Abstract
Objectives
The aims of this study were to compare clinical outcomes of early versus delayed bronchial artery embolization (BAE) for non-massive hemoptysis and to investigate predictors of recurrent hemoptysis.
Methods
From March 2018 to February 2021, 138 consecutive patients (age, 65.5 ± 12.4 years; male, 67.4%) with non-massive hemoptysis underwent BAE. The enrolled patients were divided into an early embolization (EE) group (within the first 24 h, n = 79) and a delayed embolization (DE) group (n = 59).
Results
The time to embolization ranged between 0 and 15 days and was shorter in the EE group (0.47 ± 0.5 days) than in the DE group (4.02 ± 2.8 days, p < 0.001). The in-hospital clinical outcomes were not different between the two groups, except for hospital stay and post-embolization hospital stay. The recurrence-free survival in the EE group was significantly better than that in the DE group (p = 0.018). The time to embolization (hazard ratio (HR), 1.21; 95% confidence interval (CI), 1.04–1.42; p = 0.015) and aspergilloma (HR, 6.89; 95% CI, 2.08–22.86; p = 0.002) were predictive factors for recurrent hemoptysis.
Conclusions
BAE is an effective and safe treatment modality for non-massive hemoptysis. An early interventional strategy should be considered in patients presenting with non-massive hemoptysis to reduce the length of hospital stay and early recurrence. A delayed time to embolization and the presence of aspergilloma were independent risk factors for recurrent hemoptysis.
Key Points
• Bronchial artery embolization afforded good clinical improvement for treating non-massive hemoptysis without significant complications.
• An early interventional strategy should be considered in patients presenting with non-massive hemoptysis to reduce the length of hospital stay and early recurrence.
• A delayed time to embolization and the presence of aspergilloma were independent risk factors for recurrent hemoptysis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bronchial artery embolization (BAE) has become the universally accepted first-line therapy for treating massive hemoptysis [1, 2]. The technical and clinical success rates of BAE have been reported to be high and similar between patients presenting with massive versus non-massive hemoptysis [3, 4]. While massive hemoptysis is always considered an emergency that requires prompt treatment to prevent catastrophic consequences including airway obstruction, the standard of care for non-massive hemoptysis remains conservative medical therapy. On the other hand, a recent multicenter randomized controlled trial (RCT) of patients with non-massive hemoptysis demonstrated the potential of endovascular treatment as a first-line treatment [5].
According to the American College of Radiology Appropriateness Criteria (ACR AC), BAE is considered as a definitive therapeutic option when medical treatment has failed [6]. However, medical treatment failure is difficult to define objectively and is highly variable among different centers or clinicians. To the best of our knowledge, there is no consensus on when BAE should be performed in patients with non-massive hemoptysis. While most previous studies have focused on the efficacy of BAE for non-massive hemoptysis [5, 7], our preliminary study offers insights into the importance of procedure timing as a potential prognostic factor for clinical outcomes. In the current study, we aimed to compare the clinical outcomes of early versus delayed BAE for non-massive hemoptysis and to investigate the predictors of recurrent hemoptysis.
Materials and methods
The institutional review boards of all collaborating institutions approved this retrospective study and waived written informed consent for use of clinical and imaging data. Written informed consent for interventional procedures was obtained from all patients.
Study design and data collection
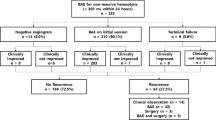
The four hospitals’ databases were queried from March 2018 to February 2021, and we identified 280 consecutive patients who were referred to interventional radiology clinics for persistent hemoptysis despite conservative medical treatment. Persistent hemoptysis was defined as at least one episode of hemoptysis after the initiation of medication during hospital stay. The exclusion criteria were as follows: massive hemoptysis, acute respiratory failure, hemodynamic instability, pulmonary arterial involvement, and traumatic hemoptysis. According to the ACR AC, massive hemoptysis was defined as bleeding amount > 100 mL of expectorated blood in 24 h on admission [6]. Acute respiratory failure was defined as a requirement of endotracheal intubation and mechanical ventilation on admission. Hemodynamic instability was defined as a systolic blood pressure < 90 mmHg or the requirement for vasopressor therapy. Pulmonary arterial involvement was defined as pulmonary arterial vasculature involvement according to a preprocedural CT angiography [5]. The study flow diagram is depicted in Fig. 1.
A total of 138 patients (age, 65.5 ± 12.4 years; male, 67.4%) were enrolled in this study. There were no patients who underwent hemostatic procedures with bronchoscopy. The enrolled patients were divided into two groups according to the timing of procedure: an early embolization (EE) group (time to embolization < 24 h, n = 79) and a delayed embolization (DE) group (n = 59). The time to embolization was defined as the time interval between the start of medical treatment and arrival at the angio-suite. A comparative analysis was performed between the two groups. The prognostic factors associated with recurrent hemoptysis were analyzed in the entire study population.
At all participating institutions, routine contrast-enhanced chest CT is performed to identify underlying causes, culprit arteries, and the extent of pulmonary diseases before the interventional procedure. The extent of the pulmonary disease on CT was graded from 0 to 6 according to the number of diseased pulmonary lobes. The following study data were collected using electronic medical records and a picture archiving and communication system: time to embolization, age, gender, volume of hemoptysis, etiology, smoking history, disease location and extent on CT, angiographic findings, and embolic material.
Procedural Techniques
Interventional procedures were performed using one of the following angiography machines: AlluraClarity, AlluraXper FD20 (Philips Healthcare), and Artis Q (Siemens Healthcare) by one of seven interventional radiologists with at least 5 years of experience. Potential culprit arteries were scrutinized on CT angiography before endovascular treatment. All procedures were performed under local anesthesia with 2% lidocaine hydrochloride. Each patient’s vital signs were monitored throughout the procedure. A 5-F vascular sheath was placed in the common femoral artery. Thoracic aortography using a 5-F pigtail catheter was performed at the discretion of the attending interventional radiologists. Selective angiograms of the bronchial and/or non-bronchial systemic collateral (NBSC) arteries were performed using 5-F diagnostic catheters and coaxial 2.0- to 3.0-Fr microcatheter systems with non-ionic contrast medium (Visipaque 320, GE Healthcare). Angiographic findings corresponding to hemoptysis were determined as follows: vascular engorgement, parenchymal hypervascularity (including tumor staining), bronchopulmonary shunting, pseudoaneurysm, and extravasation of the contrast agent (Fig. 2) [8, 9]. Selective embolization was attempted in all pathologic bronchial arteries and NBSCs. The choice of embolic agent was left to the discretion of the attending interventional radiologists.
A 71-year-old woman was presented to the emergency room for non-massive hemoptysis. a Axial CT image with lung window setting demonstrates bronchiectasis and mucoid impactions in the right lung. b Right intercostobronchial trunk angiogram shows hypertrophic right bronchial artery (white arrow) and areas of parenchymal staining (white arrowheads). c Selective intercostal angiogram reveals tortuous collateral vessel (black arrow) with parenchymal staining. d The final angiogram shows successful embolization of culprit arteries
Study endpoints
The study endpoints included in-hospital clinical outcome and recurrence. In-hospital clinical outcome was evaluated by technical and clinical success, complications, length of hospital stay, and in-hospital mortality. Technical success was defined as the complete embolization of all bronchial and NBSC arteries in which embolization was attempted. Clinical success was defined as cessation of hemoptysis or clinically significant reduction of hemoptysis volume > 50% during the index hospital stay after BAE [1, 10, 11]. The length of hospital stay was defined as the number of days from admission or presentation of hemoptysis for patients hospitalized for other reasons to hospital discharge. The length of post-embolization hospital stay was defined as the number of days from BAE to hospital discharge. Complications were classified as minor or major according to the guidelines of the Society of Interventional Radiology [3]. In-hospital mortality was categorized as hemoptysis-related mortality and other causes.
Recurrence referred to clinically significant hemoptysis occurring after discharge requiring hospital admission or repeat intervention. Recurrences were classified into early (< 3 months) and late recurrences. Recurrence-free survival was defined as the time from BAE until the onset of recurrence or death. The causes of recurrence based on angiographic findings were categorized as follows: recanalization of the previously embolized vessels or development of new NBSCs. The follow-up period referred to the time period from the date of embolization procedure to the date of last hospital visit or death.
Statistical analysis
For descriptive statistics, continuous variables are presented as the mean ± standard deviation, and categorical variables are presented as absolute and relative frequencies. For the comparative analysis, the two-sample t test or Mann-Whitney U test was used for continuous variables, and the chi-square test or Fisher’s exact test was used for categorical variables. Recurrence-free survival rates were analyzed and compared using Kaplan-Meier survival analysis. Potential prognostic factors associated with recurrent hemoptysis were analyzed with a Cox proportional-hazards model. Variables with a p value of less than 0.1 in the univariate analysis were included in the multivariate analysis. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated. To test the multicollinearity of the multivariable analysis model, we calculated the variance inflation factor (VIF). A p value < 0.05 was considered statistically significant. All statistical analyses were executed using R (version 3.6.3 software; Foundation for Statistical Computing).
Results
Baseline characteristics and procedure details
Baseline characteristics are summarized in Table 1, and no statistically significant differences were observed between the two groups except for the time to embolization and disease location of the lingular left upper lobe. A disease location of the lingular left upper lobe was more frequent in the EE group (40.5%) than in the DE group (20.34%, p = .02). The mean volume of hemoptysis over 24 h was 36.6 ± 27.3 cc (EE group, 38.8 ± 29.7 cc; DE group, 33.7 ± 23.5 cc; p = 0.607). The top two common causes of non-massive hemoptysis requiring BAE were bronchiectasis (EE group, n = 36, 45.6%; DE group, n = 28, 47.5%; p = 0.962) and tuberculosis sequelae (EE group, n = 17, 21.5%; DE group, n = 17, 28.8%; p = 0.433) in both groups.
The details of the BAE procedures are summarized in Table 2. The angiographic findings corresponding to hemoptysis included the following, presented in descending order: hypervascularity (EE group, n = 65, 82.3%; DE group, n = 46, 78%; p = 0.678), hypertrophy (EE group, n = 38, 48.1%; DE group, n = 26, 44.1%; p = 0.766), and systemic-to-pulmonary shunt (EE group, n = 37, 46.8%; DE group, n = 23, 39%; p = 0.455). There were no patients with angiographic findings of pseudoaneurysm or contrast extravasation. There were no significant differences in the number of total embolized arteries and the number of embolized NBSC arteries between the two groups (p = 0.646 and p = 0.579, respectively). The utilized embolic materials included polyvinyl alcohol (PVA) (EE group, n = 57, 72.2%; DE group, n = 39, 67.2%; p = 0.666), microspheres (EE group, n = 7, 8.9%; DE group, n = 2, 3.5%; p = 0.302), and a combination of multiple agents (EE group, n = 15, 19%; DE group, n = 17, 29.3%; p = 0.228). There were no cases where coils served as the main embolic agent.
In-hospital clinical outcomes
The in-hospital clinical outcomes are summarized in Table 3, and no statistically significant differences were observed between the two groups except for the length of hospital stay (EE group, 10.7 ± 10.4 days; DE group, 12.6 ± 11.2 days; p = 0.017) and post-embolization hospital stay (EE group, 10.2 ± 10.4 days; DE group, 8.6 ± 10.9 days; p = 0.049). Both the technical and clinical success rates were 98.7% in the EE group and 98.3% in the DE group (p > 0.99). In one patient in the EE group with technical failure, embolization could not be performed in one of two culprit arteries due to orifice stenosis, but clinical success was achieved. In another patient in the DE group with technical failure, conventional angiography failed to localize a source of bleeding, and bronchoscopic management was performed. One patient died of uncontrolled hemoptysis despite the technical success.
The complication rate was 2.5% in the EE group and 0% in the DE group (p = 0.507). No major complications were observed. Minor complications consisted of minor (< 3 cm) puncture site hematoma formation (n = 1) and subsegmental renal infarction due to migration of a glue cast (n = 1). Both patients were observed without additional treatment. The in-hospital mortality rate was 2.5% in the EE group and 8.5% in the DE group (p = 0.137). Deaths from other causes were as follows: underlying pneumonia (n = 3), underlying disease progression (n = 2), and myocardial infarction (n = 1).
Recurrences
The median follow-up time was 12.6 months (range 2–1272 days). Of the 136 patients who achieved clinical success, recurrence was observed in 19 (14%) patients (Table 4). The early recurrence rate was significantly higher in the DE group (8.6%) than in the EE group (0%, p = 0.013). Among five patients with early recurrence, three patients underwent repeat BAE to control recurrent hemoptysis. One patient experienced recurrence due to new NBSCs and two patients experienced recurrence due to both recanalization and new NBSCs. The remaining two patients underwent either bronchoscopic or surgical management without repeat angiography. The late recurrence rate was 10.3% (11.5% for the EE group, 8.6% for the DE group), and there were no significant differences between the two groups (p = 0.788). Among 14 patients with late recurrence, six patients experienced recurrence due to recanalization, six patients experienced recurrence due to new NBSCs, and two patients experienced recurrence due to both recanalization and new NBSCs.
The Kaplan–Meier survival curve stratified based on the time to embolization is shown in Fig. 3. The recurrence-free survival rate in the EE group was significantly better than that in the DE group (p = 0.033). The results of the univariable and multivariable Cox proportional hazards regression models are shown in Table 5. The time to embolization (HR, 1.21; 95% CI, 1.04–1.42; p = 0.015) and aspergilloma (HR, 6.89; 95% CI, 2.08–22.86; p = 0.002) were predictive factors associated with recurrent hemoptysis. The VIF values for the variables were < 2, indicating that there was no multicollinearity.
Discussion
BAE is increasingly used as a definitive therapeutic option for non-massive hemoptysis after failed medical treatment [5, 7]. However, the timing of endovascular treatment for patients with non-massive hemoptysis depends entirely on their physicians’ decisions. To date, no study has compared the efficacy and safety of early versus delayed BAE in patients with non-massive hemoptysis. The current study demonstrated that the time to embolization for non-massive hemoptysis ranges between 0 and 15 days. The in-hospital clinical outcomes were similar between the two groups, except for the length of hospital stay, which was longer in the DE group than in the EE group. However, patients in the EE group had better recurrence-free survival than those in the DE group, and a longer time to embolization was associated with an increased risk of recurrence.
In the current study, excellent technical and clinical success rates (98–99%) were achieved in both groups. No procedure-related major complications occurred, and only two minor complications occurred in the EE group. However, there was no significant difference between the two groups in minor complication rates, and puncture site hematoma formation and inadvertent embolization were generally not associated with the time to embolization. These findings are in accordance with those of previous studies that showed satisfactory clinical outcomes of BAE for patients with non-massive hemoptysis. However, neurological complications related to spinal cord ischemia have been reported at 0.6–4.4% and should not be overlooked [1]. Since the EE group had a shorter hospital stay than the DE group, early intervention may be more advantageous in terms of early discharge and quick return to daily activity.
In contrast to the in-hospital clinical outcomes, the long-term follow-up data for recurrent hemoptysis showed a significant difference between the two groups. The recurrence-free survival rate was higher in the EE group than in the DE group. After stratification according to the time to recurrence, the EE group showed a decreased early recurrence rate compared with the DE group; however, there was no difference in the late recurrence rate. Follow-up angiograms of patients with early recurrence revealed findings of new NBSCs and/or recanalization. According to previous studies, early recurrence after BAE is most commonly due to suboptimal embolization rather than underlying disease progression or recruitment of new arteries [1, 12]. This may explain the higher early recurrence rate in the DE group than in the EE group despite the comparable excellent in-hospital clinical outcomes in both groups.
Patients with hemoptysis usually receive medical treatment (e.g., antifibrinolytic agents or vasoconstrictors) from the time of admission, and hemoptysis can be reduced to some extent with such conservative management [13, 14]. Endogenous blood clot formation in the culprit arteries has likely already begun during medical treatment, which can help provide immediate control of hemoptysis [15]. The longer the duration of medical treatment before procedure is, the greater the likelihood of endogenous clot formation. However, endogenous clots induced by hemostatic agents provide temporary hemostasis and have the potential to recanalize earlier than embolic agents [16]. Furthermore, if a culprit artery with transient hemostasis is not identified during the initial BAE, its early recanalization may be considered as a new NBSC during the follow-up procedure. Our results are in accordance with the aforementioned RCT, where the medical treatment group demonstrated a higher recurrence rate than the BAE group [5].
In addition to the time to embolization, aspergilloma was another independent risk factor for recurrent hemoptysis in the current study. Previous studies of patients with massive hemoptysis have identified various risk factors including aspergilloma, NBSCs, systemic-to-pulmonary shunt, and tuberculosis which were associated with recurrent hemoptysis [1, 17,18,19]. A recent study of patients with non-massive hemoptysis found that tuberculosis sequelae and NBSC were associated with recurrence, which differs from the results of this study. This discrepancy can be largely attributed to stratification of target populations based on hemoptysis severity. The current study defined massive hemoptysis as bleeding exceeding 100 mL of expectorated blood in 24 h, following the ACR AC. However, Hwang et al defined massive hemoptysis as bleeding greater than 300 mL. Based on the definition of ACR AC, 25.8% of the patients included in their study had massive hemoptysis.
There are some limitations to the current study that deserve mention. The most important is the retrospective nature of the study, which leads to several biases, such as information or selection biases. The follow-up period for each patient was variable. The choice of embolic material was case-dependent, and approximately one fourth of patients underwent BAE with a combination of multiple embolic agents. However, in most cases, PVA or a microsphere was used as the primary embolic agent; these have been revealed to be effective and safe [1, 11, 20]. In addition, there was no difference in the embolic agents used between the two groups.
In conclusion, BAE is an effective and safe treatment modality for non-massive hemoptysis. An early interventional strategy should be considered in patients presenting with non-massive hemoptysis to reduce the length of hospital stay and early recurrence. A delayed time to embolization and the presence of aspergilloma were independent risk factors for recurrent hemoptysis.
Abbreviations
- ACR AC:
-
American College of Radiology Appropriateness Criteria
- BAE:
-
Bronchial artery embolization
- CI:
-
Confidence interval
- DE:
-
Delayed embolization
- EE:
-
Early embolization
- HR:
-
Hazard ratio
- NBSC:
-
Non-bronchial systemic collateral
- PVA:
-
Polyvinyl alcohol
- RCT:
-
Randomized controlled trial
- VIF:
-
Variance inflation factor
References
Panda A, Bhalla AS, Goyal A (2017) Bronchial artery embolization in hemoptysis: a systematic review. Diagn Interv Radiol 23:307–317
Burke CT, Mauro MA (2004) Bronchial artery embolization. Semin Intervent Radiol 21:43–48
Angle JF, Siddiqi NH, Wallace MJ et al (2010) Quality improvement guidelines for percutaneous transcatheter embolization: Society of Interventional Radiology Standards of Practice Committee. J Vasc Interv Radiol 21:1479–1486
Woo S, Yoon CJ, Chung JW et al (2013) Bronchial artery embolization to control hemoptysis: comparison of N-butyl-2-cyanoacrylate and polyvinyl alcohol particles. Radiology 269:594–602
Fartoukh M, Demoule A, Sanchez O et al (2021) Randomised trial of first-line bronchial artery embolisation for non-severe haemoptysis of mild abundance. BMJ Open Respir Res 8:e000949. https://doi.org/10.1136/bmjresp-2021-000949
Expert Panel on Thoracic I, Olsen KM, Manouchehr-Pour S et al (2020) ACR Appropriateness criteria(R) hemoptysis. J Am Coll Radiol 17:S148–S159
Hwang JH, Kim JH, Park S, Lee KH, Park SH (2021) Feasibility and outcomes of bronchial artery embolization in patients with non-massive hemoptysis. Respir Res 22:221
Almeida J, Leal C, Figueiredo L (2020) Evaluation of the bronchial arteries: normal findings, hypertrophy and embolization in patients with hemoptysis. Insights Imaging 11:70
Yoon W, Kim JK, Kim YH, Chung TW, Kang HK (2002) Bronchial and nonbronchial systemic artery embolization for life-threatening hemoptysis: a comprehensive review. Radiographics 22:1395–1409
Dorji K, Hongsakul K, Jutidamrongphan W, Oofuvong M, Geater S (2021) Bronchial artery embolization in life-threatening hemoptysis: outcome and predictive factors. J Belg Soc Radiol 105:5
Fu Z, Li X, Cai F et al (2021) Microspheres present comparable efficacy and safety profiles compared with polyvinyl alcohol for bronchial artery embolization treatment in hemoptysis patients. J Transl Med 19:422
Garcia-Olive I, Sanz-Santos J, Centeno C et al (2014) Predictors of recanalization in patients with life-threatening hemoptysis requiring artery embolization. Arch Bronconeumol 50:51–56
Prutsky G, Domecq JP, Salazar CA, Accinelli R (2016) Antifibrinolytic therapy to reduce haemoptysis from any cause. Cochrane Database Syst Rev 11:CD008711
Dweik RA, Stoller JK (1999) Role of bronchoscopy in massive hemoptysis. Clin Chest Med 20:89–105
Lee HN, Park HS, Hyun D et al (2021) Combined therapy with bronchial artery embolization and tranexamic acid for hemoptysis. Acta Radiol 62:610–618
Numan F, Cantasdemir M, Ozbayrak M et al (2008) Posttraumatic nonischemic priapism treated with autologous blood clot embolization. J Sex Med 5:173–179
Chun JY, Belli AM (2010) Immediate and long-term outcomes of bronchial and non-bronchial systemic artery embolisation for the management of haemoptysis. Eur Radiol 20:558–565
van den Heuvel MM, Els Z, Koegelenberg CF, Naidu KM, Bolliger CT, Diacon AH (2007) Risk factors for recurrence of haemoptysis following bronchial artery embolisation for life-threatening haemoptysis. Int J Tuberc Lung Dis 11:909–914
Le HY, Le VN, Pham NH, Phung AT, Nguyen TT, Do Q (2020) Value of multidetector computed tomography angiography before bronchial artery embolization in hemoptysis management and early recurrence prediction: a prospective study. BMC Pulm Med 20:231
Hahn S, Kim YJ, Kwon W, Cha SW, Lee WY (2010) Comparison of the effectiveness of embolic agents for bronchial artery embolization: gelfoam versus polyvinyl alcohol. Korean J Radiol 11:542–546
Funding
This work was supported by the Soonchunhyang University Research Fund. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Hyoung Nam Lee.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
“Clinical Trial Center at Soonchunhyang University College of Medicine, Cheonan Hospital” kindly provided statistical advice for this manuscript.
Informed consent
Institutional review boards of all collaborating institutions approved this retrospective study and waived written informed consent for use of clinical and imaging data. Written informed consent for interventional procedures was obtained from all patients.
Ethical approval
Institutional review board approval was obtained.
Methodology
• retrospective
• observational
• performed at four institutions
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Park, SJ., Lee, S., Lee, H.N. et al. Early versus delayed bronchial artery embolization for non-massive hemoptysis. Eur Radiol 33, 116–124 (2023). https://doi.org/10.1007/s00330-022-08993-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-08993-z