Abstract
Introduction
In planning Yttrium-90 (90Y)-radioembolizations, strategy problems arise in tumours with multiple arterial supplies. We aim to demonstrate that tumours can be treated via one main feeding artery achieving flow redistribution by embolizing accessory vessels.
Methods
One hundred 90Y-radioembolizations were performed on 90 patients using glass microspheres. In 19 lesions/17 patients, accessory branches were found feeding a minor tumour portion and embolized. In all 17 patients, the assessment of the complete perfusion was obtained by angiography and single photon emission computerized tomography–computerized tomography (SPECT–CT). Dosimetry, toxicity, and tumor response rate of the patients treated after flow redistribution were compared with the 83 standard-treated patients. Seventeen lesions in 15 patients with flow redistribution were chosen as target lesions and evaluated according to mRECIST criteria.
Results
In all patients, the complete tumor perfusion was assessed immediately before radioembolization by angiography in all patients and after the 90Y-infusion by SPECT–CT in 15 of 17 patients. In the 15 assessable patients, the response rate in their 17 lesions was 3 CR, 8 PR, and 6 SD. Dosimetric and toxicity data, as well tumour response rate, were comparable with the 83 patients with regular vasculature.
Conclusions
All embolization procedures were performed successfully with no complications, and the flow redistribution was obtained in all cases. Results in term of toxicity, median dose administered, and radiological response were comparable with standard radioembolizations. Our findings confirmed the intratumoral flow redistribution after embolizing the accessory arteries, which makes it possible to treat the tumour through its single main feeding artery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Radioembolisation is a well-known and described therapeutic procedure based on intra-arterial injection of microparticles of glass or resin containing a radioactive isotope, Yttrium-90 (90Y), which has the characteristic of being a pure beta ray emitter. The action of the particles extends for approximately 1 cm [1–6]. This feature, along with the predominantly arterial vascularisation typical of focal liver lesions and the small size of the spheres, means that microparticles carried by the arterial flow remain trapped in the arterioles and capillaries of the tumour and release their energy there. The result is highly targeted selective intra-arterial brachytherapy.
The ideal is that the treatment will cover the entire arterial circulation of the cancer, to leave no part excluded, preserving as much of the cancer-free parenchyma as possible [7–9]. For this reason, it is of fundamental importance to identify the best point for the infusion of microspheres at the angiographic workup. Usually, we treat the cancer as selectively as possible, i.e., in a segmental, multisegmental, or lobar manner depending on the extent of the tumour. When the tumour contains several nodular lesions in both hepatic lobes, our choice is always to perform treatments in different lobes at different times and closely spaced, while avoiding performing the Y infusion in the entire liver at the same time. We chose to perform lobar or selective treatments in our series of cirrhotic patients, instead of treating the whole liver as reported by other centers [10, 11], in order to avoid unnecessary treatment to the healthy liver parenchyma and therefore to prevent potential liver dysfunction. A problem arises when the tumour vasculature is complex and derived from multiple arteries, originating from the hepatic or extrahepatic arteries, so that the cancer nodule cannot be treated simultaneously from a single point of infusion, usually due to the fact that the tumour site itself is located between two lobes or peripherally or due to the presence of anatomical vascular variants. In this case, a strategy for the manner and timing of the microsphere administration must be developed.
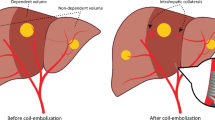
The term vascular redistribution describes a series of angiographic manoeuvres aimed at modifying the arterial circulation by the proximal occlusion of an arterial branch, whose distribution area is rehabilitated and perfused distally through collateral circulation from other arteries [12].
The purpose of this study was to evaluate our case sample of radioembolisation procedures performed for hepatocellular carcinoma (HCC) in the cirrhotic liver to determine whether the change in intra- and extrahepatic arterial flow brought about by embolisation of some arterial branches could effectively lead to a redistribution of flow that would make it possible to treat all the tumour tissue effectively by infusing the 90Y into a single artery.
Materials and Methods
Case Series
A consecutive series of 100 radioembolisation procedures were conducted at our institution on 90 patients with advanced or intermediate-advanced HCC with well compensated cirrhosis, child stage A–B7, unsuitable for curative, surgical, or ablative therapy. In 90 % of cases, the aetiology was posthepatitis HCV/HBV-related. All radioembolisation procedures were performed using TheraSphere microglass beads (BTG, UK). A single administration of 148 MBq technetium 99m (99mTc)-macroaggregated albumin particles (99mTc-MAA) for the initial angiographic study and also the TheraSpheres was made in 68 instances through the right hepatic artery and in 24 by the left hepatic artery. In the remaining eight procedures, a more selective approach was possible up the posterior branch of the right hepatic artery in four cases, the right anterior branch in three cases, and the artery for the fourth segment in one case. Complex tumour vascularisation was observed in 17 cases and defined by the presence of manifold or composite arterial blood supply to the targeted HCC lesions, judged incompatible with straightforward single lobar injection. The series examined in this study included these 17 of 90 patients treated with radioembolisation, 15 men and 2 women, with 19 lesions (one patient had three lesions in the left lobe) with an average diameter of 65 mm (range 16–152). In the 17 patients, 18 accessory arterial branches were found in addition to the main feeding artery, 9 originating from the hepatic arteries (Fig. 1) and 9 from other branches that supplied a relatively minor part of the tumour (Fig. 2). In these patients, we performed embolisation of the accessory branches to redistribute the circulation, subsequently performing an infusion into the main artery. Table 1 summarises the embolised vessels. Subsequently, all patients were treated by infusion of 90Y in the desired location. The median interval between the first angiographic workup and Y infusion was 11 days (range 10–25). In all patients, complete perfusion of the tumour was verified angiographically before administration of the particles containing Y and confirmed in 15 of 17 patients by performing single photon emission computerized tomography (SPECT)–computerized tomography (CT) after the injection of Y, using Bremsstrahlung’s effect; in 2 patients this assessment could not be performed due to technical problems that arose, which were unrelated to the procedure. In the 15 patients for whom it was possible to perform a radiological follow-up, the 17 hepatic lesions subject to flow redistribution were selected as target lesions, and the radiological response was verified by mRECIST criteria [13].
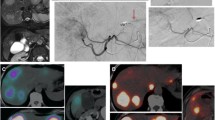
A CT scan in arterial phase shows the presence of a large HCC involving the VII–VIII segments. B Hepatic angiography shows a hypervascular mass vascularized mainly by the right hepatic artery. C Selective angiography of left hepatic artery shows two accessory arteries that supply blood to part of the tumor (arrows). These branches were occluded with coils. D Hepatic angiography after embolization of the branches of left hepatic artery (the arrows indicate the two coils). E Hepatic angiography shows the vascularization of the entire lesion due to redistribution. F SPECT image obtained after radioembolization with 90Y confirms the homogeneous uptake throughout the tumor volume
A CT scan in arterial phase shows the presence of a large HCC in the right hepatic lobe. B Selective angiography of a hypertrophic right phrenic artery with an accessory branch that supply blood to part of the tumor. This branch was occluded with two coils. C Right hepatic angiography shows the vascularization of the entire hypervascular lesion after redistribution. We can see coils in the right phrenic artery (arrow). D SPECT/CT performed after the infusion of 99mTc-MAA in the right hepatic artery. There is homogeneous uptake of the tumor
Toxicity data were recorded at 7, 14, 30, 60, and 90 days after treatment and included clinical examinations, laboratory data, and registration of adverse events according to Common Terminology Criteria for Adverse Events (CTCAE 4.0) [14].
Dosimetry, toxicity, and tumor response rate (15 patients) of the 17 patients treated after flow redistribution were compared to the 83 patients treated in the standard way to assess if there were significant differences or clinical problems due to the embolization [15, 16].
Patients in the present series were included in a prospective phase 2 protocol on radioembolisation in advanced and intermediate-advanced HCC, which was approved by our Ethics Committee. All patients signed an informed consent.
Angiographic Technique and 90Y Microsphere Radioembolization Protocol
All patients underwent CT of thorax and abdomen at the enrollment in the protocol, to stage tumor burden accurately, assessing the hepatic extension and to exclude extrahepatic metastasis. Patients were imaged using a dual-source, dual-energy CT scanner (Somatom Definition Flash, Siemens Medical Solutions). All CT scans, before and after treatment, were performed with the same protocol without and with 120–140 ml of intravenous contrast medium (Iopamiro 370, Bracco, Italy) injected at a rate of 4 ml/s using bolus tracking technique with image acquisitions in arterial, portal, and late phases. In all patients, a detailed mapping of the liver and tumor vascularity was obtained, with particular attention to extrahepatic arterial supply to the liver parenchyma. Then, angiograms of the visceral vessels were performed using a Siemens Axiom angiographic unit with digital flat panel, including abdominal aortography, the superior mesenteric artery (SMA), and celiac trunk. Diagnostic angiography was first performed with a 5-French catheter (Cobra or Simmons 1 shaped, Imager II, Boston Scientific, MA, USA). After the injections into the main trunks, selective angiograms were performed using a microcoaxial catheter (27-mm Progreat Terumo, Japan). At this stage, we studied the vascular anatomy of the liver, including all its possible variants. In particular, we had to look for accessory arteries for hepatic circulation with an external origin, both from the aorta and from other branches. The selective arteriographies always included the common hepatic artery, both lobar hepatic arteries and all accessory vessels. All angiographic embolization procedures on the accessory vessels were conducted using a microcatheter and one or more pushable microcoils (VortX-18, Boston Scientific) or detachable microcoils (Azur, Terumo) to obtain the complete arrest of arterial circulation. At the end of the first angiographic workup, macroaggregates of albumin labelled with 99mTc (99mTc-MAA) were injected into the site chosen for the administration of Y for the execution of the liver scintigraphy using SPECT, considering, for this purpose, a behaviour and distribution pattern similar to that of the particles containing Y. MAA labelling was specifically performed for each patient not more than 20 min before the intra-arterial administration, using 148 MBq of 99mTc-MAA in 5 ml. This was injected as a bolus. Planar and tomographic acquisition was performed with a dual-head gamma camera, the VG™ Infinia II by General Electric.
SPECT and CT images were then digitally coregistered. This step is important and complementary to angiography, because it is necessary to select correctly patients who may have benefit from the treatment, to identify extrahepatic accumulation of radiopharmaceutical, and to evaluate the lung shunt fraction. The lung absorbed dose limits are 25–30 Gy for a single administration or a total of 50 Gy for multiple doses. The presence of extrahepatic shunts, especially in intestinal structures, is a contraindication, so if it is not possible to eliminate the shunt by changing the injection point or embolising the arteries involved, it was exclude from treatment.
The SPECT/CT images also make it possible to quantify the degree of radiopharmaceutical uptake by the malignant lesions and by the heathy liver, both for providing a check on the correct injection site and, above all, to perform a dosimetric treatment planning. Three-dimensional regions of interest drawn on the tumor and on the nontumoral parenchyma provide SPECT counts, which allow the pretreatment calculation of the absorbed doses to these two regions. On these bases, the individually optimized 90Y activity (GBq) to be administered is chosen by the nuclear medicine specialist in order to deliver less than 70 Gy averaged on the whole healthy parenchyma and aiming at more than 500 Gy to the tumor [17].
The second angiography session, performed for the administration of microparticles containing 90Y, was performed by positioning the microcatheter in the artery previously chosen for the infusion. Before administration of the Y, an angiogram was always taken to verify the vascular situation and the outcome of redistributing the arterial circulation in the tumour.
To confirm the correct radiopharmaceutical administration, a planar and tomographic acquisition was performed 24 h posttreatment with Bremsstrahlung effect: a planar scan static liver image was obtained, with a 256 × 256 matrix size and high-energy collimators. An SPECT scan was acquired with 60 projections, 15 s per projection, and a 128 × 128 matrix size. At the end of the procedure, SPECT images were manually coregistered to arterial phase CT images [18].
Results
All angiographic and embolization procedures were performed successfully and without complications. During the first angiographic workup, it was immediately possible to identify accessory vessels and embolize them for redistribution in 12 of 17 patients, in whom complete perfusion of the tumour was verified by performing SPECT–CT after the infusion of 99mTc-MAA. In 5 of 17 patients, during the first angiographic stage, it was not possible to understand immediately and fully the distribution of the arterial circulation within the malignant lesion, although it was assumed that the presence of an accessory artery was causing a perfusion defect. 99mTc-MAA scintigraphy therefore was performed, which confirmed the angiographic suspicion of a perfusion defect, showing a cold area, which corresponds to a different artery perfusion. A second angiographic investigation was performed, with identification of accessory vessels causing a heterogonous radiopharmaceutical distribution and subsequent embolization. In these five patients, a new SPECT–CT with 99mTc-MAA was repeated after embolization to demonstrate a homogenous 99mTc-MAA distribution in the lesion.
In all 17 patients, the angiographic study performed immediately before infusion of the Y appeared satisfactory, with confirmation of the imaged pathological circulation evenly distributed throughout the tumour. In the 15 cases in which SPECT–CT was performed after Y, perfusion of the tumour appeared complete and homogeneous.
Two of 17 patients with liver flow redistribution, with advanced HCC with portal vein thrombosis, developed remote metastases and died before to have radiological evaluation 3 months after radioembolization; therefore, these 2 patients are not evaluable for tumour response in the liver. In both of these patients, the SPECT–CT after Y revealed that the distribution of microspheres was very satisfactory, because the lesion appeared to capture the radiopharmaceutical homogeneously and intensely.
Fifteen of 17 patients who could be evaluated in terms of radiological response, carriers of 17 lesions, had an average follow-up of 6 months (range 3–20). Three of the 17 lesions had a CR, 8 a PR, and 6 an SD according to mRECIST criteria, with an overall response rate (CR + PR + SD) of 100 % of lesions and an objective response (CR + PR) of 11 of 17 lesions (64.7 %) in 15 patients evaluable. These results confirm that the entire target lesion was treated, and they were similar to those obtained in patients with standard procedure. In fact, the tumour necrosis radiologically observed was homogeneous over the entire volume of target lesions, including the parts subjected to flow redistribution. Even the dosimetric values calculated as median activity delivered and as median dose administered to the treatment site were comparable and not less than that obtained in patients with regular vasculature. Finally, the toxicity observed was very limited and comparable between the two groups of patients. Table 2 summarises these results of the two groups.
Discussion
The idea of modifying the hepatic arterial flow embolizing one or more vessels derived from the experience gained since the 1970s, based on experimental observations [19–21], from locoregional injection of chemotherapy agents into the artery [22–24], from the experience of embolization for bleeding resulting from operations, such as percutaneous biliary drainage [25–28]. Lastly, in 2009 Bilbao et al. [12] and in 2011 Abdelmaksoud et al. [10, 11] reported studies on patients who were candidates for radioembolisation in whom the change of intrahepatic circulation resulting in redistribution of the flow was found to increase the safety and efficacy of radioembolization and in some instances has allowed to treat the whole liver in a single session.
In our experience of 100 radioembolization procedures performed in 90 patients, we found in 17 patients that pathological vascularisation was supported by several arteries, including one main artery, which therefore could be considered the main feeding artery, and one or more accessory arteries. In all these cases, it was impossible to infuse the microspheres containing 90Y from a single point of infusion simultaneously into all the arteries involved. The need to identify a single main point of infusion in these cases also seemed to us important, due to several considerations. First of all, it is extremely difficult to calculate the dose accurately in the case of a malignant lesion vascularised by several arteries with different sizes and flow rates, and therefore achieve an accurate subdivision of the administered activity. Thus, how could the percentage doses be established in each artery? Based on the same consideration, at least two scintigraphic studies should be performed to evaluate the presence of shunts and to perform a correct calculation of the administered doses to the tumour and normal parenchyma. In our institute, an individualized planning treatment is encouraged, in order to be effective and safe. The assessment of tumour volume and the calculation of the radiopharmaceutical distribution into tumour and healthy liver, can be determined by SPECT/CT [15, 17]. Furthermore, the injection of 90Y microsphere can be very dangerous or impossible into extrahepatic vessels, such as the phrenic arteries.
In 5 of 17 patients, the suspicion of an accessory artery revealed during in the first angiographic workup was confirmed by the 99mTc-MAA–SPECT/CT study. In these circumstances, a C-arm cone-beam CT (CACT) could be useful, providing important information on the distribution of flow and intratumour perfusion [29, 30]. However this option is not available in our angiography department. The treatment response evaluated in the target lesions were very satisfactory, with an overall response rate (CR + PR + SD) of 100 % of lesions and an objective response (CR + PR) of 11 of 17 lesions (64.7 %). These findings confirm that the entire target lesion was treated. The calculation of the adsorbed dose to the tumour in the 17 cases with flow redistribution is comparable to that of the 83 standard cases. Also for that reason the flow redistribution can be considered an efficient procedure to obtain a good objective response. Moreover, in any case we find correspondence between the residual living part of the lesion and the vascular bed of the embolized accessory artery. The angiographic and scintigraphic studies confirmed that flow redistribution within the tumour subsequent to embolization of accessory arterial branches took place in all cases, allowing the treatment of the entire tumour through a single main feeding artery.
Limitations of our series are multiple and intrinsic to the study. We have a limited number of patients, and our good results need to be confirmed in larger series of patients. Another limitation is the lack of CACT, which is currently not available in our Department of Radiology; the use of CACT could improve the treatment planning detecting a better tumour perfusion. In fact, it can determine an improvement of the 99mTc-MAA delivery to targeted lesion and consequently a more accurate dosimetric study.
In conclusion, the strategy of embolizing accessory arterial branches to achieve a redistribution of blood flow that makes it possible to treat the entire tumour from a single main vessel was found to be relatively simple to perform and safe. It may be proposed as a clinical practice in selected cases where tumours are vascularised by several arteries, including one main artery and one or more accessory arteries feeding a smaller part of the tumour, into which 90Y-microspheres cannot be infused simultaneously.
References
Geschwind JF, Salem R, Carr BI et al (2004) Yttrium-90 microspheres for the treatment of hepatocellular carcinoma. Gastroenterology 127:S194–S205
Carr BI (2004) Hepatic arterial 90-yttrium glass microspheres (TheraSphere) for unresectable hepatocellular carcinoma: interim safety and survival data on 65 patients. Liver Transplant 10:S107–S110
Salem R, Lewandowski RJ, Atassi B et al (2005) Treatment of unresectable hepatocellular carcinoma with use of 90Y microspheres (TheraSphere): safety, tumor response, and survival. J Vasc Interv Radiol 16:1627–1639
Hilgard P, Hamami M, Fouly AE et al (2010) Radioembolization with Yttrium-90 glass microspheres in hepatocellular carcinoma: European experience on safety and long-term survival. Hepatology 52(5):1741–1749
Mazzaferro V, Sposito C, Bhoori S et al (2013) Yttrium (90) radioembolization for intermediate-advanced hepatocarcinoma: a phase II study. Hepatology 57(5):1826–1837
Ibrahim SM, Lewandowski RJ, Sato KT et al (2008) Radioembolization for the treatment of unresectable hepatocellular carcinoma: a clinical review. World J Gastroenterol 14:1664–1669
Young JY, Rhee TK, Atassi B et al (2007) Radiation dose limits and liver toxicities resulting from multiple Yttrium-90 radioembolization treatments for hepatocellular carcinoma. J Vasc Interv Radiol 18:1375–1382
Riaz A, Lewandowski RJ, Kulik LM et al (2009) Complications following radioembolization with Yttrium-90 microspheres: a comprehensive literature review. J Vasc Interv Radiol 20(9):1121–1130
Kennedy A, Nag S, Salem R et al (2007) Recommendations for radio-embolization of hepatic malignancies using yttrium-90 microsphere brachytherapy: a consensus panel report from the Radio-embolization Brachytherapy Oncology Consortium (REBOC). Int J Radiat Oncol Biol Phys 68:13–23
Abdelmaksoud MH, Louie JD, Kothary N et al (2011) Embolization of parasitized extrahepatic arteries to re-establish intrahepatic arterial supply to tumors before yttrium-90 radioembolization. J Vasc Interv Radiol 22(10):1355–1362
Abdelmaksoud MH, Louie JD, Kothary N et al (2011) Consolidation of hepatic arterial inflow by embolization of variant hepatic arteries in preparation for yttrium-90 radioembolization. J Vasc Interv Radiol 22(10):1364–1371
Bilbao JI, Garrastachu P, Maria J, Herraiz MJ et al (2010) Safety and efficacy assessment of flow redistribution by occlusion of intrahepatic vessels prior to radioembolization in the treatment of liver tumors. Cardiovasc Interv Radiol 33(3):523–531
Lencioni R, Llovet JM (2010) Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 30(1):52–60
National Cancer Institute (29 May 2009) Common Terminology Criteria for Adverse Events v4.0 NCI, NIH, DHHS. NIH publication number 09-7473
Chiesa C, Maccauro M, Romito R, Spreafico C et al (2011) Need, feasibility and convenience of dosimetric treatment planning in liver selective internal radiation therapy with 90Y microspheres: the experience of National Cancer Institute of Milan. Q J Nucl Med Mol Imaging 55:168–197
Salem R, Lewandowski RJ, Kulik L et al (2011) Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology 140(2):497–507
Chiesa C, Mira M, Maccauro M, Romito R, Spreafico C, Sposito C, Bhoori S, Morosi C, Pellizzari S, Negri A, Civelli E, Lanocita R, Camerini T, Bampo C, Carrara M, Seregni E, Marchianò A, Mazzaferro V, Bombardieri E (2012) A dosimetric treatment planning strategy in radioembolization of hepatocarcinoma with 90Y glass microspheres. Q J Nucl Med Mol Imaging 56(6):503–508
Sebastian AJ, Szyszko T, Al-Nahhas A, Nijran K, Tait NP (2008) Evaluation of hepatic angiography procedures and Bremsstrahlung imaging in selective internal radiation therapy: a two-year single-center experience. Cardiovasc Interv Radiol 31:643–649
Redman HC, Reuter SR (1970) Arterial collaterals in the liver hilus. Radiology 94:575–579
Chuang VP, Wallace S (1980) Hepatic arterial redistribution for intraarterial infusion of hepatic neoplasms. Radiology 135:295–299
Charnsangavej C, Chuang VP, Wallace S, Soo CS, Bowers T (1982) Angiographic classification of hepatic arterial collaterals. Radiology 144:485–494
Arai Y, Yakeuchi Y, Inaba Y et al (2007) Percutaneous catheter placement for hepatic arterial infusion chemotherapy. Tech Vasc Interv Radiol 10:30–37
Ganeshan A, Upponi S, Hon LQ, Warakaulle D, Uberoi R (2008) Arterial infusion of chemotherapy: the role of diagnostic and interventional radiology. Ann Oncol 19:847–851
Tanaka T, Arai Y, Inaba Y et al (2003) Radiologic placement of side-hole catheter with tip fixation for hepatic arterial infusion chemotherapy. J Vasc Interv Radiol 14:63–68
Cho A, Gunji H, Koike N et al (2007) Intersegmental arterial communication between the medial and left lateral segments of the liver. Dig Surg 24:328–330
Gunji H, Cho A, Tohma T et al (2006) The blood supply of the hilar bile duct and its relationship to the communicating arcade located between the right and left hepatic arteries. Am J Surg 192:276–280
Tohma T, Cho A, Okazumi S et al (2005) Communicating arcade between the right and left hepatic arteries: evaluation with CT and angiography during temporary balloon occlusion of the right or left hepatic artery. Radiology 237:361–365
Miyazaki M, Ito H, Nkagawa K et al (2000) Unilateral hepatic artery reconstruction is unnecessary in biliary tract carcinomas involving lobar hepatic artery: implications of interlobar hepatic artery and its preservation. Hepatogastroenterology 47:1526–1530
Sone M, Kato K, Hirose A et al (2008) Impact of multislice CT angiography of radiological catheter placement for hepatic arterial infusion chemotherapy. Cardiovasc Interv Radiol 31:91–97
Wallace MJ, Murthy R, Kammat PP et al (2007) Impact of C-arm CT on hepatic arterial interventions for hepatic malignancies. J Vasc Interv Radiol 18:1500–1507
Conflict of interest
Carlo Spreafico, Carlo Morosi, Marco Maccauro, Raffaele Romito, Rodolfo Lanocita, Enrico M. Civelli, Carlo Sposito, Sherrie Bhoori, Carlo Chiesa Ph, Laura F. Frigerio, Alice Lorenzoni, Tommaso Cascella, Alfonso Marchianò and Vincenzo Mazzaferro have no conflict of interest
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Spreafico, C., Morosi, C., Maccauro, M. et al. Intrahepatic Flow Redistribution in Patients Treated with Radioembolization. Cardiovasc Intervent Radiol 38, 322–328 (2015). https://doi.org/10.1007/s00270-014-0921-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-014-0921-2