rates, and lower cost, it can be assumed that US-FNA, when performed by experienced operators, should be considered as the first diagnostic step for the invasive characterization of unresectable solid pancreatic lesions.
An alternative to FNA is biopsy, which is performed with larger needles that collect enough tissue for histological examination. Several authors have evaluated the sensitivity and accuracy of pancreatic biopsy. The largest published series [33–38] reported sensitivity and accuracy of 86 –99.2 % and 86 –93 %, respectively, therefore comparable to those of FNA. It must be otherwise noted that the complication rate of biopsy reported in these studies was higher than that of FNA (3.3–21 %). Yang et al. [39] reported similar diagnostic values for core biopsy, FNA, and core biopsy + FNA of pancreatic lesions, with sensitivity of 92.6 %, 92.3 %, and 92.3 %, and accuracy of 93.3 %, 92.3 %, and 93.3 %, respectively. Interestingly, four of the five non-diagnostic samples in this study were core biopsies, and the remaining case was FNA performed without the presence of a cytologist. Most complications developed after core biopsies. Pancreatic FNA, therefore, seems to have rates of sensitivity and accuracy similar to those of biopsy, but lower complication rates.
This study has several limitations. First, as this series was collected in two highly specialized centres for pancreatic diseases, local expertise might have contributed to favourable results.
Moreover, differences between the centres in modality of sample collection and evaluation may have contributed to better diagnostic results in centre 1 than centre 2.
In addition, the short-term follow-up period derived from the retrospective nature of this study did not allow the evaluation of long-term complications, such as tumoral seeding. However, most patients had undergone US-FNA for a metastatic or locally advanced disease, with short life expectancy: the presence of tumour seeding would therefore have likely had minimal impact on their survival.
Conclusions
Percutaneous US-FNA is a sensitive, accurate and safe procedure for the diagnosis and management of solid pancreatic neoplasms. The present data support the use of percutaneous US-FNA over EUS-FNA or biopsy as the first tool for the invasive characterization of unresectable solid pancreatic lesions. The on-site presence of a cytologist and the use osf aspiration needles may lead to a high acquisition rate of diagnostic-quality samples, thus reducing the need for repetition of FNA.
References
Di Stasi M, Lencioni R, Solmi L et al (1998) Ultrasound-guided fine needle biopsy of pancreatic masses: results of a multicenter study. Am J Gastroenterol 93:1329–1333
Zamboni GA, D’Onofrio M, Idili A et al (2009) Ultrasound-guided percutaneous fine needle aspiration of 545 focal pancreatic lesions. AJR Am J Roentgenol 193:1691–1695
Horwhat JD, Paulson EK, McGrath K et al (2006) A randomized comparison of EUS-guided FNA versus CT or US-guided FNA for the evaluation of pancreatic mass lesions. Gastrointest Endosc 63:966–975
David O, Green L, Reddy V et al (1998) Pancreatic masses: a multi-institutional study of 364 fine-needle aspiration biopsies with histopathologic correlation. Diagn Cytopathol 19:423–427
Linder S, Blasjo M, Sundelin P, von Rosen A (1997) Aspects of percutaneous fine-needle aspiration biopsy in the diagnosis of pancreatic carcinoma. Am J Surg 174:303–306
Bhatia P, Srinivasan R, Rajwanshi A et al (2008) 5-year review and reappraisal of ultrasound-guided percutaneous transabdominal fine needle aspiration of pancreatic lesions. Acta Cytol 52:523–529
Garre Sanchez MC, Rendon Unceta P, Lopez Cano A et al (2007) Ultrasound-guided biopsy of the pancreas: a multicenter study. Rev Esp Enferm Dig 99:520–524
Affolter KE, Schmidt RL, Matynia AP, Adler DG, Factor RE (2013) Needle size has only a limited effect on outcomes in EUS-guided fine needle aspiration: a systematic review and meta-analysis. Dig Dis Sci 58:1026–1034
Brugge W (2004) Pancreatic fine needle aspiration: to do or not to do? JOP 5:282–288
Hartwig W, Schneider L, Diener MK, Bergmann F, Buchler MW, Werner J (2009) Preoperative tissue diagnosis for tumours of the pancreas. Br J Surg 96:5–20
Layfield LJ, Schmidt RL, Hirschowitz SL, Olson MT, Ali SZ, Dodd LL (2014) Significance of the diagnostic categories “atypical” and “suspicious for malignancy” in the cytologic diagnosis of solid pancreatic masses. Diagn Cytopathol 42:292–296
Hébert-Magee S, Bae S, Varadarajulu S et al (2013) The presence of a cytopathologist increases the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration cytology for pancreatic adenocarcinoma: a meta-analysis. Cytopathology 24:159–171
DelMaschio A, Vanzulli A, Sironi R et al (1991) Pancreatic cancer versus chronic pancreatitis: diagnosis with CA 19-9 assessment, US, CT, and CT-guided fine-needle biopsy. Radiology 178:95–99
Zhou JQ, Zhang JW, Zhan WW et al (2014) Comparison of fine-needle aspiration and fine-needle capillary sampling of thyroid nodules: a prospective study with emphasis on the influence of nodule size. Cancer Cytopathol 122:266–273
Hopper KD, Grenko RT, Fisher AI, TenHave TR (1996) Capillary versus aspiration biopsy: effect of needle size and length on the cytopathologic specimen quality. Cardiovasc Intervent Radiol 19:341–344
Hopper KD, Abendroth CS, Sturtz KW, Matthews YL, Shirk SJ (1992) Fine-needle aspiration biopsy for cytopathologic analysis: utility of syringe handles, automated guns, and the nonsuction method. Radiology 185:819–824
Collins BT, Murad FM, Wang JF, Bernadt CT (2013) Rapid on-site evaluation for endoscopic ultrasound-guided fine-needle biopsy of the pancreas decreases the incidence of repeat biopsy procedures. Cancer Cytopathol 121:518–523
Spier BJ, Johnson EA, Gopal DV et al (2009) Predictors of malignancy and recommended follow-up for patients with negative endoscopic ultrasound-guided fine-needle aspiration of suspected pancreatic lesions. Can J Gastroenterol 34:279–286
Caturelli E, Rapaccini GL, Anti M, Fabiano A, Fedeli G (1985) Malignant seeding after fine-needle aspiration biopsy of the pancreas. Diagn Imaging Clin Med 54:88–91
Frolich E, Fruhmorgen P, Seeliger H (1986) Cutaneous implantation metastasis after fine needle puncture of a pancreatic cancer. Ultraschall Med 7:141–144
Johnson DE, Pendurthi TK, Balshem AM et al (1997) Implications of fine-needle aspiration in patients with resectable pancreatic cancer. Am Surg 63:675–679
Hernandez LV, Bhutani MS, Eisner M et al (2009) Non-surgical tissue biopsy among patients with advanced pancreatic cancer: effect on survival. Pancreas 38:289–292
Katanuma A, Maguchi H, Hashigo S et al (2012) Tumor seeding after endoscopic ultrasound-guided fine-needle aspiration of cancer in the body of the pancreas. Endoscopy 44:UCTN:E160–E161
Chong A, Venugopal K, Segarajasingam D, Lisewski D (2011) Tumor seeding after EUS-guided FNA of pancreatic tail neoplasia. Gastrointest Endosc 74:933–935
Hirooka Y, Goto H, Itoh A et al (2003) Case of intraductal papillary mucinous tumor in which endosonography-guided fine-needle aspiration biopsy caused dissemination. J Gastroenterol Hepatol 18:1323–1324
Yoon WJ, Daglilar ES, Fernandez-del Castillo C, Mino-Kenudson M, Pitman MB, Brugge WR (2014) Peritoneal seeding in intraductal papillary mucinous neoplasm of the pancreas who underwent endoscopic ultrasound-guided fine-needle aspiration: the PIPE Study. Endoscopy 46:382–387
Puli SR, Bechtold ML, Buxbaum JL, Eloubeidi MA (2013) How good is endoscopic ultrasound-guided fine-needle aspiration in diagnosing the correct etiology for a solid pancreatic mass? A meta-analysis and systematic review. Pancreas 42:20–26
Hewitt MJ, McPhail MJ, Possamai L, Dhar A, Vlavianos P, Monahan JJ (2012) EUS-guided FNA for diagnosis of solid pancreatic neoplasms: a meta-analysis. Gastrointest Endosc 75:319–331
Abdelgawwad MS, Alston E, Eltoum IA (2013) The frequency and cancer risk associated with the atypical cytologic diagnostic category in endoscopic ultrasound-guided fine-needle aspiration specimens of solid pancreatic lesions: a meta-analysis and argument for a Bethesda System for Reporting Cytopathology of the Pancreas. Cancer Cytopathol 121:620–628
Siddiqui AA, Kowalski TE, Shahid H et al (2011) False-positive EUS-guided FNA cytology for solid pancreatic lesions. Gastrointest Endosc 74:535–540
Schwartz DA, Unni KK, Levy MJ, Clain JE, Wiersema MJ (2002) The rate of false-positive results with EUS-guided fine-needle aspiration. Gastrointest Endosc 56:868–872
Gleeson FC, Kipp BR, Caudill JL et al (2010) False positive endoscopic ultrasound fine needle aspiration cytology: incidence and risk factors. Gut 59:586–593
Woolf KM, Liang H, Sletten ZJ, Russell DK, Bonfiglio TA, Zhou Z (2013) False-negative rate of endoscopic ultrasound-guided fine-needle aspiration for pancreatic solid and cystic lesions with matched surgical resections as the gold standard: one institution’s experience. Cancer Cytopathol 121:449–458
Brandt KR, Charboneau JW, Stephens DH, Welch TJ, Goellner JR (1993) CT- and US-guided biopsy of the pancreas. Radiology 187:99–104
Matsubara J, Okusaka T, Morizane C, Ikeda M, Ueno H (2008) Ultrasound-guided percutaneous tumor biopsy in pancreatic cancer: a comparison with metastatic liver tumor biopsy, including sensitivity, specificity, and complications. J Gastroenterol 43:225–232
Xu K, Zhou L, Liang B et al (2012) Safety and accuracy of percutaneous core needle biopsy in examining pancreatic neoplasms. Pancreas 41:649–651
Amin Z, Theis B, Russell RC, House C, Novelli M, Lees WR (2006) Diagnosing pancreatic cancer: the role of percutaneous biopsy and CT. Clin Radiol 61:996–1002
Jennings PE, Donald JJ, Coral A, Rode J, Lees WR (1989) Ultrasound-guided core biopsy. Lancet 17;1:1369–1371
Yang RY, Ng D, Jaskolka JD, Rogalla P, Sreeharsha B (2014) Evaluation of percutaneous ultrasound-guided biopsies of solid mass lesions of the pancreas: a center’s 10-year experience. Clin Imaging. doi:10.1016/j.clinimag.2014.06.010
Acknowledgments
The scientific guarantor of this publication is Roberto Pozzi Mucelli. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. No complex statistical methods were necessary for this paper. Institutional review board approval was not required due to the retrospective nature of the study. Written informed consent was obtained from all subjects (patients) in this study. A total of 544 study subjects were previously reported in Zamboni GA et al., AJR 2009; 193:1691–1695.
Methodology: retrospective, observational, multicentre study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
D’Onofrio, M., De Robertis, R., Barbi, E. et al. Ultrasound-guided percutaneous fine-needle aspiration of solid pancreatic neoplasms: 10-year experience with more than 2,000 cases and a review of the literature. Eur Radiol 26, 1801–1807 (2016). https://doi.org/10.1007/s00330-015-4003-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-015-4003-x