Abstract
Background
Bronchial artery embolisation (BAE) is recommended for the treatment of massive haemoptysis in cystic fibrosis (CF), but there are no randomised controlled trials of this therapy and its role in sub-massive haemoptysis is unclear. This study aimed to determine the outcomes and safety of BAE in adults with CF.
Materials and Methods
All patients with CF undergoing BAE at our centre between March 2011 and January 2015 were identified at the time of the procedure. Patient records were reviewed at hospital discharge, death or one month post-procedure (whichever was soonest). Follow-up continued to January 2016. Severity of haemoptysis was classified as: massive (>240 ml/24 h or >100 ml/day for ≥2 days), moderate–severe (>20 ml/24 h) or mild (<20 ml/24 h).
Results
Twenty-seven patients underwent 51 BAE procedures over a median follow-up period of 26 months (range 1–54). Ten patients (37%) required more than one BAE during the study. BAE was performed for massive haemoptysis in 18 cases (35%). Haemoptysis recurred after 31 (61%) of BAE procedures with no difference in recurrence rates between massive and sub-massive haemoptysis. Side effects were reported after 61% of procedures with chest pain the most common adverse event . Mortality after first BAE in the study was 3.9% at 30 days and 14.8% at 12 months. No significant predictors of mortality were identified.
Conclusions
BAE is often effective in controlling haemoptysis but is associated with considerable morbidity and high recurrence rates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Haemoptysis is a common and potentially life-threatening complication of cystic fibrosis (CF). Bronchial artery embolisation (BAE) is accepted as the treatment of choice in massive haemoptysis [1, 2], but there remains a paucity of robust, prospective data to inform clinicians on the optimal use of this technique. Particular uncertainties exist over the safety and efficacy of BAE in sub-massive haemoptysis, although a small retrospective study of eight patients suggested that early BAE may reduce rates of haemoptysis recurrence [3].
Data from the US CF Foundation registry suggest an incidence of haemoptysis in CF of 9.1% over a five-year period [4]. Thompson et al. [5] reported a higher incidence of haemoptysis in a pooled population of patients participating in clinical trials 8% of patients experiencing haemoptysis over a median of 8 months of follow-up. In the majority of cases, haemoptysis in CF is relatively minor and self-limiting [5]. Massive haemoptysis is fortunately much less common with an annual incidence of <1% but is associated with substantially increased morbidity and mortality [4].
We prospectively identified all patients with CF undergoing BAE at our adult CF centre over a 46-month period. Using this population, we performed a single-centre observational study to investigate the safety and effectiveness of BAE among adults with CF. Some of the results have been presented in abstract form previously [6].
Materials and Methods
All adult patients with CF who underwent BAE at our centre between March 2011 and January 2015 were included in the study. The patient population attending our centre increased from 376 patients in 2011 to 435 patients in 2015. Patients were prospectively identified at the time of each BAE procedure. Data were collected from the clinical records at discharge from hospital, death or one month after the BAE procedure, whichever occurred first. Baseline forced expiratory volume in 1 s (FEV1) was taken as the best value in the 12 months prior to entry to the study. The indication for BAE was classified according to severity of haemoptysis: massive (>240 ml/24 h or >100 ml/day for ≥2 days) [1], moderate–severe (>20 ml/24 h) or mild (<20 ml/24 h). For subsequent analysis, cases of moderate–severe and mild haemoptysis were pooled as sub-massive haemoptysis. Details of any adjuvant treatments for haemoptysis given prior to or following BAE were recorded. Follow-up was continued through to January 2016. Complications of BAE were classified according to Society of Interventional Radiology criteria.
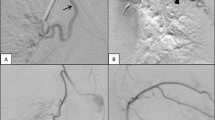
BAE procedures were performed via a femoral arterial approach. The thoracic aorta was explored using a curved Cobra or Headhunter catheter. Target vessels were selectively catheterised with the use of a 2.7-Fr microcatheter where necessary. Subclavian angiography to detect additional sources of bleeding was performed in cases of recurrent or refractory haemoptysis but not as standard for all procedures. Aberrant or actively bleeding vessels were embolised to stasis with 355–500 μm polyvinyl alcohol (PVA) particles.
Statistical analysis was performed using GraphPad Prism (San Diego, CA). Unpaired two-tailed t-tests or Fisher’s exact test was used as appropriate. Kaplan–Meier analysis was performed to investigate survival. Significance was assumed at the 5% level. The study was an evaluation of the current BAE service in routine clinical use, and appropriate institutional policies were followed. Formal research ethics committee approval and specific patient consent were therefore not deemed necessary.
Results
Twenty-seven patients underwent 51 BAE procedures during the study period (mean 1.89 (SD 1.63) BAEs per patient). Patients underwent follow-up for a median period of 26 months (range 1–54). Baseline patient demographics are listed in Table 1. Five patients had previously undergone a BAE procedure prior to the study. Five patients had a CT angiogram examination during the study period.
The indication for BAE was massive haemoptysis in 18 cases (35.2%), moderate–severe haemoptysis in 27 cases (52.9%) and mild haemoptysis in 4 cases (7.8%). Volume of haemoptysis was not determined in 2 cases (3.9%). Table 2 shows details of adjunctive treatments given alongside BAE.
Overall, haemoptysis recurred after 31/51 (60.8%) procedures with no significant difference in recurrence rates following BAE for massive or sub-massive haemoptysis (61.1% vs 60.6%). Ten patients (37.0%) required more than one BAE during the study (range 2–7). Median time to first repeat BAE was 279 days (range 18–682).
Adverse events were common and occurred after 31/51 (60.8%) of procedures. Of these, 26/31 (83.9%) complications were classified as minor and 5/31 (16.1%) as major. Chest pain was the most common side effect and was reported after 14 (27.4%) of BAE procedures. Paraesthesia occurred after five BAEs and limb weakness after two procedures; in each case, these symptoms resolved spontaneously. One case of upper limb ischaemia was observed which was managed conservatively. A full breakdown of adverse events documented is listed in Table 3.
Nine patients (33.3%) died during the study. The cause of death was respiratory failure due to advanced CF in 6/9 (66.7%), metastatic cancer in 2/9 (22.2%) and massive haemoptysis in 1/9 (11.1%) cases. Thirty-day mortality following first BAE during the study was 3.7% and mortality at 12 months was 14.8% (see Fig. 1). There were no statistically significant factors associated with increased mortality following BAE. Table 4 lists the key clinical parameters of patients who died during the study in comparison with survivors.
Discussion
Our experience suggests that the occurrence of haemoptysis requiring BAE is associated with a relatively high mortality at one year among patients with CF. Our experience also suggests that BAE, while potentially life-saving, is associated with considerable morbidity. These factors and the high rate of eventual recurrence of haemoptysis in our cohort illustrate the pressing need for large-scale prospective studies to guide the management of this dramatic complication of CF. The data from this study, however, help highlight the difficult balance to be made when considering the use of BAE in CF-related haemoptysis.
In our cohort, the majority of patients experienced recurrence of haemoptysis during the follow-up period. This is in keeping with the findings of previous studies in both paediatric and adult CF populations as well as in other patient groups [7–12]. Reported rates of recurrent haemoptysis following BAE have varied from 46% [7] to as high as 100% [3, 10]. However, the relatively long average period between first and repeat BAEs in our study suggests that these procedures may afford a period of stability which may be crucial in the realm of lung transplant assessment and listing. The impact of BAE on transplant suitability or outcomes is unclear from our data as none of the patients included in our analysis underwent lung transplantation.
Of particular importance in our study is the relatively high incidence of adverse events and side effects of BAE. Other investigators have reported varying rates of such incidents, with Antonelli et al. [3] for instance, observing no significant complications at all in their small cohort. Such differences are likely to be due to differing methodologies in recording adverse events. Other groups have documented significant side effects including stroke, spinal artery infarction and other neurological consequences [8, 12]. Fortunately, all neurological and vascular events in our series resolved spontaneously or with conservative therapy only. However, the significant morbidity associated with haemoptysis and BAE procedures is important to note, for both clinicians and patients.
It is highly unlikely that a randomised controlled trial of BAE will ever be conducted in massive haemoptysis given the emergency nature of such presentations and the lack of proven alternative therapies. There is scope, however, for the further work to determine optimal outcomes from BAE in massive haemoptysis. For instance, there is uncertainty over the most appropriate investigations to be performed prior to the BAE procedure itself. Our practice has not included mandatory cross-sectional imaging or bronchoscopy prior to BAE to target the source of bleeding. Conceivably, such tests could enhance the impact of BAE but this needs to be tempered with the risk of complications such as contrast-induced nephropathy. The role of CT scanning and bronchoscopy prior to BAE requires more research. Technical aspects of BAE, for instance choice of embolisation material, the number of vessels targeted and the benefit of including subclavian angiography, would all benefit from further investigation. In our study, PVA particles were used as the embolic material and it is possible that other techniques, for instance vascular coils, may lead to different outcomes. Randomised controlled trials of alternative embolising agents would be highly informative.
The role of BAE in sub-massive haemoptysis in CF remains unclear. Antonelli et al. [3] reported improved outcomes with early BAE in this population but the small size of their study makes it difficult to draw clear conclusions. It is notable that all eight patients treated with BAE in their cohort experienced recurrent haemoptysis over a three-year follow-up period. Similarly, Efrati et al. [10] reported that all five patients undergoing BAE in their observational study experienced recurrent haemoptysis, although none within 24 h. Further studies are required to clarify whether intervention with BAE for sub-massive haemoptysis has benefits beyond simply control of haemoptysis which is likely to be temporary.
A further area of uncertainty in the management of CF-related haemoptysis centres on optimal conservative management. As given in Table 2, the vast majority of our cohort received intravenous antibiotics and tranexamic acid. Tranexamic acid is a prothrombotic agent that has been used in a variety of settings including major trauma, elective orthopaedic surgery and menorrhagia [13–15]. There is no definitive evidence to show that tranexamic acid or other antifibrinolytic drugs are of benefit in the setting of haemoptysis [16, 17]. Within the CF literature, there are case reports of the successful use of tranexamic acid in the management of haemoptysis [18] but there is a lack of definitive evidence to support its use. Concern exists over the risk of thrombosis with the use of agents such as tranexamic acid, and it is notable that one patient in our study experienced SVC thrombosis associated with an implanted intravenous catheter. The role of other conservative and prophylactic strategies also requires further investigation, including the use of beta blockers for prevention of recurrence of haemoptysis [19].
Our study has a number of limitations including the lack of a control group and the lack of a defined protocol for the BAE procedures itself. The observational nature of the study also means that participants were followed up for variable durations which impairs the study’s ability to fully assess long-term prognosis following BAE. However, the single-centre, prospective nature of the study with its complete follow-up data for the entire cohort is significant strengths and contributes to the current evidence base.
In summary, our data reveal the real-world impact and effectiveness of BAE for haemoptysis in CF. BAE in adults with CF has a significant burden of morbidity but is frequently able to afford control of haemoptysis. There is a clear need for robust studies to investigate interventional radiological techniques to maximise the effectiveness of BAE and reduce the risk of complications. Randomised trials of different embolic agents should be considered as a priority. Pending such studies, our findings are of importance not just to the CF population but also to patients at risk of haemoptysis due to myriad other pulmonary diseases including lung cancer, bronchiectasis and chronic pulmonary aspergillosis [20, 21].
References
Flume PA, Mogayzel PJ, Robinson KA, Rosenblatt RL, Quittell L, Marshall BC, et al. Cystic fibrosis pulmonary guidelines: pulmonary complications: hemoptysis and pneumothorax. Am J Respir Crit Care Med. 2010;182(3):298–306.
Smyth AR, Bell SC, Bojcin S, Bryon M, Duff A, Flume P, et al. European cystic fibrosis society standards of care: best practice guidelines. J Cyst Fibros. 2014;13(Suppl 1):S23–42.
Antonelli M, Midulla F, Tancredi G, Salvatori FM, Bonci E, Cimino G, et al. Bronchial artery embolization for the management of nonmassive hemoptysis in cystic fibrosis. Chest. 2002;121(3):796–801.
Flume PA, Yankaskas JR, Ebeling M, Hulsey T, Clark LL. Massive hemoptysis in cystic fibrosis. Chest. 2005;128(2):729–38.
Thompson V, Mayer-Hamblett N, Kloster M, Bilton D, Flume PA. Risk of hemoptysis in cystic fibrosis clinical trials: a retrospective cohort study. J Cyst Fibros. 2015;14(5):632–8.
Flight WG, Barry PJ, Bright-Thomas RJ, Butterfield S, Ashleigh R, Jones AM. S53 Outcomes following bronchial artery embolisation for haemoptysis in adults with cystic fibrosis. Thorax. 2015;70(Suppl 3):A33.
Barben JU, Ditchfield M, Carlin JB, Robertson CF, Robinson PJ, Olinsky A. Major haemoptysis in children with cystic fibrosis: a 20-year retrospective study. J Cyst Fibros. 2003;2(3):105–11.
Brinson GM, Noone PG, Mauro MA, Knowles MR, Yankaskas JR, Sandhu JS, et al. Bronchial artery embolization for the treatment of hemoptysis in patients with cystic fibrosis. Am J Respir Crit Care Med. 1998;157(6 Pt 1):1951–8.
Sweezey NB, Fellows KE. Bronchial artery embolization for severe hemoptysis in cystic fibrosis. Chest. 1990;97(6):1322–6.
Efrati O, Harash O, Rivlin J, Bibi H, Meir MZ, Blau H, et al. Hemoptysis in Israeli CF patients–prevalence, treatment, and clinical characteristics. J Cyst Fibros. 2008;7(4):301–6.
Mal H, Rullon I, Mellot F, Brugière O, Sleiman C, Menu Y, et al. Immediate and long-term results of bronchial artery embolization for life-threatening hemoptysis. Chest. 1999;115(4):996–1001.
Pathak V, Stavas JM, Ford HJ, Austin CA, Aris RM. Long-term outcomes of the bronchial artery embolization are diagnosis dependent. Lung India. 2016;33(1):3–8.
Ker K, Roberts I, Shakur H, Coats TJ. Antifibrinolytic drugs for acute traumatic injury. Cochrane Database Syst Rev. 2015. doi:10.1002/14651858.CD004896.pub4.
Sukeik M, Alshryda S, Haddad FS, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br. 2011;93(1):39–46.
Bonnar J, Sheppard BL. Treatment of menorrhagia during menstruation: randomised controlled trial of ethamsylate, mefenamic acid, and tranexamic acid. BMJ. 1996;313(7057):579–82.
Prutsky G, Domecq JP, Salazar CA, Accinelli R. Antifibrinolytic therapy to reduce haemoptysis from any cause. Cochrane Database Syst Rev. 2012. doi:10.1002/14651858.CD008711.pub2.
Moen CA, Burrell A, Dunning J. Does tranexamic acid stop haemoptysis? Interact Cardiovasc Thorac Surg. 2013;17(6):991–4.
Hurley M, Bhatt J, Smyth A. Treatment massive haemoptysis in cystic fibrosis with tranexamic acid. J R Soc Med. 2011;104(Suppl 1):S49–52.
Moua J, Nussbaum E, Liao E, Randhawa IS. Beta-blocker management of refractory hemoptysis in cystic fibrosis: a novel treatment approach. Ther Adv Respir Dis. 2013;7(4):217–23.
Conlan AA, Hurwitz SS, Krige L, Nicolaou N, Pool R. Massive hemoptysis. Review of 123 cases. J Thorac Cardiovasc Surg. 1983;85(1):120–4.
Jean-Baptiste E. Clinical assessment and management of massive hemoptysis. Crit Care Med. 2000;28(5):1642–7.
Authors’ Contributions
All authors contributed to the study design and manuscript development. WGF and PJB undertook the data analysis. WGF wrote the first draft of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author confirms that there is no conflict of interest.
Informed Consent
Informed consent does not apply as this was an evaluation of routine clinical practice with no patient identifiable data included.
Rights and permissions
About this article
Cite this article
Flight, W.G., Barry, P.J., Bright-Thomas, R.J. et al. Outcomes Following Bronchial Artery Embolisation for Haemoptysis in Cystic Fibrosis. Cardiovasc Intervent Radiol 40, 1164–1168 (2017). https://doi.org/10.1007/s00270-017-1626-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-017-1626-0