Abstract
Purpose
The purpose of this study was to analyze the intrahepatic perfusion redistribution after embolization of hepatic arterial variants during percutaneous arterial port catheter placement as well as to investigate the treatment efficacy of intraarterial chemotherapy in perfusion redistribution-dependent compared to redistribution-independent liver areas.
Materials and Methods
This retrospective study included 62 patients (67.7% males, mean age of 56 ± 12 years). A replaced left hepatic artery was encountered in 36/62 (58.1%), a replaced right hepatic artery in 19/62 (30.6%) and a replaced left and right hepatic artery in 7/62 of patients (11.3%), respectively. Subjective perfusion analysis was performed on digital subtracted angiography and computed tomography (CT)/cone-beam computed tomography (CBCT) images evaluating the visibility of the main, segmental and subsegmental branches of the embolized variant hepatic artery, re-perfused from intrahepatic arterial anastomoses. For objective perfusion analysis ROI measurements on CT/CBCT images were taken in the redistribution-dependent and redistribution-independent liver lobe. Response analysis according to RECIST 1.1 was separately calculated for the redistribution-dependent and redistribution-independent liver lobe.
Results
Intrahepatic reperfusion of the embolized variant hepatic artery was observed immediately after embolization with visualization of the subsegmental branches in 95.2% of patients. ROI measurements on CT/CBCT images (right lobe mean 76 ± 30.2 HU, left lobe mean 74.4 ± 30.5, p-value 0.88) did not show any differences. Treatment response after intraarterial chemotherapy did not differ between the redistribution-dependent and redistribution-independent liver lobes.
Conclusion
Embolization of hepatic arterial variants during percutaneous arterial port catheter placement results in effective intrahepatic perfusion redistribution and does not compromise treatment efficacy of intraarterial chemotherapy in the redistribution-dependent liver lobe.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hepatic arterial infusion chemotherapy (HAIC) via a surgically or percutaneously placed catheter/port system is a technique that allows high-dose drug delivery to liver metastases. This technique has been extensively studied in colorectal liver metastases with promising results in terms of local response rates and overall survival [1,2,3]. It is used in currently ongoing clinical trials in France, in a neo-adjuvant setting to convert initially unresectable liver metastases to resectable disease [4], as a treatment alternative for patients with progressive disease after standard systemic chemotherapy [5] as well as in an adjuvant setting after curative liver surgery to reduce the risk of hepatic recurrences [6].
Besides surgical hepatic arterial infusion pumps, percutaneous placements have been described with better results in terms of catheter functionality at a comparable complication rate [7]. For both, a fundamental principle of HAIC is that the entire liver is supplied by a single artery to ensure perfusion of the entire liver through a single catheter. However, hepatic artery variants are found in about 25 to 45% of the population [8, 9]. During catheter placement, these additional arteries have to be ligated/embolized in order to have whole-liver perfusion via a single hepatic artery. This strategy requires efficient perfusion redistribution via intrahepatic collaterals to ensure that the applied chemotherapy reaches all metastases in a proportional fashion. The concept of intrahepatic perfusion redistribution has been studied in radioembolization as well as hepatic arterial infusion chemotherapy in several case series [10,11,12,13,14,15,16]. Response analysis in redistribution-dependent liver areas after embolization of the anatomic variants has only been investigated in few studies [17,18,19].
Hence, the purpose of this study was to analyze the intrahepatic perfusion redistribution after embolization of hepatic arterial variants during percutaneous arterial port catheter placement as well as to investigate the treatment efficacy in redistribution-dependent compared to redistribution-independent liver areas after intraarterial chemotherapy.
Materials and Methods
This retrospective study was approved by the institutional review board and ethics committee (IRB N° 2022–144). Written informed consent requirement was waived.
Patients
Between December 2003 and March 2020, a total of 359 patients underwent percutaneous arterial port catheter placement for intraarterial chemotherapy of liver metastases at a single tertiary referral center. Out of these 359 patients, 62 patients (17.3%) were found to have an anatomical variant of the hepatic arteries that represented the study population. Patients were predominantly male in 67.7% with a mean age of 56 ± 12 years (Table 1). Liver metastases were all histologically proven. Colorectal liver metastases were encountered in 59/62 patients (95.2%), gastroesophageal liver metastases in 2/62 patients (3.2%) and thymic cancer liver metastases in 1/62 patient (1.6%) (Table 1). All patients underwent angio-CT upfront intervention, and diagnosis of an anatomic variant was made before catheter placement. The treatment decision was made after multidisciplinary tumor board discussion. Most of the patients were enrolled in various prospective trials [5,6,7, 20].
Anatomical Variants
Anatomical variants were classified according to Michels et al. [9] (Fig. 1). Type 2 anatomy (replaced left hepatic artery arising from the left gastric artery) was encountered in 36/62 patients (58.1%) (Fig. 2), type 3 anatomy (replaced right hepatic artery arising from the superior mesenteric artery) in 19/62 patients (30.6%) and type 4 anatomy (replaced right hepatic artery arising from the superior mesenteric artery and replaced left hepatic artery arising from the left gastric artery) in 7/62 patients (11.3%) (Fig. 3).
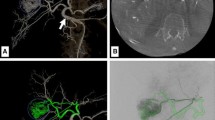
Percutaneous arterial port catheter placement in a 59-year-old patient with a variant hepatic artery anatomy type II according to Michels et al. [9] with a replaced left hepatic artery (Panel a white star). After coil embolization of the replaced left hepatic artery (Panel b white dotted arrow) immediate arterial redistribution via intrahepatic collaterals (Panel b white arrows) is noted on digital subtraction angiography. Panel c shows the CT scan after contrast medium injection via the port system with homogenous perfusion of the right and left liver. A highly arterialized liver metastasis is visualized in the redistribution-dependent liver segment II/III
Percutaneous arterial port catheter placement in a 49-year-old patient with a variant hepatic artery anatomy type IV according to Michels et al. [9] with a replaced right (Panel a white star), middle hepatic artery arising from the common hepatic artery (Panel b white star) and replaced left hepatic artery (Panel c white star). After coil embolization of the middle hepatic artery and replaced left hepatic artery (Panel d/e white dotted arrows) immediate arterial redistribution via intrahepatic collaterals (Panel d/e white arrow) is noted on digital subtracted angiography (Panel d) and CT arteriogram (Panel e). Panel f shows the CT scan after contrast medium injection via the port system with homogenous perfusion of the right and left liver and visualization of subsegmental arterial branches in both lobes
Catheter Placement
All interventions took place in a fully equipped angiography room under conscious sedation and local anesthesia (Xylocaine 1%; Astra Zeneca, Rueil-Malmaison, France). Percutaneous intraarterial catheters were either placed via the common femoral artery (59/62 patients, 95.2%), the thoraco-acromial artery (2/62 patients, 3.2%) or the brachial artery (1/62 patient, 1.6%). After retrograde puncture of the appropriate access artery, a 5F Cobra catheter (Cook, Bjaeverskov, Denmark) was directly introduced without using a sheath. Subsequently, digital subtraction angiography (DSA) of the coeliac trunk and the superior mesenteric artery was performed to assess the arterial supply of the liver. Different catheter placement strategies were used depending on the variant of hepatic arteries encountered. To avoid problems for future liver surgery, in case of major therapy response and eligibility for curative resection, all cases were discussed with hepatic surgeons beforehand. Placement of choice was the “fixed catheter tip technique” first described by Arai et al. [21]. For this technique the catheter tip is placed in the gastroduodenal artery and a side hole in the proper hepatic artery to allow perfusion of the liver via the catheter. Finally, the catheter is “fixed” by coil embolization of the gastroduodenal artery. If this was inconvenient for future surgery, the catheter was placed directly into the variant hepatic artery. According to the anatomy encountered, the variant hepatic arteries were subsequently embolized using 0.018-inch or 0.035-inch steel coils (Cook, Bjaeverskov, Denmark). All arteries supplying extrahepatic structures, such as the right gastric artery, were embolized using 0.018-inch or 0.035-inch steel coils (Cook, Bjaeverskov, Denmark) to avoid extrahepatic perfusion after catheter placement. Catheterization of the corresponding arteries was performed using a 5F Cobra catheter (Cook, Bjaeverskov, Denmark) or a 2.7F / 2.4F microcatheter (Progreat, Terumo, Tokyo, Japan). Coil embolization was performed proximally in the corresponding arteries. Finally, the placed catheter (5-F ST-305C catheter, B. Braun Medical, Center Valley, Pennsylvania) was connected to a subcutaneously implanted port system (CELSITE ST-305C, B. Braun Medical, Center Valley, Pennsylvania).
Perfusion Analysis
After successful catheter implantation, the port system was directly punctured using a 19-gauge Huber needle (Perouse, Ivry-le-Temple, France) and a DSA was performed in all patients (n = 62, 100%) using a standardized protocol of 10 ml of contrast medium injected at an injection rate of 1.5 ml/sec. The catheters were systematically opacified before each cycle of intraarterial chemotherapy. In addition, during the first control, a computed tomography scan (CT scan) or cone-beam computed tomography (CBCT) was performed in 51/62 patients (82.3%) with injection of the contrast medium directly through the port (20 ml of contrast medium at an injection rate of 1.5 ml/sec; scan delay of 10 s). Furthermore, single-photon emission computed tomography (SPECT) was performed in 20/62 patients (32.2%) with injection of 8 ml of technetium-99 macroaggregate albumin injected into the chamber to analyze tracer distribution in the liver and exclude extrahepatic perfusion.
Subjective Perfusion Analysis
Perfusion analysis was performed by an experimented interventional radiologist (5-year experience in intraarterial liver interventions) on postoperative DSA from the intraarterial catheter, already in place. Analysis of the vascularization of the liver, from the skeletonized, leftover hepatic artery was based on whether the main branch, segmental branches and subsegmental branches of the embolized variant hepatic artery were re-perfused from intrahepatic arterial anastomoses. Identical analysis was performed on CT/CBCT, when available.
Objective Perfusion Analysis
Objective perfusion analysis was performed using the CT/CBCT scan. Region of interest (ROI) measurements (20 mm of diameter) were taken in the left and right lobes of the liver in an area with normal liver parenchyma without tumor involvement (Fig. 4). Mean Hounsfield units (HU) and standard deviation were noted. Same ROI measurements were taken on SPECT images in the right and left lobe, respectively, obtaining gamma count measurements.
Objective perfusion analysis on a computed tomography image after contrast injection via the percutaneous arterial port catheter. Region of interest measurements (20 mm of diameter) were performed in the left and right lobe of the liver in an area with normal liver parenchyma without tumor involvement
Follow-up and Response Analysis
Follow-up consisted of clinical visits during every administration of intraarterial chemotherapy by the treating oncologists. Standard protocol of intraarterial chemotherapy consisted of drug administration every two weeks. Number of intraarterial chemotherapy courses administered per patient was noted. First re-staging was performed by contrast-enhanced CT 3 months after initiation of intraarterial chemotherapy. Treatment response was evaluated according to RECIST 1.1 criteria [22]. Image analysis was performed by two readers (5 years and 7 years of experience in abdominal radiology, respectively). In both lobes three target metastases were defined on pre-treatment CT images in consensus by the two readers. Response analysis according to RECIST 1.1 was calculated for the right and left liver lobe separately as well as for the whole liver using the mean from the measurements of the two readers. Response was classified as complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD).
Statistical Analysis
For descriptive data, mean values and standard deviations are provided. Continuous variables were compared using independent 2-sample t test; categorical variables were compared using χ2 test. Statistical significance was defined as p-value < 0.05. Statistical analyses were performed with a commercially available software (IBM, SPSS® Statistics v. 25, Chicago, IL).
Results
Intervention
Catheter placement was performed in 62 patients. The catheter was placed into the gastroduodenal artery in 52/62 patients (83.9%) using the “fixed catheter tip technique.” The replaced left hepatic artery or replaced right hepatic artery was used for catheter placement in 2/62 patients (3.2%) and 8/62 patients (12.9%), respectively. A replaced left, replaced right and proper hepatic artery were embolized in 41/62 patients (66.1%), 18/62 patients (29%) and 10/62 patients (16.1%), respectively. In total, 69 aberrant arteries were embolized which is due to the seven patients (7/62, 11.3%) with a type 4 anatomy.
Perfusion Analysis
Subjective Perfusion Analysis
Intrahepatic reperfusion of the embolized variant hepatic artery was observed immediately after embolization in 96.8% of patients for the main branch, 96.8% of patients for the segmental branches and 95.2% of patients for the subsegmental branches.
On CT/CBCT images, 98% of patients showed intrahepatic reperfusion of the main, segmental and subsegmental branches of the occluded variant hepatic artery (Table 2).
Objective Perfusion Analysis
ROI measurements on CT/CBCT images (right lobe mean 76 ± 30.2 HU, left lobe mean 74.4 ± 30.5, p-value 0.88) and scintigraphy images (right lobe mean 1721.7 ± 1959.9, left lobe mean 1523.4 ± 1584.1, p-value 0.86) did not show any difference between the right and left liver lobe (Table 2).
Response Analysis
Mean time to first evaluation was 3.5 ± 1.1 months. Re-staging CT at follow-up was available for all patients. A mean number of 4.5 ± 1.6 cycles of hepatic intraarterial chemotherapy was administered. For bilobar comparative response analysis, 13/62 patients (21%) had to be excluded as unilobar metastases were observed in 6/62 (9.6%) patients, 2/62 (3.2%) patients underwent hepatectomy before 3-month follow-up, 3/62 (4.9%) patients received hepatic intraarterial chemotherapy in an adjuvant setting after complete resection of all liver metastases, 1/62 (1.6%) catheter was not used for intraarterial chemotherapy due to hepatic artery thrombosis 10 days after placement, and 1/62 (1.6%) catheter was not used due to missing intrahepatic perfusion redistribution. Finally, response analysis according to RECIST 1.1 was performed in 49/62 patients (79%).
Whole-liver response analysis according to RECIST 1.1 showed PD in 9/49 patients (18.4%), SD in 16/49 patients (32.7%), PR in 23/49 patients (46.9%) and CR in 1/49 patient (2%). Separate response analysis of the left and right liver lobe did not show any significant difference in treatment response after intraarterial chemotherapy (PD right lobe n = 9 (18.4%) vs. left lobe n = 8 (16.3%), p-value = 0.8; SD right lobe n = 16 (32.7%) vs. left lobe n = 17 (34.7%), p-value = 0.86; PR right lobe n = 23 (46.9%) vs. left lobe n = 23 (46.9%), p-value = 1; CR right lobe n = 1 (2%) vs. left lobe n = 1 (2%), p-value = 1) (Fig. 5). Only in one patient, with embolization of a replaced left hepatic artery, discordant tumor response between both lobes was observed with progressive disease in the right lobe and stable disease in the left lobe. For this single patient, reperfusion of the left replaced embolized hepatic artery was satisfactory on both subjective (subsegmental branches opacification) and objective (HU on CT scan) perfusion analysis.
Discussion
The present study confirms that coil embolization of hepatic arterial variants during percutaneous arterial port catheter placement results in effective intrahepatic reperfusion and does not compromise treatment efficacy in the redistribution-dependent liver lobes to hepatic arterial infusion chemotherapy compared to the redistribution-independent liver lobes.
HAIC is a well-known technique, especially in patients with colorectal liver metastases with progressive disease after standard systemic chemotherapy, as well as in a first-line and adjuvant setting [2,3,4]. However, for patients with anatomic variants of the hepatic arteries few data exist to demonstrate that HAIC is equally efficient. We therefore report the outcomes of patients with hepatic artery variants from a high-volume center with more than a decade of experience with percutaneously placed arterial port catheters.
First, intrahepatic perfusion redistribution was subjectively and objectively analyzed. Of the 62 patients included patients, only two patients did not experience intrahepatic perfusion redistribution via intrahepatic collaterals. Both patients presented with a replaced right hepatic artery, used for catheter placement, and coil embolization of the proper hepatic artery. Both patients were found to have reperfusion of the occluded proper hepatic artery by peripancreatic collaterals. In one patient, super-selective embolization of these collaterals was possible and homogenous perfusion of the whole liver was finally achieved via the placed catheter. In the other patient, intraarterial chemotherapy administration was not possible due to missing perfusion of the left lobe. This confirms that intrahepatic collaterals usually form after embolization of an additional hepatic artery, unless there is competitive flow after insufficient embolization or recanalization by parasitic arterial branches.
For surgically placed intraarterial catheters, perfusion redistribution after ligation of variant hepatic arteries has been analyzed in several studies with homogenous whole-liver perfusion after ligation of these arteries in almost all patients [14, 23,24,25]. For percutaneously placed arterial port catheters, Chuang et al. were the first to analyze intrahepatic perfusion redistribution after endovascular coil embolization of variant hepatic arteries [15]. In their series, intrahepatic collaterals immediately developed for all ten patients and whole-liver perfusion was achieved by the placed catheter. In a study conducted by Yamagami et al., a total of 76 patients with variant hepatic arteries, undergoing coil embolization and percutaneous arterial port catheter placement, homogenous whole-liver perfusion on CT were found in 64 patients (84.2%) [16]. In five patients, collateral flow via the inferior phrenic artery was found and homogenous whole-liver perfusion was achieved after embolization. This underlines that, in case of missing intrahepatic perfusion redistribution, collateral flow via parasitic arteries has to be looked for.
Nevertheless, successful intrahepatic perfusion redistribution does not equate to a successful therapeutic response in the redistribution-dependent areas, assumed that the liver metastases respond to the applied chemotherapeutic agent. Therefore, in a second step in the present study, the treatment response according to RECIST 1.1 was analyzed and compared separately for the redistribution-dependent and redistribution-independent liver lobes.
There is a case series with 13 patients published by Burke et al. in 1995 analyzing the effect of ligation of variant hepatic arteries during surgical intraarterial catheter placement on tumor response in the redistribution-dependent area [19]. Despite intrahepatic perfusion redistribution to the ligated liver segments, confirmed via intraoperative injection of methylene blue, no significant reduction in median tumor volume was observed in the redistribution-dependent area after HAIC with floxuridine compared to significant reduction in the other liver segments. This is in contrast to a study conducted by Meijer et al., analyzing treatment efficiency of percutaneous intraarterial hepatic perfusion in 12 patients with liver metastases from uveal melanoma after endovascular coil embolization of variant hepatic arteries [17]. The authors did not find any difference in terms of treatment response in the redistribution-dependent and redistribution-independent liver segments and concluded that embolization of variant hepatic arteries does not compromise treatment efficiency. This is in accordance with the herein presented results. The present study did not find any difference in treatment response in the redistribution-dependent and redistribution-independent liver lobes. Only one patient was found to have a discordant tumor response between both lobes, with PD in the right lobe and SD in the left. This specific patient underwent embolization of a replaced left hepatic artery. With a “better” treatment response in the redistribution-dependent lobe, thus the discordant treatment response cannot be explained by inefficient perfusion redistribution of intrahepatic flow. However, another possible explanation might be a better treatment response due to an ischemic effect as a result of an insufficient perfusion redistribution.
These findings are of major importance, as other techniques such as radioembolization or infusion of novel therapeutics such as immunotherapies [26] may rely on the same principal.
Another important finding is that we did not observe a difference in intrahepatic reperfusion or response to treatment depending on which hepatic artery was embolized. Hence, coil embolization and catheter placement must be performed individually in each patient taking into account anatomical considerations, such as steep angles of the celiac trunk or the superior mesenteric artery making the catheter placement difficult. Furthermore, one has to consider the tumor burden in the right and left liver lobe and discuss with your hepatic surgeon which surgical approach will be possible in case of major tumor response to intraarterial chemotherapy.
We must acknowledge some study limitations. First, it is a retrospective single-center study, but to limit this bias, all consecutive patients were included and analyzed. Furthermore, it must be assumed that results might be different in less experienced teams as this study was performed in a single high-volume center with special expertise in these complex patients. Second, all HAIC protocols consisted of an intraarterial chemotherapy combined with a systemically administrated chemotherapeutic agent. Thus, it cannot be completely excluded that the systemically administered chemotherapeutic agent can interfere with bilobar treatment response evaluation. Third, assessment of treatment efficacy might be limited due to the short mean follow-up of 3.5 ± 1.1 months.
Conclusion
In conclusion, intrahepatic perfusion redistribution after embolization of hepatic arterial variants during percutaneous arterial port catheter placement occurs almost always. If not, reperfusion of the occluded variant hepatic artery by parasitic arteries has to be looked for. More importantly, treatment efficacy of intraarterial chemotherapy in the redistribution-dependent liver lobe is not compromised by embolization of variant hepatic arteries.
Abbreviations
- AE:
-
Adverse event
- CT:
-
Computed tomography
- CBCT:
-
Cone-beam computed tomography
- DSA:
-
Digital subtraction angiography
- HAIC:
-
Hepatic arterial infusion chemotherapy
- HU:
-
Hounsfield unit
- MRI:
-
Magnetic resonance imaging
- ROI:
-
Region of interest
- SPECT:
-
Single-photon emission computed tomography
References
Kwan J, Pua U. Review of intra-arterial therapies for colorectal cancer liver metastasis. Cancers. 2021;13:1371.
Zhao JJ, Tan E, Sultana R, Syn NL, Zhuang KD, Leong S, et al. Intra-Arterial therapy for unresectable colorectal liver metastases: a meta-analysis. J Vasc Interv Radiol. 2021;32:1536-1545.e38.
Chapelle N, Matysiak-Budnik T, Douane F, Metairie S, Rougier P, Touchefeu Y. Hepatic arterial infusion in the management of colorectal cancer liver metastasis: current and future perspectives. Digest Liver Dis. 2018;50:220–5.
Boilève A, Maillard A, Wagner M, Dromain C, Laurent C, Bierre ED, et al. Treatment intensification with hepatic arterial infusion chemotherapy in patients with liver-only colorectal metastases still unresectable after systemic induction chemotherapy: a randomized phase II study–SULTAN UCGI 30/PRODIGE 53 (NCT03164655)-study protocol. BMC Cancer. 2020;20:74.
Pernot S, Pellerin O, Mineur L, Monterymard C, Smith D, Lapuyade B, et al. Phase III randomized trial comparing systemic versus intra-arterial oxaliplatin, combined with LV5FU2 +/- irinotecan and a targeted therapy, in the first-line treatment of metastatic colorectal cancer restricted to the liver (OSCAR): PRODIGE 49. Digest Liver Dis. 2022;54:324–30.
Goéré D, Pignon J-P, Gelli M, Elias D, Benhaim L, Deschamps F, et al. Postoperative hepatic arterial chemotherapy in high-risk patients as adjuvant treatment after resection of colorectal liver metastases - a randomized phase II/III trial–PACHA-01 (NCT02494973). BMC Cancer. 2018;18:787.
Deschamps F, Elias D, Goere D, Malka D, Ducreux M, Boige V, et al. Intra-arterial hepatic chemotherapy: a comparison of percutaneous versus surgical implantation of port-catheters. Cardiovasc Inter Rad. 2011;34:973–9.
Favelier S, Germain T, Genson P-Y, Cercueil J-P, Denys A, Krausé D, et al. Anatomy of liver arteries for interventional radiology. Diagn Interv Imaging. 2015;96:537–46.
Michels NA. Newer anatomy of the liver and its variant blood supply and collateral circulation. Am J Surg. 1966;112:337–47.
Spreafico C, Morosi C, Maccauro M, Romito R, Lanocita R, Civelli EM, et al. Intrahepatic flow redistribution in patients treated with radioembolization. Cardiovasc Inter Rad. 2015;38:322–8.
Bilbao JI, Garrastachu P, Herráiz MJ, Rodríguez M, Iñarrairaegui M, Rodríguez J, et al. Safety and efficacy assessment of flow redistribution by occlusion of intrahepatic vessels prior to radioembolization in the treatment of liver tumors. Cardiovasc Inter Rad. 2010;33:523–31.
Ezponda A, Rodríguez-Fraile M, Morales M, Vivas I, Torre MDL, Sangro B, et al. Hepatic flow redistribution is feasible in patients with hepatic malignancies undergoing same-day work-up angiography and yttrium-90 microsphere radioembolization. Cardiovasc Inter Rad. 2020;43:987–95.
Karunanithy N, Gordon F, Hodolic M, Al-Nahhas A, Wasan HS, Habib N, et al. Embolization of hepatic arterial branches to simplify hepatic blood flow before yttrium 90 radioembolization: a useful technique in the presence of challenging anatomy. Cardiovasc Inter Rad. 2011;34:287–94.
Allen PJ, Stojadinovic A, Ben-Porat L, Gonen M, Kooby D, Blumgart L, et al. The management of variant arterial anatomy during hepatic arterial infusion pump placement. Ann Surg Oncol. 2002;9:875–80.
Chuang VP, Wallace S. Hepatic arterial redistribution for intraarterial infusion of hepatic neoplasms. Radiology. 1980;135:295–9.
Yamagami T, Yoshimatsu R, Matsumoto T, Nishimura T. Redistribution of multiple hepatic arteries into a single hepatic artery to perform repeated hepatic arterial infusion chemotherapy. Acta Radiol. 2008;49:513–20.
Meijer TS, Geus-Oei L-F de, Martini CH, Netherlands D of A Leiden University Medical Center, Leiden, the, Tijl FGJ, Netherlands D of ECC Leiden University Medical Center, Leiden, et al. Embolization of variant hepatic arteries in patients undergoing percutaneous hepatic perfusion for unresectable liver metastases of ocular melanoma. Diagn Interv Radiol. 2019;25:451–8.
Ikeda O, Tamura Y, Nakasone Y, Shiraishi S, Kawanaka K, Tomiguchi S, et al. Evaluation of intrahepatic perfusion on fusion imaging using a combined CT/SPECT system: influence of anatomic variations on hemodynamic modification before installation of implantable port systems for hepatic arterial infusion chemotherapy. Cardiovasc Inter Rad. 2007;30:383–91.
Burke D, Earlam S, Fordy C, Allen-Mersh TG. Effect of aberrant hepatic arterial anatomy on tumour response to hepatic artery infusion of floxuridine for colorectal liver metastases. Brit J Surg. 1995;82:1098–100.
Lévi FA, Boige V, Hebbar M, Smith D, Lepère C, Focan C, et al. Conversion to resection of liver metastases from colorectal cancer with hepatic artery infusion of combined chemotherapy and systemic cetuximab in multicenter trial OPTILIV. Ann Oncol. 2016;27:267–74.
Arai Y, Takeuchi Y, Inaba Y, Yamaura H, Sato Y, Aramaki T, et al. Percutaneous catheter placement for hepatic arterial infusion chemotherapy. Tech Vasc Interv Radiol. 2007;10:30–7.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer Oxf Engl. 1990;2009(45):228–47.
Walker BS, Sutton TL, Eil RL, Korngold EK, Kolbeck KJ, Billingsley KG, et al. Conventional hepatic arterial anatomy? Novel findings and insights of a multi-disciplinary hepatic arterial infusion pump program. Am J Surg. 2021;221:1188–94.
Curley SA, Chase JL, Roh MS, Hohn DC. Technical considerations and complications associated with the placement of 180 implantable hepatic arterial infusion devices. Surgery. 1993;114:928–35.
Rayner AA, Kerlan RK, Stagg RJ, Price DC, Hohn DC. Total hepatic arterial perfusion after occlusion of variant lobar vessels: implications for hepatic arterial chemotherapy. Surgery. 1986;99:708–15.
Sato T, Eschelman DJ, Gonsalves CF, Terai M, Chervoneva I, McCue PA, et al. Immunoembolization of malignant liver tumors, including uveal melanoma, using granulocyte-macrophage colony-stimulating factor. J Clin Oncol. 2008;26:5436–42.
Funding
This study was not supported by any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Consent for Publication
For this type of study consent for publication is not required.
Ethical Approval
For this type of study formal consent is not required.
Informed Consent
For this type of study informed consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kobe, A., Deschamps, F., Meyblum, L. et al. Coil Embolization of Variant Hepatic Arteries During Percutaneous Arterial Port Catheter Placement for Intraarterial Chemotherapy: Analysis of Intrahepatic Perfusion Redistribution and Treatment Efficacy. Cardiovasc Intervent Radiol 46, 69–79 (2023). https://doi.org/10.1007/s00270-022-03303-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-022-03303-y