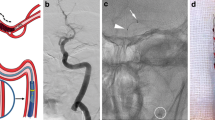
Abstract
Background and Purpose
Direct thrombus aspiration is increasingly used as a first-line therapy in acute ischemic stroke with large vessel occlusion. We assessed the performance and safety of a novel aspiration catheter available: the 6-French AXS Catalyst catheter.
Materials and Methods
We conducted a cohort study from a prospective clinical registry of consecutive stroke patients treated by mechanical thrombectomy between March 2016 and July 2016. Baseline clinical and imaging characteristics, recanalization rates, complications, and clinical outcomes were analyzed.
Results
Among the 60 patients included, 30 were treated using aspiration alone, 14 were treated using aspiration and then stent retriever as a rescue therapy, and 16 were treated using aspiration combined with a stent retriever straightaway. Successful recanalization (mTICI2b/3) was achieved in 85% patients and functional independence in 48.3%. We observed one intracranial perforation and one vertebral artery dissection. Symptomatic intracranial hemorrhage occurred in 5% and mortality in 21.7%.
Conclusion
Endovascular stroke therapy using the AXS Catalyst catheter seems safe and effective, with similar performance than other reperfusion catheters.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recent guidelines include mechanical thrombectomy with stent retriever in the early management of patients with acute ischemic stroke with large vessel occlusion (AIS-LVO) [1]. Fewer data on safety and efficacy are available on other endovascular techniques such as direct aspiration. However, some authors reported high recanalization rates using a Direct Aspiration First Pass Technique (ADAPT) [2], based on a first-line large-bore aspiration catheter advanced in direct contact with the clot. Other studies reported shorter recanalization time and reduced cost when using ADAPT [3]. Other endovascular strategies have been described using a combined approach with a stent retriever and a distal aspiration catheter, such as the stent-retriever-assisted vacuum-locked extraction (SAVE) or the aspiration-retriever technique for stroke (ARTS) techniques [4, 5]. The 6-French AXS Catalyst (Stryker Neurovascular, Mountain View, CA, USA) is a large-bore intermediate and aspiration catheter designed for endovascular management of acute ischemic stroke. It combines a long rigid stainless-steel reinforced shaft and a flexible Nitinol braided distal part to provide enhanced push and trackability for challenging cases with unstable catheterization of tortuous vessels. We aimed to assess the performance and safety of the 6-French AXS Catalyst as an aspiration catheter in first-line endovascular treatment of AIS-LVO.
Methods
Study Design
We conducted a cohort study from the prospective clinical registry of consecutive stroke patients treated by MT in our comprehensive stroke center. The ethical committee classified the study as observation on March 9, 2010, and the patient’s personal data-protecting committee approved the study on December 21, 2010 (n° 10.677). Patients and their relatives gave their informed consent to participate.
Population
All consecutive AIS-LVO patients who underwent MT with the 6-French AXS Catalyst catheter between March 2016 and July 2016 at a single academic comprehensive stroke center were included for analysis. Eligibility criteria for IV thrombolysis (IVT) and MT were previously published [6]. Upon admission, patients underwent brain MRI with intracranial time-of-flight MR-angiography (TOF-MRA) as first-line imaging (or CT and CTA, if presenting a MR contraindication). A follow-up brain MRI with contrast-enhanced MR-angiography (CE-MRA), or CT and CTA, if contraindication, was routinely performed 24–36 h after treatment.
Endovascular Procedure
MT procedures were usually performed within 8 h of symptom onset, under conscious sedation, using a transfemoral approach. An initial diagnostic cerebral angiogram was performed to assess intracranial clot location (middle cerebral artery, intracranial internal carotid artery, or basilar artery). A 6-French long introducer sheath (Flexor Shuttle Select, Cook, Bloomington, Indiana, USA) was placed in the target cervical artery (internal carotid artery or vertebral artery). The 6-French AXS Catalyst catheter was placed in contact with the clot in a wedge position over a 0.021″ microcatheter (Prowler Select Plus, Cerenovus, or Headway, Microvention) and a 0.014″ guidewire (Synchro, Stryker or Radifocus, Terumo). Clot removal was performed using contact aspiration as the first-line treatment (aspiration first group) or in conjunction with a stent retriever (aspiration and stent-retriever group) depending on the operator’s choice. Aspiration was always performed using a dedicated mechanical pump. During contact aspiration, thrombus perforation was avoided whenever possible, and both microcatheter and guidewire were retrieved to improve suction force. In case of tandem occlusion, treatment of the extracranial ICA could involve balloon angioplasty or stenting as a retrograde approach whenever possible.
Clinical Data Collection
Basic demographic characteristics, including vascular risk factors and vascular history, were prospectively collected. The National Institutes of Health Stroke Scale (NIHSS) was evaluated by stroke neurologists upon admission and 24 h after recanalization attempt. Times of symptom onset, IVT, groin puncture, and recanalization were recorded. The etiology of stroke was determined using the TOAST classification [7]. Symptomatic intracerebral hemorrhage (sICH) was defined according to ECASS-2 criteria [8]. Scores on the modified Rankin scale (mRS) were assessed at 3 months by a stroke neurologist through formal, structured in-person interviews [9]. For patients who did not undergo the visit with the neurologist, a mRS score was obtained by telephone contact with the patient or the closest caregiver.
Imaging Data Collection
All images were reviewed by neuroradiologists blinded to clinical data. The extent of ischemia was estimated using DWI-ASPECT (or CT-ASPECT) scores on baseline. Intracerebral hemorrhage (ICH) on day-1 imaging was analyzed according to the ECASS criteria adapted to MRI [8, 10]: hemorrhagic infarction (HI-1 or HI-2) and parenchymal hematoma (PH-1 or PH-2). Recanalization was rated on the basis of findings on post-procedural conventional angiography using the modified Thrombolysis In Cerebral Ischemia (mTICI) scale. Successful recanalization was defined as a grade of 2b (50–99% reperfusion), 2c (near-complete reperfusion with remnant distal cortical vascular defect), or 3 (complete reperfusion) on the mTICI scale [11]. First-pass effect was defined as a complete mTICI3 recanalization of the downstream territory after a single pass of a device and no use of rescue therapy. Procedure-related adverse events were recorded. Anatomical difficulties (tortuosity, loops) and clot location (M1-MCA, M2-MCA, intracranial ICA or basilar artery) were assessed on conventional angiography images and reports. Extracranial tortuosity was defined as the presence of tortuosity (S- or C-shaped elongation), kinking (acute angulation of the ICA < 60°), or coiling (exaggerated S-shaped curve or a circular configuration) [12]. The presence of cavernous ICA tortuosity was assessed subjectively on DSA.
Outcomes
The primary outcomes were the rate of successful recanalization of the target intracranial artery defined as a mTICI score of 2b, 2c or 3, and the incidence of procedure-related complications within 24 h after the procedure. Secondary outcomes were the rate of functional independence at three mounts (mRS 0–2): parenchymal hematoma, sICH, and mortality.
Statistics
Continuous variables were expressed as mean (standard deviation, SD) or as median (interquartile range, IQR) in case of non-normal distribution. Normality of distributions was assessed using histograms and the Shapiro–Wilk test. Categorical variables were expressed as frequencies (percentages). Statistical analyses were performed using SPSS version 25 (SPSS Inc., Chicago, IL, USA).
Results
Population
During the study period, among the 89 patients eligible for inclusion, 29 were excluded: 14 patients with target artery catheterization failure, 12 patients treated with another thrombectomy device, and three patients treated by ICA angioplasty/stenting without intracranial thrombectomy. A total of 60 consecutive patients treated initially with the 6-French AXS Catalyst catheter were included for analysis. Patients’ demographics and basic clinical baseline characteristics are reported in Table 1. Overall, the mean age was 69 years (SD 17.5), 60% were female (36/60), and mean initial NIHSS was 17 (SD 6.2). The intracranial occlusion site was M1 middle cerebral artery (M1-MCA) in 37/60 (61.7%), M2 middle cerebral artery (M2-MCA) in 7/60 (11.7%), intracranial carotid artery (ICA) in 10/60 (16.7%), and basilar artery in 6/60 (10%) patients. Out of 60 patients, 9 (15%) presented with tandem occlusions. IV thrombolysis was administered to 42/60 (70%) patients prior to MT.
Patient Outcome
At 3 months, 29/60 (48.3%) patients achieved functional independence (mRS 0-2), and 13/60 (21.7%) patients died. Hemorrhagic transformation occurred in 27/60 (%) patients: eight HI-1, eight HI-2, six PH-1, four PH-2. Symptomatic intracranial hemorrhage (sICH) occurred in 3/60 (5%) patients.
Endovascular Procedure
Out of 60 patients, 44 (73.3%) were treated using 6-F Catalyst aspiration as first line, achieving successful recanalization in 39/44 patients (88.6%) in a mean procedural time of 30 min.
Among these patients, 30/44 were treated using aspiration alone achieving successful recanalization in 83.3%, with a 60% first-pass effect, in a mean procedural time of 22 min.
However, a stent retriever was required as a rescue device in 14/44 patients, achieving successful recanalization in all cases in a mean procedural time of 42 min.
Out of 60 patients, 16 (26.7%) were treated using aspiration combined with a stent retriever straightaway, achieving successful recanalization in 12/16 patients (75%), with a 25% first-pass effect, in a mean procedural time of 43 min.
Overall, successful recanalization (mTICI ≥ 2b) was achieved in 86.7% (52/60) of patients and complete recanalization (mTICI 3) in 31/60 (51.7%) patients. The 6-F AXS Catalyst was successfully advanced in contact with the clot in 95.0% (57/60). It crossed the ophthalmic segment of the ICA in 96.3% (53/55) of patients with anterior circulation occlusion. Tortuosity of the extracranial or intracranial vessels was observed in 55.0% (33/60) of patients. The median time from symptom to groin puncture was 224 min (IQR 150–286), median time from groin puncture to clot (when achieved) was 15 min (IQR 10–23), and median time of procedure was 32 min (IQR 20–50). Table 2 shows angiographic outcomes.
Complications
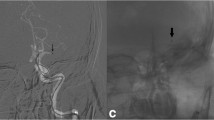
No erratic embolus in a new territory was detected on the final angiograms. A perforation of a distal M2 branch requiring coil embolization occurred in one patient (presenting major vascular tortuosity). One vertebral artery dissection was evidenced on DSA.
Discussion
Our general results are in accordance with the previously published literature which reported similar success rate, especially with the aspiration group. In the ASTER trial, Lapergue et al. reported 85.4% of successful recanalization with the same technique [2]. However, we observed a slightly lower success rate in the aspiration and stent-retriever group where successful revascularization was achieved in only 75% of patients versus 86.2% in the ASTER trial. This difference may be explained by the small sample size in this group and the higher prevalence of ICA occlusions than in the direct aspiration group (37.5% vs 9.1%), which may be considered as more challenging cases.
The Hermes meta-analysis [13] reported 29% of failed recanalization (mTICI0-2a). In our study, among the 9/60 (15%) patients who did not achieve TICI2b/3, four had a pre-stroke mRS > 2 and one patient was recovering from a recent stroke (mRS = 2). The operators might have treated these patients with less commitment knowing the predictable poor outcome as pre-stroke mRS has been shown as a marker of poor prognosis [14]. Amid the four other patients, one suffered from intracranial perforation which resulted in arterial coiling. Moreover, recent studies have reported poor recanalization rates with fibrin-rich or calcified clots [15, 16]. We supposed that such challenging clots could be responsible for most recanalization failures.
The performance of the aspiration catheter in the aspiration and stent-retriever group is difficult to analyze since the performance of each device cannot be separated. The recanalization could be strongly correlated to the presence of a stent retriever. Moreover, since no randomization was performed, this study is exposed to selection bias.
The 6-F AXS Catalyst catheter has a distal inner diameter of 0.060 in. (1.52 mm) and is then considered as a large-bore catheter. It provides with a distal inner diameter similar to the ACE 68 reperfusion catheter (Penumbra) and the Sofia Plus (Microvention) that have, respectively, a distal inner diameter of 0.068″ (1.73 mm) and 0.070″ (1.78). This difference in diameter is probably responsible for a slight decrease in suction force which did not translate into lower recanalization rate. Its distal part is reinforced with Nitinol wiring which gives it more push and trackability. A classical challenge when performing contact aspiration is to successfully bypass the ophthalmic artery which was achieved in 96.3% patients in this study.
As found in the literature, we recorded quicker recanalization time using aspiration only as a first-line treatment. We observed several hemorrhagic complications in our cohort: 10/60 (16.7%) patients had a PH-1 or PH-2 on day-1 MRI. This finding might be explained by the small sample size and the ICH rating on MRI [17] which might overrate HI-2 to PH-1. Moreover, we observed similar rate of symptomatic ICH than in the published literature. In the Hermes pooled data meta-analysis, Goyal et al. reported 4.4% sICH in the intervention group versus 5% in this study [18]. Further, the occurrence of ICH has been shown to be higher in patients with tandem occlusions or large infarct core, which were not considered as exclusion criteria in our analysis [19]. Five percent of patients developed a sICH, whereas Mocco et al. [20] in the therapy trial observed 9.3% in a group treated with aspiration and thrombolysis. In terms of mortality, 21.7% of our patients were found dead at three months which did not differ in comparison with the ADAPT trial, where mortality rate was 20% [21].
We observed one distal M2-MCA perforation. Two steps could be responsible: distal guidewire perforation and contact aspiration with a large-bore 6-F catheter in a distal M2-MCA artery. The vertebral artery dissection occurred while attempting to reach the clot with the 6-F Catalyst catheter via a hypoplastic vertebral artery. In a recently published article, Sallustio et al. [22] reported a distal perforation and an embolus using the 6-F Catalyst for first-line aspiration mechanical thrombectomy. We observed no embolus in a different territory, which is probably due to the small sample size, but this also gives insight into the performance and safety of this device.
As described in the literature, we observed a reduced time of procedure in the aspiration only group with a median time of 22 min per procedure versus 43 min in the aspiration and stent-retriever group; Turk et al. manage to obtain mTICI2b/3 recanalization in a mean time of 37 min in an aspiration only group with another catheter (3).
This study is mainly limited by its observational design and by its sample size leading to an exposure to strong selection bias.
Conclusion
Endovascular stroke therapy with the 6-French AXS Catalyst catheter seems safe and effective. It provided recanalization rates similar to other reperfusion catheters in competitive procedural times.
Abbreviations
- AIS:
-
Acute ischemic stroke
- ADAPT:
-
A Direct Aspiration First Pass Technique
- HI:
-
Hemorrhagic infarction
- ICA:
-
Internal carotid artery
- IVT:
-
Intravenous thrombolysis
- LVO:
-
Large vessel occlusion
- MCA:
-
Middle cerebral artery
- MT:
-
Mechanical thrombectomy
- mTICI:
-
Modified Thrombolysis In Cerebral Infarction
- PH:
-
Parenchymal hemorrhage
- sICH:
-
Symptomatic intracerebral hemorrhage
References
Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018. https://doi.org/10.1161/STR.0000000000000158.
Lapergue B, Blanc R, Gory B, Labreuche J, Duhamel A, Marnat G, et al. Effect of endovascular contact aspiration vs stent retriever on revascularization in patients with acute ischemic stroke and large vessel occlusion: the ASTER randomized clinical trial. JAMA. 2017;318:443–52.
Turk AS, Frei D, Fiorella D, Mocco J, Baxter B, Siddiqui A, et al. ADAPT FAST study: a direct aspiration first pass technique for acute stroke thrombectomy. J Neurointerv Surg. 2014;6:260–4.
Massari F, Henninger N, Lozano JD, Patel A, Kuhn AL, Howk M, et al. ARTS (aspiration-retriever technique for stroke): initial clinical experience. Interv Neuroradiol. 2016;22:325–32.
Maus V, Behme D, Kabbasch C, Borggrefe J, Tsogkas I, Nikoubashman O, et al. Maximizing first-pass complete reperfusion with SAVE. Clin Neuroradiol. 2017;28:1–12.
Ferrigno M, Bricout N, Leys D, Estrade L, Cordonnier C, Personnic T, et al. Intravenous recombinant tissue-type plasminogen activator: influence on outcome in anterior circulation ischemic stroke treated by mechanical thrombectomy. Stroke. 2018;49:1377–85.
Adams HP, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke. 1993;24:35–41.
Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet Lond Engl. 1998;352:1245–51.
van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–7.
Neeb L, Villringer K, Galinovic I, Grosse-Dresselhaus F, Ganeshan R, Gierhake D, et al. Adapting the computed tomography criteria of hemorrhagic transformation to stroke magnetic resonance imaging. Cerebrovasc Dis Extra. 2013;3:103–10.
Zaidat OO, Yoo AJ, Khatri P, Tomsick TA, von Kummer R, Saver JL, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke. 2013;44:2650–63.
Togay-Işikay C, Kim J, Betterman K, Andrews C, Meads D, Tesh P, et al. Carotid artery tortuosity, kinking, coiling: stroke risk factor, marker, or curiosity? Acta Neurol Belg. 2005;105:68–72.
Goyal M, Menon BK, Van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723–31.
Quinn TJ, Taylor-Rowan M, Coyte A, Clark AB, Musgrave SD, Metcalf AK, et al. Pre-stroke modified rankin scale: evaluation of validity, prognostic accuracy, and association with treatment. Front Neurol. 2017;8:275.
Dobrocky T, Piechowiak E, Cianfoni A, Zibold F, Roccatagliata L, Mosimann P, et al. Thrombectomy of calcified emboli in stroke. Does histology of thrombi influence the effectiveness of thrombectomy? J NeuroInterv Surg. 2018;10:345–50.
Gunning GM, McArdle K, Mirza M, Duffy S, Gilvarry M, Brouwer PA. Clot friction variation with fibrin content; implications for resistance to thrombectomy. J Neurointerv Surg. 2017;10:34–8.
Kidwell CS, Chalela JA, Saver JL, Starkman S, Hill MD, Demchuk AM, et al. Comparison of MRI and CT for detection of acute intracerebral hemorrhage. JAMA. 2004;292:1823–30.
Goyal M, Menon BK, van Zwam WH, Dippel DWJ, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723–31.
Vora NA, Gupta R, Thomas AJ, Horowitz MB, Tayal AH, Hammer MD, et al. Factors predicting hemorrhagic complications after multimodal reperfusion therapy for acute ischemic stroke. Am J Neuroradiol. 2007;28:1391–4.
Mocco J, Zaidat OO, von Kummer R, Yoo AJ, Gupta R, Lopes D, et al. Aspiration thrombectomy after intravenous alteplase versus intravenous alteplase alone. Stroke. 2016;47:2331–8.
Blanc R, Redjem H, Ciccio G, Smajda S, Desilles J-P, Orng E, et al. Predictors of the aspiration component success of a direct aspiration first pass technique (ADAPT) for the endovascular treatment of stroke reperfusion strategy in anterior circulation acute stroke. Stroke. 2017;48:1588–93.
Sallustio F, Pampana E, Davoli A, Merolla S, Koch G, Alemseged F, et al. Mechanical thrombectomy of acute ischemic stroke with a new intermediate aspiration catheter: preliminary results. J NeuroInterv Surg. 2018. https://doi.org/10.1136/neurintsurg-2017-013679.
Funding
This work was supported by Stryker Neurovascular. The funding source was not involved in study design, monitoring, data collection, statistical analyses, interpretation of results, or manuscript writing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Consent for Publication
Consent for publication was obtained for every individual person’s data included in the study.
Rights and permissions
About this article
Cite this article
Bretzner, M., Estrade, L., Ferrigno, M. et al. Endovascular Stroke Therapy with a Novel 6-French Aspiration Catheter. Cardiovasc Intervent Radiol 42, 110–115 (2019). https://doi.org/10.1007/s00270-018-2093-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-018-2093-y