Abstract
The article is part of the series of articles on radiation protection. You can find further articles in the special section of the CVIR issue. In addition to the risks from fluoroscopic-guided interventional procedures of tissue injuries, recent studies have drawn attention to the risk of stochastic effects. Guidelines exist for preprocedural planning and radiation management during the procedure. The concept of a substantial radiation dose level (SRDL) is helpful for patient follow-up for tissue injury. The uncommon nature of tissue injuries requires the interventionalist to be responsible for follow-up of patients who receive substantial radiation doses. Dose management systems for recognizing and avoiding higher patient exposures have been introduced. The European Directive provides a legal framework and requirements for equipment, training, dose monitoring, recording and optimization that are helpful in radiation risk management.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fluoroscopically guided interventional (FGI) procedures have a well-established and important role in the management of a variety of health conditions. It is well known that some FGI procedures can impart high-radiation doses to patients, and that these doses can result in radiation-induced skin injuries, which may be severe and extend into subcutaneous tissues and bone [1, 2]. Reports of patients with these injuries began appearing in the early 1990s and continue to appear [3,4,5,6,7,8,9,10,11,12]. These injuries also include hair loss, which is more commonly seen with procedures that irradiate the head and neck region, as the scalp is relatively sensitive for epilation [4, 13]. Injury location depends on the entrance location of the x-ray beam. For example, skin injuries due to cardiac interventions occur on the trunk. Similarly, skin injuries due to neuroradiology interventions involve the head and neck region. Radiation-induced skin injuries are tissue reactions (also called deterministic effects) [14]. In contrast to the carcinogenic effects of radiation, which are stochastic in nature, tissue injuries have a threshold. Many injuries can be avoided by adjusting imaging parameters to keep the skin dose below the threshold for a tissue effect (Table 1) [15, 16]. Radiation-induced cataract formation is also considered a tissue reaction by the International Commission on Radiological Protection (ICRP), though there is some evidence that it may be a stochastic effect [14, 17].
Preprocedural Planning and Radiation Management During the Procedure.
Some FGI procedures have the potential to be high-dose procedures, and some patients are more radiosensitive than the typical individual [18]. Procedures that have the potential to result in high radiation doses include embolization, angioplasty and stent placement, and transjugular intrahepatic portosystemic shunt creation (TIPS), among others [3, 16, 19, 20]. Some procedures in obese patients have the potential to require high radiation doses due to increased body part thickness [20, 21]. Patients may have increased radiosensitivity due to a genetic disorder (e.g., ataxia telangiectasia, Fanconi anemia) or an autoimmune or connective tissue disorder [4]. Hyperthyroidism and diabetes mellitus are also associated with increased radiosensitivity [4, 22]. Areas of previously irradiated skin may also demonstrate increased radiosensitivity. If the patient has had previous FGI procedures, examination of the skin in the area of the beam entrance should be performed, as there may be skin changes due to those earlier procedures.
If the FGI procedure is expected to be low dose, there is little likelihood that the threshold for a tissue reaction will be reached, unless the patient is radiosensitive. Communication of radiation risk may not be necessary on this basis, but may be required by national legislation or regulation. However, if the FGI procedure is potentially high dose (as discussed further in Sect. 5), the pre-procedure discussion needs to include the risk of a tissue reaction. For potentially high-dose procedures, and especially when these procedures are to be performed on patients at a greater risk of a skin injury, a discussion of the radiation risk should be part of the informed consent process. It should involve consideration of benefit of the procedure, risks of not doing the procedure, and the risks of alternative procedures.
Although tissue injury is the principal risk, some patients who receive a high-radiation dose should also be informed of the cancer risk [23, 24]. An understanding of cancer risk on the part of interventionalists can help in communicating with the patient. This includes the likelihood of carcinogenesis based on radiation dose and the latency period, as well as the resultant reduced probability of radiation-induced carcinogenesis in older patients. Effective communication of stochastic risk can be difficult [25,26,27] and need be done only for high-dose procedures that result in an effective dose of a few tens of mSv or more. There is a lack of guidance regarding at what level of dose this should be done [20].
Principles of Cone-Beam CT and its Application in Interventional Fluoroscopy
Cone-beam computed tomography (CBCT) is a radiographic imaging method that allows accurate, three-dimensional imaging, especially useful for highly attenuating/hard tissue or high contrast structures. CBCT can be performed on a C-arm fluoroscopy system but requires dedicated software. It is available as an option on all modern interventional fluoroscopy equipments. CBCT uses the X-ray tube and detector array rotating simultaneously around the patient through an arc of 180°–360° to collect data. This technique provides projection data similar to computed tomography, which can be post-processed by dedicated software programs to generate volumetric data that can be reconstructed in any projection, including oblique planes. The images provided by CBCT have lower contrast resolution as compared with images from standard CT scanners but are usually adequate for most intraoperative purposes [28]. CBCT allows three-dimensional display of vascular anatomy, enables early detection of certain intraprocedural complications and can decrease the risk of repeat interventions [29]. CBCT can sometimes replace digital subtraction angiography (DSA) acquisitions, with a decrease in DSA-related skin dose and may help limit peak skin dose (PSD) in complex and lengthy interventional procedures [30]. However, routine use of cone-beam CT can increase the risk of stochastic effects because of the higher total radiation dose [31].
How Fluoroscopy, Angiographic Series and Cone-Beam CT Contribute to Radiation Exposure of Patients, and How to Optimize Practice
Thorough knowledge of the fluoroscopic equipment, adequate training in radiation protection and an awareness of the potential for radiation injury are needed to ensure optimal benefit and safety for the patient [32]. Radiation should always be optimized according to the ALARA principle, i.e., ‘as low as reasonably achievable’ [33]. Imaging should be performed only when necessary and with no more radiation than needed to provide adequate image quality. Positioning of the patient with respect to the X-ray tube and the detector is very important, not only for optimum visualization of the anatomy of interest but also for minimizing radiation exposure [34, 35]. Steep angulation of the C-arm can increase exposure substantially due to the increased length of the radiation path through the patient. Orientations that result in high-dose rates should be avoided when possible. In potentially high-dose FGI procedures, it can be helpful, when possible, to reposition the beam entrance site on the patient frequently to avoid irradiation of the same part of the skin. All fluoroscopy equipment allows the user to adjust the fluoroscopy dose rate. It is advisable to always start the procedure in low-dose fluoroscopy mode and switch to a higher dose rate only if image quality is inadequate. The patient couch and image receptor should be positioned to keep the X-ray tube as far away from the patient and the image receptor as close to the patient as possible. Additional tools should be used to minimize radiation exposure, including added filtration, pulsed fluoroscopy, collimation, real-time digital fluoroscopy processing, lower DSA frame-rate settings, avoiding magnification and using last image hold and fluoroscopy loops. By implementing these techniques properly, providers can reduce patient radiation dose substantially [36, 37].
Fluoroscopy systems in modern interventional suites have the capability to perform DSA, rotational angiography, road mapping and CBCT [38]. Fluoroscopy contributes a relatively small fraction of the total radiation dose from imaging (measured as kerma-area product (PKA, also abbreviated as KAP) [39,40,41] administered during many vascular interventional procedures. For some procedures, nearly 70% of the total PKA comes from the acquisition of radiographic frames (DSA runs) [42,43,44]. Novel imaging options such as rotational angiography, road mapping and CBCT have unique advantages and are suited for different clinical situations, but all these techniques use ionizing radiation. They provide additional anatomic and diagnostic information but may lead to higher cumulative radiation exposures [45,46,47,48]. However, as these imaging tools provide better depiction of the anatomy of interest, and better guidance for interventional procedures, their intelligent use can result in more efficient procedures, with less fluoroscopy time, fewer DSA runs and a reduction in radiation exposure [31].
Staff working with fluoroscopy should have adequate knowledge of radiation protection [39]. The major contributor to exposure of medical personnel comes from scattered radiation from the patient. Every effort to reduce radiation dose to the patient has a corresponding effect on workers. The intensity of scattered radiation is greatest at the x-ray tube side of the C-arm, so examinations should be performed with an under-couch tube. This will reduce the amount of scattered radiation to the head and chest of medical personnel operating the equipment. When a lateral projection is used, staff should stand on the detector side of the C-arm.
The Concept of Substantial Radiation Dose Levels (SRDL) and Trigger Levels
Proper management of patient radiation dose during FGI procedures requires that the amount of radiation being administered is monitored and that the interventionalist is aware of this amount. Since the interventionalist should be concentrating on the clinical requirements of the interventional procedure, it is appropriate to assign this responsibility to another person, typically a radiographer or nurse. That individual should notify the interventionalist when certain dose levels (Table 2) have been reached [16]. These notifications can act as a trigger to the interventionalist to consider the radiation dose already delivered and the additional radiation likely necessary to complete the procedure [49]. Thought should be given to ways in which the radiation dose can be managed to keep the PSD as low as possible [6]. PSD is still not routinely reported on angiographic equipment and in the future, it will probably be available. Only in rare circumstances should a therapeutic procedure be stopped if the only reason is the radiation dose—if the clinical purpose has not been accomplished the patient receives no benefit from the radiation already administered.
Table 2 provides notification values for four different radiation dose quantities, PSD (Dskin,max), cumulative dose at the interventional reference point (Ka,r also known as cumulative air kerma and reference air kerma), PKA and fluoroscopy time. These values have been recommended by U.S. and European interventional radiology societies including CIRSE [49, 50]. Since the principal clinical concern is avoidance of a tissue reaction, Dskin,max is the most useful of these quantities, followed by Ka,r and PKA. Fluoroscopy time should not be relied on unless no other dose metric is available. With modern fluoroscopy equipment, this should never be the case.
The SRDL, the rightmost column in Table 2, is a level that, if reached, should trigger additional dose management and certain follow-up actions after the procedure. In can be thought of as a trigger level to initiate follow-up of a radiation dose that might produce a clinically relevant injury in an average patient [49, 51]. The SRDL values shown in Table 2 for various dose quantities are based on skin radiobiology [4]. The SRDL value can vary depending on patient parameters and should be reduced if known sensitizing factors are present. A lower SRDL value should be used when high levels of radiation have been administered to the same skin region in previous FGI procedures or when there is previous or planned radiation therapy in the same anatomical region. If the appropriate SRDL value is uncertain, initiating follow-up may be appropriate regardless of the final value of the radiation dose quantity.
Dose Management Systems for Recognizing and Avoiding Higher Patient Exposures
Dose management systems (DMS) have been introduced in the past decade. They are not used for managing dose during the procedure—they are essentially a quality assurance tool. They monitor dose rather than managing it, but the term ‘dose management system’ is more commonly used. A DMS uses as input data from the radiation dose-structured report (RDSR) generated by the imaging device that, for fluoroscopy, includes dose parameters for all fluoroscopic and radiographic exposures during interventional procedures. These include PKA and Ka,r. The RDSR also includes information on C-arm angulation, table position and field size for each exposure. DMS eliminates the manual effort of tracking, collecting, collating and analyzing doses from different imaging procedures. They can provide temporal trends for doses for different interventional procedures and exposure histories for individual patients, a concept that has been gaining momentum [23, 52, 53]. The information from DMS can be utilized by the medical physicist to help in quality control and patient safety activities. It has been shown that the data provided by the DMS can help in reducing the number of cases with high patient radiation doses [52]. However, the high cost of DMS has created obstacles to greater adoption. Since bitmap dose reports are analyzed by using OCR, and this may introduce errors, their use is not recommended for the future.
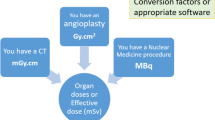
The DMS can use the information in the RDSR to provide an estimate of organ doses and effective dose E, but this estimate is based on typical anatomy and typical procedures, as the data in the RDSR do not define the location and extent of irradiation of specific internal organs and there is no ‘typical’ example for most FGI procedures. The uncertainty in the relative values of E for a reference patient can be as much as about 40% [54]. Use of the latest ICRP phantom is advisable to reduce some of these inaccuracies [55]. Even if E estimation is not available, DMS data on Ka,r can help with tissue injury risk assessment and DMS data on PKA may be useful in assessing the risk of a stochastic effect.
How to Manage Patients Exposed at or Above Trigger Levels and How to Communicate Risks Associated with Radiation to the Patient?
Radiation dose information should always be recorded in the patient’s medical record after completion of the procedure. For patients who have received a radiation dose greater than the SRDL during an FGI procedure, additional actions are necessary. The interventionalist should write an appropriate note in the patient’s medical record indicating that an SRDL has been exceeded and providing the reason. Because these patients should be followed clinically after the procedure, arrangements for radiation follow-up should be made before the patient leaves the facility. The patient should be told that the SRDL has been exceeded and should be given written radiation follow-up instructions in addition to their other discharge instructions. These instructions should include information on potential skin effects and their likely location. This will help the patient understand any skin changes that may develop. Patients, care givers and responsible healthcare professionals should be made aware of the possible radiologic etiology of relevant signs and symptoms. Patients must be informed of who to call if skin changes are observed.
The concept that the main radiation risk from interventional procedures is tissue injury is based on several factors: if the radiation dose is high enough, a tissue reaction is relatively immediate and (depending on dose and individual radiosensitivity) both predictable and certain; for older individuals, the latency period for clinical appearance of radiogenic cancer is such that cancer is unlikely to occur before death from some other cause, and the risk of a stochastic effect is much less than the medical risks of the FGI procedure or of alternative treatments. However, a recent study analyzing data from a single large tertiary care hospital over 8 years has shown that 4% patients received relatively high E (> 100 mSv) from some high-dose interventional procedures [24].
Management of Possible Tissue Reactions
Experience has shown that patients with radiation-induced skin injuries see many physicians before the correct diagnosis is established. Due to the uncommon nature of the injury and the time interval between the FGI procedure and the appearance of the skin injury, physicians, including dermatologists, may not consider radiation as the cause of the injury [5, 7,8,9,10,11,12]. For this reason, both ICRP and NCRP state that the interventionalist is responsible for patient follow-up for possible tissue injury until the likelihood of a reaction has passed [15, 16]. The patient should be instructed to notify the interventionalist of the results of self-examination of the irradiated area (either positive or negative). Telephone contact can be sufficient if no tissue reactions are reported. Clinical follow-up is arranged if the examination is positive. Although not all skin injuries related to FGI procedures are due to radiation [11], all suspicious findings should be treated as a probable radiation effect unless an alternative diagnosis is established. If radiation is suspected as the cause, it is essential to refer the patient to a physician experienced in managing radiation injuries (i.e., injuries from radiation oncology).
Legal Basis of Guidelines (European Basic Safety Standards)
The current European Basic Safety Standards Directive (Council Directive 2013/59/ EURATOM) [56] forms the legal basis for radiation protection in Europe. Countries in the European Union are supposed to transpose the Directive into their national regulatory framework. The points that pertain to interventional fluoroscopy practice are: use and regular review of diagnostic reference levels (DRLs), not only in diagnostic but also in interventional procedures; responsibilities for optimization; the role of medical physics experts in diagnostic and interventional procedures; new requirements for equipment; clinical audit and registry and analysis of accidental or unintended exposures of the patients, and requirements for competence in radiation protection of individuals involved.
The use and regular review of DRLs is a new requirement [57] and many European countries have DRLs for at least some FGI procedures. For interventional practice, it poses a challenge due to the wide range of complexity characteristic of interventional procedures [58,59,60,61]. The involvement of medical physicists in the optimization process is also required. However, the limitations of DRLs should be kept in view [62] and the use of certain percentile values (10, 25, 50, 75, 95th) can help in better optimization [23, 63].
There are equipment requirements in the directive. Any equipment used for interventional radiology shall have a device or a feature that provides information on the quantity of radiation produced by the equipment during the procedure and also shall have the capacity to transfer this dosimetric information to the record of the examination (equipment installed prior to February 6, 2018, may be exempted from this requirement). Member States shall ensure that “Information relating to patient exposure forms part of the report of the medical radiological procedure.”
There are requirements for provision of information to the referrer, the practitioner and the patient or their representative about clinically significant unintended or accidental exposures and the results of the analysis. These events are required to be declared to the competent authority as soon as possible. In the case of accidental or unintended exposures, a report should be produced for the Quality Assurance programme, together with an educational note to avoid the repetition of incidents. Communication with referrer and their awareness of this information needs to be strengthened [25, 26, 64].
Abbreviations
- CBCT:
-
Cone-beam computed tomography
- CT:
-
Computed tomography
- DMS:
-
Dose management systems, also called dose monitoring systems
- DRL:
-
Diagnostic reference level
- DSA:
-
Digital subtraction angiography
- EURATOM:
-
European atomic energy community
- FGI:
-
Fluoroscopically guided intervention
- ICRP:
-
International commission on radiological protection
- IR:
-
Interventional radiology
- NCRP:
-
National council on radiation protection and measurements
- PSD:
-
Peak skin dose
- RDSR:
-
Radiation dose-structured report
- SRDL:
-
Substantial radiation dose level
- TIPS:
-
Transjugular intrahepatic portosystemic shunt creation
References
López Aventín D, Gil I, López González DM, Pujol RM. Chronic scalp ulceration as a late complication of fluoroscopically guided cerebral aneurysm embolization. Dermatol Basel Switz. 2012;224(3):198–203. https://doi.org/10.1159/000338891.
Balter S, Miller DL. Patient skin reactions from interventional fluoroscopy procedures. AJR Am J Roentgenol. 2014;202(4):W335–42. https://doi.org/10.2214/AJR.13.12029.
Koenig TR, Mettler FA, Wagner LK. Skin injuries from fluoroscopically guided procedures: part 2, review of 73 cases and recommendations for minimizing dose delivered to patient. AJR Am J Roentgenol. 2001;177(1):13–20. https://doi.org/10.2214/ajr.177.1.1770013.
Balter S, Hopewell JW, Miller DL, Wagner LK, Zelefsky MJ. Fluoroscopically guided interventional procedures: a review of radiation effects on patients’ skin and hair. Radiology. 2010;254(2):326–41. https://doi.org/10.1148/radiol.2542082312.
Guesnier-Dopagne M, Boyer L, Pereira B, Guersen J, Motreff P, D’Incan M. Incidence of chronic radiodermatitis after fluoroscopically guided interventions: a retrospective study. J Vasc Interv Radiol JVIR. 2019;30(5):692-98.e13. https://doi.org/10.1016/j.jvir.2019.01.010.
Miller DL, Balter S, Noonan PT, Georgia JD. Minimizing radiation-induced skin injury in interventional radiology procedures. Radiology. 2002;225(2):329–36. https://doi.org/10.1148/radiol.2252011414.
Wei K-C, Yang K-C, Mar GY, Chen LW, Wu CS, Lai CC, et al. STROBE–radiation ulcer: An overlooked complication of fluoroscopic intervention: a cross-sectional study. Medicine (Baltimore). 2015;94(48):e2178. https://doi.org/10.1097/MD.0000000000002178.
Tsapaki V, Rehani MM. I perform more than 100 interventional procedures every year but have never seen radiation-induced skin injury: am I missing something? AJR Am J Roentgenol. 2014;203(5):W462–3. https://doi.org/10.2214/AJR.13.11765.
Srimahachota S, Udayachalerm W, Kupharang T, Sukwijit K, Krisanachinda A, Rehani M. Radiation skin injury caused by percutaneous coronary intervention, report of 3 cases. Int J Cardiol. 2012;154(2):e31–3. https://doi.org/10.1016/j.ijcard.2011.05.016.
Kostova-Lefterova D, Vassileva J, Rehani MM. Lessons from two cases of radiation induced skin injuries in fluoroscopic procedures in Bulgaria. J Radiol Prot. 2017;37(4):938–46. https://doi.org/10.1088/1361-6498/aa8ce7.
Ramirez M, Ravichandran S, Ronald L, et al. Recognition and management of dermatologic complications from interventional radiology procedures. Diagn Interv Imaging. 2019;100(11):659–70. https://doi.org/10.1016/j.diii.2019.06.007.
Rehani MM, Srimahachota S. Skin injuries in interventional procedures. Radiat Prot Dosimetry. 2011;147(1–2):8–12. https://doi.org/10.1093/rpd/ncr257.
Geleijns J, Wondergem J. X-ray imaging and the skin: radiation biology, patient dosimetry and observed effects. Radiat Prot Dosimetry. 2005;114(1–3):121–5. https://doi.org/10.1093/rpd/nch544.
Stewart FA, Akleyev AV, Hauer-Jensen M, Hendry JH, Kleiman NJ, Macvittie TJ, et al. ICRP publication 118: ICRP statement on tissue reactions and early and late effects of radiation in normal tissues and organs–threshold doses for tissue reactions in a radiation protection context. Ann ICRP. 2012;41(1–2):1–322. https://doi.org/10.1016/j.icrp.2012.02.001.
Cousins C, Miller DL, Bernardi G, Rehani MM, Schofield P, Vañó E, et al. ICRP publication 120: radiological protection in cardiology. Ann ICRP. 2013;42(1):1–125. https://doi.org/10.1016/j.icrp.2012.09.001.
NCRP Report 168. Radiation dose management for fluoroscopically- guided interventional medical procedures|NCRP|Bethesda, MD. Accessed May 16, 2020. https://ncrponline.org/publications/reports/ncrp-report-168/
NCRP Commentary No. 26: Guidance on radiation dose limits for the lens of the eye (2016) |NCRP|Bethesda, MD. Accessed May 16, 2020. https://ncrponline.org/shop/commentaries/commentary-no-26-guidance-on-radiation-dose-limits-for-the-lens-of-the-eye-2016/
Human Radiosensitivity: Report of the Independent Advisory Group on Ionising Radiation. Health Protection Agency; 2013.
Miller DL, Balter S, Cole PE, et al. Radiation doses in interventional radiology procedures: the RAD-IR study: part II: skin dose. J Vasc Interv Radiol JVIR. 2003;14(8):977–90. https://doi.org/10.1097/01.rvi.0000084601.43811.cb.
Rehani MM, Ciraj-Bjelac O, Vañó E, et al. ICRP Publication 117. Radiological protection in fluoroscopically guided procedures performed outside the imaging department. Ann ICRP. 2010; 40(6):1–102. doi:https://doi.org/10.1016/j.icrp.2012.03.001
Bryk SG, Censullo ML, Wagner LK, Rossman LL, Cohen AM. Endovascular and interventional procedures in obese patients: a review of procedural technique modifications and radiation management. J Vasc Interv Radiol JVIR. 2006;17(1):27–33. https://doi.org/10.1097/01.RVI.0000186953.44651.19.
Koenig TR, Wolff D, Mettler FA, et al. Skin injuries from fluoroscopically guided procedures. Part 1. Characteristics of radiation injury. AJR Am J Roentgenol. 2001;177:3–11.
Li X, Hirsch JA, Rehani MM, Yang K, Liu B. Effective dose assessment for patients undergoing contemporary fluoroscopically guided interventional procedures. Am J Roentgenol. 2019;214(1):158–70. https://doi.org/10.2214/AJR.19.21804.
Li X, Hirsch JA, Rehani MM, Ganguli S, Yang K, Liu B. Radiation effective dose above 100 mSv from fluoroscopically guided intervention: frequency and patient medical condition. Am J Roentgenol. 2020. https://doi.org/10.2214/AJR.19.22227.
NCRP Report No. 185. Evaluating and Communicating Radiation Risks for Studies Involving Human Subjects: Guidance for Researchers and Institutional Review Boards (2020) |NCRP|Bethesda, MD. Accessed May 23, 2020. https://ncrponline.org/shop/reports/report-no-185-evaluating-and-communicating-radiation-risks-for-studies-involving-human-subjects-guidance-for-researchers-and-institutional-review-boards-2020/
WHO Communicating radiation risks in paediatric imaging. WHO. Accessed May 23, 2020. https://www.who.int/ionizing_radiation/pub_meet/radiation-risks-paediatric-imaging/en/
Rehani MM. Radiation effects and risks: overview and a new risk perception index. Radiat Prot Dosimetry. 2015a;165(1–4):7–9. https://doi.org/10.1093/rpd/ncv117.
Eide KR, Ødegård A, Myhre HO, Lydersen S, Hatlinghus S, Haraldseth O. DynaCT during EVAR–a comparison with multidetector CT. Eur J Vasc Endovasc Surg Off J Eur Soc Vasc Surg. 2009;37(1):23–30. https://doi.org/10.1016/j.ejvs.2008.09.017.
Dijkstra ML, Eagleton MJ, Greenberg RK, Mastracci T, Hernandez A. Intraoperative C-arm cone-beam computed tomography in fenestrated/branched aortic endografting. J Vasc Surg. 2011;53(3):583–90. https://doi.org/10.1016/j.jvs.2010.09.039.
Kothary N, Abdelmaksoud MHK, Tognolini A, et al. Imaging guidance with C-arm CT: prospective evaluation of its impact on patient radiation exposure during transhepatic arterial chemoembolization. J Vasc Interv Radiol. 2011;22(11):1535–43. https://doi.org/10.1016/j.jvir.2011.07.008.
Bartal G, Vano E, Paulo G, Miller DL. Management of patient and staff radiation dose in interventional radiology: current concepts. Cardiovasc Intervent Radiol. 2014;37(2):289–98. https://doi.org/10.1007/s00270-013-0685-0.
Rehani MM. Challenges in radiation protection of patients for the 21st century. Am J Roentgenol. 2013;200(4):762–4. https://doi.org/10.2214/AJR.12.10244.
Prasad KN, Cole WC, Haase GM. Radiation protection in humans: extending the concept of as low as reasonably achievable (ALARA) from dose to biological damage. Br J Radiol. 2004;77(914):97–9. https://doi.org/10.1259/bjr/88081058.
Jones AK, Dixon RG, Collins JD, Walser EM, Nikolic B. Society of interventional radiology health and safety committee best practice guidelines for CT-guided interventional procedures. J Vasc Interv Radiol JVIR. 2018;29(4):518–9.
Posters and leaflets about radiation protection. Published July 26, 2017. Accessed June 13, 2020. https://www.iaea.org/resources/rpop/resources/posters-and-leaflets
Jones AK, Balter S, Rauch P, Wagner LK. Medical imaging using ionizing radiation: optimization of dose and image quality in fluoroscopy. Med Phys. 2014;41(1):014301. https://doi.org/10.1118/1.4835495.
Heidbuchel H, Wittkampf FHM, Vano E, et al. Practical ways to reduce radiation dose for patients and staff during device implantations and electrophysiological procedures. EP Eur. 2014;16(7):946–64. https://doi.org/10.1093/europace/eut409.
Orth RC, Wallace MJ, Kuo MD. C-arm cone-beam CT: general principles and technical considerations for use in interventional radiology. J Vasc Interv Radiol. 2009;20(7):S538–44. https://doi.org/10.1016/j.jvir.2009.04.026.
Vañó E, Rosenstein M, Liniecki J, Rehani MM, Martin CJ, Vetter RJ. ICRP Publication 113. Education and training in radiological protection for diagnostic and interventional procedures. Ann ICRP. 2009;39(5):7–68. doi:https://doi.org/10.1016/j.icrp.2011.01.002
Rehani MM, Gupta R, Bartling S, et al. Radiological protection in cone-beam computed tomography (CBCT). ICRP Publication 129. Ann ICRP. 2015;44(1):9–127. doi:https://doi.org/10.1177/0146645315575485
Rehani MM. Radiological protection in computed tomography and cone-beam computed tomography. Ann ICRP. 2015b;44(1 Suppl):229–35. https://doi.org/10.1177/0146645315575872.
Pitton MB, Kloeckner R, Schneider J, Ruckes C, Bersch A, Düber C. Radiation exposure in vascular angiographic procedures. J Vasc Interv Radiol JVIR. 2012;23(11):1487–95. https://doi.org/10.1016/j.jvir.2012.05.048.
Vano E, Gonzalez L, Fernandez JM, Prieto C, Guibelalde E. Influence of patient thickness and operation modes on occupational and patient radiation doses in interventional cardiology. Radiat Prot Dosimetry. 2006;118(3):325–30. https://doi.org/10.1093/rpd/nci369.
Tsapaki V, Vano E, Muavrikou I, et al. Comparison of patient dose in two-dimensional carotid arteriography and three-dimensional rotational angiography. Cardiovasc Intervent Radiol. 2008;31(3):477–82. https://doi.org/10.1007/s00270-007-9190-7.
Schulz B, Heidenreich R, Heidenreich M, et al. Radiation exposure to operating staff during rotational flat-panel angiography and C-arm cone-beam computed tomography (CT) applications. Eur J Radiol. 2012;81(12):4138–42. https://doi.org/10.1016/j.ejrad.2012.01.010.
Rehani MM, Yang K, Melick ER, et al. Patients undergoing recurrent CT scans: assessing the magnitude. Eur Radiol. 2020;30(4):1828–36. https://doi.org/10.1007/s00330-019-06523-y.
Iwazawa J, Ohue S, Mitani T, et al. Identifying feeding arteries during TACE of hepatic tumors: comparison of C-arm CT and digital subtraction angiography. AJR Am J Roentgenol. 2009;192(4):1057–63. https://doi.org/10.2214/AJR.08.1285.
Brambilla M, Vassileva J, Kuchcinska A, Rehani MM. Multinational data on cumulative radiation exposure of patients from recurrent radiological procedures: call for action. Eur Radiol. 2020;30(5):2493–501. https://doi.org/10.1007/s00330-019-06528-7.
Jaschke W, Bartal G, Martin CJ, Vano E. Unintended and accidental exposures, significant dose events and trigger levels in interventional radiology [published online ahead of print, 2020 May 20]. Cardiovasc Intervent Radiol. 2020;https://doi.org/10.1007/s00270-020-02517-2. doi:https://doi.org/10.1007/s00270-020-02517-2
Stecker MS, Balter S, Towbin RB, et al. Guidelines for patient radiation dose management. J Vasc Interv Radiol JVIR. 2009;20(7 Suppl):S263–73. https://doi.org/10.1016/j.jvir.2009.04.037.
Vano E, Miller DL, Martin CJ, Rehani MM, Kang K, Rosenstein M, et al. ICRP publication 135: diagnostic reference levels in medical imaging. Ann ICRP. 2017;46(1):1–144. https://doi.org/10.1177/0146645317717209.
Liu B, Hirsch JA, Li X, et al. Radiation dose monitoring for fluoroscopically guided interventional procedures: effect on patient radiation exposure. Radiology. 2019;290(3):744–9. https://doi.org/10.1148/radiol.2019180799.
Seuri R, Rehani MM, Kortesniemi M. How tracking radiologic procedures and dose helps: experience from Finland. Am J Roentgenol. 2013;200(4):771–5. https://doi.org/10.2214/AJR.12.10112.
Martin CJ. Effective dose: how should it be applied to medical exposures. Br J Radiol. 2007;80(956):639–47.
Martin CJ, Harrison JD, Rehani MM. Effective dose from radiation exposure in medicine. Past, present and future. Phys Med 2020. https://doi.org/10.1016/j.ejmp.2020.10.020.
Council Directive 2013/59/Euratom of 5 December 2013 laying down basic safety standards for protection against the dangers arising from exposure to ionising radiation, and repealing Directives 89/618/Euratom, 90/641/Euratom, 96/29/Euratom, 97/43/Euratom and 2003/122/Euratom. :73.
Compagnone G, Padovani R, D’Ercole L, et al. Provision of Italian diagnostic reference levels for diagnostic and interventional radiology. Radiol Med (Torino). 2020. https://doi.org/10.1007/s11547-020-01165-3.
Fetterly KA, Lennon RJ, Bell MR, Holmes DR, Rihal CS. Clinical determinants of radiation dose in percutaneous coronary interventional procedures: influence of patient size, procedure complexity, and performing physician. JACC Cardiovasc Interv. 2011;4(3):336–43. https://doi.org/10.1016/j.jcin.2010.10.014.
D’Ercole L, Thyrion FZ, Bocchiola M, Mantovani L, Klersy C. Proposed local diagnostic reference levels in angiography and interventional neuroradiology and a preliminary analysis according to the complexity of the procedures. Phys Medica PM Int J Devoted Appl Phys Med Biol Off J Ital Assoc Biomed Phys AIFB. 2012;28(1):61–70. https://doi.org/10.1016/j.ejmp.2010.10.008.
Ruiz-Cruces R, Vano E, Carrera-Magariño F, et al. Diagnostic reference levels and complexity indices in interventional radiology: a national programme. Eur Radiol. 2016;26(12):4268–76. https://doi.org/10.1007/s00330-016-4334-2.
International Atomic Energy Agency. Establishing Guidance Levels in X Ray Guided Medical Interventional Procedures: A Pilot Study. International Atomic Energy Agency; 2009.
Rehani MM. Limitations of diagnostic reference level (DRL) and introduction of acceptable quality dose (AQD). Br J Radiol. 2014;88(1045):20140344. https://doi.org/10.1259/bjr.20140344.
Roch P, Célier D, Dessaud C, Etard C, Rehani MM. Long-term experience and analysis of data on diagnostic reference levels: the good, the bad, and the ugly. Eur Radiol. 2020;30(2):1127–36. https://doi.org/10.1007/s00330-019-06422-2.
Rehani MM, Berris T. International Atomic Energy Agency study with referring physicians on patient radiation exposure and its tracking: a prospective survey using a web-based questionnaire. BMJ Open. 2012;2(5):e001425. https://doi.org/10.1136/bmjopen-2012-001425.
Funding
This study was not supported by any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Consent for Publication
For this type of review, article consent for publication is not required.
Ethical Approval
This article does not contain any studies with human participants performed by any of the authors. Being a review article, IRB approval is not required.
Informed Consent
For this type of review article, informed consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rehani, M.M., Miller, D.L. & Baliyan, V. High-Dose Fluoroscopically Guided Procedures in Patients: Radiation Management Recommendations for Interventionalists. Cardiovasc Intervent Radiol 44, 849–856 (2021). https://doi.org/10.1007/s00270-020-02703-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-020-02703-2