Abstract
Purpose
Intermediate-stage hepatocellular carcinoma (HCC) is usually treated with locoregional therapy using transarterial chemoembolization (TACE). Transarterial radioembolization (TARE) using β-emitting yttrium-90 integral to the glass matrix of the microspheres is an alternative to TACE. This retrospective case-control study compared the outcomes and safety of TARE versus TACE in patients with unresectable HCC.
Materials and Methods
Patients with unresectable HCC without portal vein thrombosis treated with TARE between 2005 and 2008 (n = 61) were retrospectively frequency-matched by age, sex, and liver dysfunction with TACE-treated patients (n = 55) in the Mayo Clinic Hepatobiliary Neoplasia Registry. Imaging studies were reviewed, and clinical and safety outcomes were abstracted from the medical records.
Results
Complete tumor response was more common after TARE (12 %) than after TACE (4 %) (p = 0.17). When complete response was combined with partial response and stable disease, there was no difference between TARE and TACE. Median survival did not differ between the two groups (15.0 months for TARE and 14.4 months for TACE; p = 0.47). Two-year survival rates were 30 % for TARE and 24 % for TACE. TARE patients received fewer treatments (p < 0.001). Fifty-nine (97 %) TARE patients received outpatient treatment. In contrast, 53 (98 %) TACE patients were hospitalized for ≥1 day (p < 0.001). Compared with TACE, TARE was more likely to induce fatigue (p = 0.003) but less likely to cause fever (p = 0.02).
Conclusion
There was no significant difference in efficacy between TARE and TACE. TARE patients reported more fatigue but had less fever than TACE patients. Treatment with TARE required less hospitalization than treatment with TACE. These findings require confirmation in randomized trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the third most common cause of death from cancer worldwide and is the second most rapidly increasing type of cancer in the United States [1, 2]. Most patients with HCC have underlying liver disease with cirrhosis due to chronic hepatitis B, chronic hepatitis C, alcoholic cirrhosis, or nonalcoholic steatohepatitis (NASH) [3, 4]. HCC is most frequently diagnosed at an intermediate or advanced stage when ablative therapies or curative surgeries, including liver transplantation, are no longer feasible [5]. HCC is relatively resistant to chemotherapy, and a substantial proportion of patients have poor liver reserve and/or compromised portal vein flow; consequently, untreated unresectable HCC has a poor prognosis [6].
Prospective studies have shown that locoregional therapy using transcatheter arterial chemoembolization (TACE) provides a survival advantage compared with supportive care for patients with intermediate stage HCC, and the American Association for the Study of Liver Diseases (AASLD) recommends TACE for treatment of unresectable intermediate-stage HCC [7–9]. A systematic review of randomized trials for unresectable HCC showed that arterial chemoembolization improved 2-year survival compared with controls [10]. Unfortunately, patients with compromised liver function due to cirrhosis have a poor tolerance for TACE [11, 12]. During the last few years, transarterial radioembolization (TARE), using β-emitting yttrium-90 integral to the glass matrix or in resin microspheres, has been used as an alternative to TACE for locoregional therapy. The microspheres are administered by way of hepatic intra-arterial injection, which enables lobar, segmental, and subsegmental treatment. Several cohort studies have reported promising results from TARE, and the lower incidence of embolic symptoms, such as fever and abdominal pain, compared with TACE permits treatment on an outpatient basis [6, 13–15]. Recent studies assessing the efficacy and safety of cohorts of patients receiving TARE with yttrium-90 versus TACE suggest comparable tumor response and survival rates in patients with unresectable HCC [13, 16–23]. This study was designed to compare the clinical outcomes and safety of TARE using yttrium-90 integral to the matrix of glass microspheres to TACE in patients with unresectable HCC. Outcomes evaluated include overall survival, response to treatment, side effects, and length of hospitalization.
Materials and Methods
Patient Population
This retrospective case-control study was approved by the Mayo Clinic Institutional Review Board. We studied patients with HCC who were initially deemed not to be candidates for liver transplant, surgical resection, or local ablation and who received treatment with either TARE with yttrium-90 integral to the glass matrix of the microspheres (TheraSphere; Nordion, ON, Canada) or TACE.
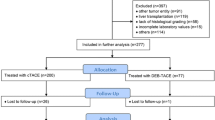
TARE patients were treated between April 2005 and February 2008 and met the following inclusion criteria: (1) ≥18 years of age of either sex; (2) histological proof of primary liver cancer or HCC diagnosis based on AASLD and European Association for the Study of the Liver (EASL) guidelines; (3) life expectancy ≥3 months; (4) postmenopausal if female or nonpregnant with acceptable contraception if premenopausal; (5) >4 weeks since previous radiation, systemic chemotherapy, or previous surgery; (6) Eastern Cooperative Oncology Group (ECOG) performance status ≤2; (7) serum bilirubin <2 mg/dl except for patients with Gilbert’s syndrome; and (8) Barcelona Clinic Liver Cancer (BCLC) stage A patients deemed not to be surgical or local ablation candidates due to technical considerations such as tumor location or comorbidities. Patients were excluded from TARE if they had (1) any contraindication to angiography or selective visceral catheterization; (2) evidence of potential delivery of >16.5 mCi (30 Gy absorbed dose) of radiation to the lungs; (3) evidence of any detectable technetium-99m macroaggregated albumin (MAA) flow to the stomach or duodenum after application of established angiographic techniques to stop such flow; (4) significant extrahepatic disease representing an imminent life-threatening outcome; (5) severe liver dysfunction or pulmonary insufficiency; (6) active uncontrolled infection; and (7) significant underlying medical or psychiatric illness. Medical records were reviewed to assess demographic information, results of cross-sectional imaging studies [e.g., computed tomography (CT) and magnetic resonance imaging (MRI) scans] and other laboratory results. Of the 136 patients evaluated for treatment with TARE, 46 could not receive yttrium-90 TARE treatment because they did not meet inclusion criteria [due to high lung shunt, poor ECOG performance status, high tumor burden, and/or poor liver function (bilirubin >2 except for patients with Gilbert’s syndrome)]. A total of 90 patients were treated; 29 were excluded because they had portal vein thrombosis; and the remaining 61 met inclusion criteria for this study (Fig. 1). Because the yttrium-90 glass microspheres do not occlude the microvasculature, patients treated with the maximum permissible radiation dose by TARE who had disease progression, or those with limited disease in the contralateral liver lobe, were subsequently treated with TACE when technically feasible. Of the 61 TARE patients, 7 were subsequently treated with TACE.
TACE patients were identified by reviewing the medical records of 252 patients from the Mayo Clinic Hepatobiliary Neoplasia Registry who received TACE between April 1998 and August 2007 to identify a patient cohort with baseline inclusion criteria consistent with that of the TARE patients. Two patients were excluded because they had portal vein thrombosis. The remaining 250 TACE patients were examined to select a group of patients matched to the TARE group by age, sex, and degree of liver dysfunction as assessed by Model for End-Stage Liver Disease (MELD) score. One hundred ninety-five TACE patients were excluded because they had different baseline characteristics compared with TARE patients, leaving a matched TACE group of 55 patients (Fig. 1). Because TACE occludes the tumor microvasculature and precludes adequate arterial perfusion for delivery of yttrium-90 glass microspheres, TARE is not usually considered in the same vascular distribution after TACE. However, patients receiving TACE who develop progression of disease with clear evidence of perfusion by new vasculature on angiography would be considered for TARE. None of the matched TACE patients were subsequently treated with TARE.
TACE Treatment
Eligible patients underwent chemoembolization after confirmation of adequate liver function. Lobar, segmental, or superselective TACE were performed as needed based on the tumor size and number of tumors. Subsequent chemoembolizations were performed for viable tumors as evidenced by persistent or recurrent contrast enhancement on imaging studies (CT or MRI) or an increasing serum alpha-fetoprotein (AFP) concentration, which prompted a hepatic angiogram with chemoembolization only being performed if angiography confirmed the presence of viable tumor. The embolization solution contained mitomycin-C 10 mg and doxorubicin 50 mg, radiopaque contrast agent Conray 60 (Mallinckrodt Medical, St. Louis, MO) or nonionic contrast agent Omnipaque 300 (GE Health care, Waukesha, WI), and Contour® PVA embolization particles (250–355 μm) (Boston Scientific, Marlborough, MA). The mixture of the chemotherapeutic agents and Ivalon particles was suspended in 10 ml of contrast. Follow-up arteriogram was performed immediately after delivery of the embolization solution to confirm satisfactory stasis in the tumor blood supply. Patients were routinely admitted for control of pain and pyrexia, including treatment with intravenous analgesics if needed, for the first 24 h after chemoembolization and were discharged on oral antibiotics and analgesics. Lipiodol was not used for TACE, allowing reliable assessment of tumor necrosis and loss of vascular enhancement in follow-up imaging studies.
Yttrium-90 TARE Treatment
Eligible patients received yttrium-90 microsphere treatment in the outpatient setting. Planning hepatic angiogram was first performed to confirm eligibility. In 35 of 61 (57 %) patients, hepatic arterial branches were identified at the time of the planning angiogram that would lead to extrahepatic deposition of the microspheres (most commonly the right gastric or gastroduodenal arteries), and these were occluded using standard angiographic techniques. Technetium-99m MAA study was then performed to calculate the dose of radiation that would be shunted to the lungs. TARE was performed by way of lobar infusion in all cases. The target dose of yttrium-90 was 80–150 Gy. After treatment, patients were observed until they were stable and a physician determined that discharge was safe.
All patients were evaluated at approximately 1 month after treatment to ensure that patients did not have deterioration of liver function after treatment of the first liver lobe. If liver tests were satisfactory, patients with untreated disease in the contralateral lobe received a second treatment approximately 2 weeks after the 1-month follow-up visit. Cross-sectional imaging was performed 30 days after initial radioembolization if additional treatment of the contralateral lobe was planned; otherwise, it was performed 60–90 days after treatment. Subsequently, patients were seen approximately every 2 months for follow-up. Patients with disease progression received additional TARE treatment to the involved lobe if this was feasible without exceeding the lung dose limit; otherwise, provided their liver function was acceptable, they received additional treatment with TACE.
Clinical Follow-Up
All patients, regardless of treatment type, received a follow-up phone call within 2 weeks of initial treatment. Additional follow-up was performed at 60–90 days after the first treatment and any subsequent treatments. These evaluations included a medical history, physical examination, and laboratory tests. Side effects were assessed based on the Common Terminology Criteria for Adverse Events v.4 (http://evs.nci.nih.gov/ftp1/CTCAE/About.html). Cross-sectional studies with a CT scan and/or MRI were performed during follow-up to assess tumor response within 30–90 days after treatment and every 2–3 months thereafter. All available imaging studies were reviewed for assessment of response to therapy. Imaging studies were reviewed by a radiologist with experience in the application and interpretation of both TACE and TARE images based on modified RECIST (mRECIST) criteria as recommended by the most recent guidelines [16, 24]. According to mRECIST criteria, (1) complete response (CR) corresponds to the disappearance of any intratumoral arterial enhancement; (2) partial response (PR) corresponds to at least a 30 % decrease in the sum of the diameters of viable target lesions (as assessed by enhancement in the arterial phase) taking as reference the baseline sum of the diameters of target lesions; (3) stable disease (SD) corresponds to any case that does not qualify for CR, PR, or progressive disease (PD); and (4) PD corresponds to an increase of at least 20 % in the sum of the diameters of viable (enhancing) target lesions taking as reference the smallest sum of the diameters of viable (enhancing) target lesions recorded since treatment started. If there was irregular nodular enhancement or central necrosis of the lesion, the longest region of contiguous enhancement was measured. Up to five largest lesions were assessed for response, and only treated lesions were assessed for response after a particular treatment [24–26]. Vital status information was obtained from the medical record, from the Social Security Death Index, or through direct contact with the patient or a family member as of July 16, 2012; thus, a minimum follow-up of 52 months (February 2008 through June 2012) was achieved.
Statistical Methods
Nominal variables (vital status, sex, ethnicity, BCLC stage, ECOG status, Cancer of the Liver Italian Program (CLIP) stage, number of tumors, extrahepatic metastases, Child-Pugh score, source of diagnosis, esophageal varices, and risk factors for HCC) were expressed as proportions. Chi square or Fisher’s exact test, as appropriate, was used to compare discrete variables between treatments. Wilcoxon signed-rank test was used to compare continuous measures, pretreatment to posttreatment, within treatment groups. Wilcoxon rank sum test was also used to compare continuous measures, including ordered response, between treatment groups. The primary end point for this study was overall survival from initial treatment until last follow-up or death. Patient survival probabilities were estimated using Kaplan–Meier method and compared using log-rank test. Statistical analyses were conducted using SAS (SAS Institute Inc., Cary, NC) or GraphPad Prism (GraphPad Software, Inc., La Jolla, CA) software and p <0.05 was considered statistically significant.
Results
Patients
Baseline characteristics of both groups of patients are listed in Table 1. Consistent with the matched study design, TARE and TACE patients were similar regarding age, sex, and degree of liver dysfunction based on MELD score. CLIP stage was comparable between the two groups, but the frequency of early or intermediate BCLC stage disease (BCLC stage A or B) was greater (77 %) in TARE patients than in TACE patients (65 %). Maximum tumor size was essentially identical in the two groups (median and 25th and 75th percentiles of 5.0, 3.3, and 8.4 cm for TARE patients and 5.0, 3.2, and 8.5 cm for TACE patients; p = 0.99) (Fig. 2). Most patients in both groups had Child-Pugh class A cirrhosis. Chronic alcohol consumption was the most common cause of liver disease for both TARE and TACE patients (20 and 24 %, respectively). Chronic hepatitis C infection affected 13 % in both groups, whereas NASH was the underlying liver disease in 8 % of TARE patients and 18 % of TACE patients. No primary risk factor was found after evaluation for causes of chronic liver disease in 21 and 18 % of TARE and TACE patients, respectively. TARE was performed with a lobar infusion in all cases. When more than one TARE session was performed, it was for retreatment of a single involved lobe in 11 cases and for treatment of the contralateral lobe in 8 patients with bilobar disease. One patient received a single TARE treatment to the entire liver. Seven patients received TACE subsequent to TARE. Two patients were treated with TARE after previously having received TACE to a different part of the liver. No patients received TARE after TACE to the same part of the liver.
Clinical Outcomes
Response Rates
Initial tumor response rates were assessed from the first posttreatment cross-sectional imaging study using modified RECIST criteria. For four TARE and eight TACE patients, the radiologist was unable to report tumor response because of insufficient information (e.g., outside pretreatment imaging not available, patients did not return for follow-up, or no CT scans or MRIs were available after treatment to assess tumor response) (Table 2). A greater frequency of TARE (12 %) than TACE (4 %) patients had CR, but the difference was not statistically significant (p = 0.17). The response rate for combined CR and PR was identical for TARE (51 %) and TACE (51 %) patients (p = 1.00). There was no significant difference in overall response between the two groups when CR, PR, and SD were considered together (89 % for TARE vs. 85 % for TACE; p = 0.56). Pretreatment AFP was >200 ng/mL in 13 TARE patients, of whom 3 had a >50 % decrease after treatment, for a total AFP response of 23 %. Pretreatment AFP was >200 ng/mL in 21 TACE patients, of whom 7 had a >50 % decrease posttreatment, for a total AFP response of 33 %.
Overall Survival
The last follow-up for patients included in the study was July 16, 2012, for both groups. Median survival was similar for both groups: 451 days (15.0 months) for TARE and 432 days (14.4 months) for TACE; log-rank test; p = 0.47; Fig. 3). Two-, 3-, and 5-year survival rates were also similar: 30, 21, and 9 % for TARE and 24, 16, and 5 % for TACE.
Overall survival was not different between TARE and TACE in a stratified analysis based on BCLC stage. In patients with early stage HCC (BCLC stage A), median survival was 717 (23.9 months) and 557 days (18.6 months) for TARE- and TACE-treated patients, respectively (p = 0.40). In patients with intermediate HCC (BCLC stage B), median survival was 503 days (16.8 months) and 390 days (13 months), favoring TARE, but not statistically significantly different (p = 0.16). In patients with advanced HCC (BCLC stage C), median survival was 252 days (8.4 months) and 303 days (10.1 months) for TARE- and TACE-treated patients, respectively (p = 0.47) (Fig. 3). Additional analysis was performed for which the seven TARE patients who subsequently received TACE were censored at the time of TACE treatment. This did not result in a substantial change in the survival outcomes (Supplemental Fig. 1).
Hospitalization and Number of Unique Treatment Days
Our clinical experience is that because TARE does not have a significant embolic effect in the hepatic arterial distribution, TARE patients usually have no significant immediate side effects and do not require routine overnight hospital observation after the procedure. In contrast, many TACE patients develop pain or pyrexia after the procedure, and we therefore routinely hospitalize them overnight to treat these symptoms. Ninety-seven percent of TARE patients received treatment as an outpatient procedure; in contrast, only 1 (2 %) TACE patient received treatment as an outpatient; 42 (78 %) were hospitalized for 1 day, and 11 (20 %) for 2–12 days (p < 0.001) (Table 3). Regarding unique days, a greater proportion of TARE (72 %) versus TACE (40 %) patients had a single treatment. No TARE patient received more then two TARE treatments (Table 4; Fig. 4). The total number of treatments was significantly greater in the TACE group (p < 0.001; Table 4).
Safety Issues
Overall, the frequency of any side effect was not different between the TARE and TACE groups (p = 0.85). Fatigue was significantly more common in the TARE group (p = 0.003); fever was significantly more common in the TACE group (p = 0.02); and there was a trend toward more frequent abdominal pain in the TACE group (p = 0.11) (Table 5).
There were no significant differences regarding liver tests between TARE and TACE patients after treatment: aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase, INR, and creatinine (Table 6). Although there were statistically significant differences in TACE patients between pretreatment and posttreatment hemoglobin (TACE 12.8 vs. 11.6; p < 0.001) and in TARE patients between pretreatment and posttreatment leucocyte levels (TARE 6 vs. 5.2; p = 0.001), and between pretreatment and posttreatment platelet counts (TARE 159 vs. 156; p = 0.009), clinically the mean posttreatment levels remained in an acceptable range, and the differences were not clinically meaningful. Creatinine levels in TARE patients significantly decreased after treatment; however, this was likely not a clinically meaningful change (TARE 1.0 vs. 0.9; p = 0.022). Comparisons within groups showed that both groups had significant decreases in albumin levels after treatment (TARE 3.5 vs. 3.2, p = 0.004; TACE 3.6 vs. 3.2; p < 0.001). Both groups were evaluated for total bilirubin levels before treatment and at 30 days and 6 months after treatment [27]. There were no significant differences in bilirubin levels between groups (Table 7).
Discussion
The aim of this study was to compare the efficacy and safety of treatment with TARE and TACE for patients with unresectable HCC. We compared radiologic response, overall survival rates, length of hospitalization, number of treatments, and side effects.
Survival rates and response to treatment were similar for both groups. This is consistent with a recent report by Salem et al. [22]. The median survival was slightly better in that study (21 vs. 17 months for TARE and TACE group, respectively), which is probably attributable to differences in the distribution of BCLC stages in the two studies: 90 % of the patients in the Salem et al. study were BCLC stages A or B, whereas 71 % of the patients in this study were BCLC stages A or B. Another report by Kooby et al. [19], which used yttrium-90 resin beads, showed median survivals of 6 months in both the TARE and TACE groups [19]. BCLC stage information was not provided for the study by Kooby et al. [19]; therefore, it is impossible to tell whether the lower median survivals in this study occurred because patients had more advanced disease, although this is likely, because a substantial proportion of the patients had macroscopic vascular invasion. Comparison of MELD scores shows a mean MELD score of 10 for both TARE and TACE groups in the study by Kooby et al. [19], whereas the median MELD score was 9 for both groups in our study.
Patients treated with TACE had a similar number of side effects overall compared with patients treated with TARE. When analyzing each side effect separately, fever was significantly greater in patients treated with TACE, whereas fatigue was significantly greater in patients treated with TARE. Because of its low side effect profile, TARE treatment is performed as an outpatient procedure, and virtually all patients meet criteria for discharge on the day of the procedure. In contrast, patients are typically hospitalized at least overnight after TACE treatment. In this study, 20 % of patients who received TACE remained in the hospital ≥2 days for management of the side effects. Regarding the number of treatments performed, 72 % of patients received a single treatment with TARE compared with 40 % of patients treated with TACE. Sixty percent of patients treated with TACE were treated multiple times, which meant that patients were exposed to the hospital environment and its inconveniences and risks more frequently than patients treated with TARE. The corresponding disadvantage of TARE treatment is the need for two angiographic procedures, i.e., (1) the planning angiogram procedure to determine eligibility and occlude arterial branches that may lead to extrahepatic deposition of microspheres before treatment and then (2) the second angiogram at which time the treatment is performed.
Seven patients (11 %) received TACE subsequent to TARE, whereas no patients received TARE subsequent to TACE to the same part of the liver. TACE can be used if HCC progresses after patients have received the maximum amount of radiation they can receive by TARE or if patients have relatively limited disease in the second lobe of the liver that can be treated with limited subselective administration of TACE. In contrast, TARE is not usually used after TACE because TACE is embolic and precludes effective subsequent delivery of the radioactive microspheres, except in patients in whom there is progressive disease supplied by new vasculature.
It has been previously reported that TARE can result in substantial increases in serum bilirubin levels [11]. In another report of a cohort of 65 patients, 30.5 % had an increase by >200 % of total bilirubin during the first 6 months after therapy, but there was only a transient increase in 18 of these patients [28]. It has also been reported that the use of TARE after extensive previous chemotherapy, whole-liver treatment, and treatment with a high amount of radioactivity relative to the targeted liver volume were associated with the development of radioembolization-induced liver disease [27]. We therefore evaluated total bilirubin levels before treatment and at 1 and 6 months after treatment in both groups. None of the analyses showed a statistically significant difference between the TARE and TACE groups, suggesting that TARE can safely be administered to Child-Pugh class A and B patients.
Evidence of the efficacy and safety of TARE, including recent reports from both single-center and multicenter observational cohorts [13, 15, 22, 23], is accumulating in the literature. Although TARE with yttrium-90 integral to the matrix of glass microspheres is approved by the United States Food and Drug Administration for the treatment of HCC under a humanitarian device-exemption status, the AASLD, EASL, and European Organisation for Research and Treatment of Cancer do not currently endorse TARE as a standard treatment for HCC, primarily due to the lack of randomized controlled trial data supporting the efficacy and safety of TARE compared with TACE [16, 25]. For the same reason, TARE is not recommended as a standard treatment in the current BCLC staging system [26]. Therefore, there is a need for randomized controlled trials to confirm the clinical utility of TARE and to clearly define the optimal patient populations in whom this treatment should be used.
One of the main strengths of this study was the matching of patients treated with TARE and TACE for equivalent severity of liver disease. TARE is often indicated in patients with occlusive portal vein thrombosis when TACE is contraindicated. Because these patients were considered to have a different progression and prognosis from patients with patent portal veins, we only included patients with patent portal veins. In addition, because of concerns raised about a possible tendency of TARE to lead to deterioration in liver function, we matched patients for underlying liver dysfunction. Another strength was that tumor response was re-evaluated by the same radiologists for both treatment groups using uniform criteria.
The main limitation of this study is the retrospective design, which resulted in incomplete data for a number of variables. Despite matching for severity of liver disease, BCLC staging was different between the two groups of patients: A larger proportion of TACE patients were BCLC stage A (42 % TACE vs. 20 % TARE), whereas a larger proportion of TARE patients were BCLC stages B or C (80 % TARE vs. 58 % TACE). Consistent with this, a larger proportion of TARE patients had three or more tumor nodules (69 % TARE vs. 42 % TACE). This was likely because physicians preferentially use TACE if a more selective approach seems feasible. To partly address this, we performed subgroup analyses to compare the overall survival between the two groups of patients within different strata of BCLC stage. This subgroup analyses confirmed the comparable overall survival between TARE- and TACE-treated patients within each strata of BCLC stage. In addition, the number of patients in this study was relatively small; however, our study has validated previous studies showing comparable effectiveness of TARE and TACE in patients with HCC [13, 15, 22, 23]. This was not a cost-effectiveness study; thus, studies oriented to this kind of analysis are needed comparing TARE versus TACE. Last, our study only included patients treated with TheraSphere in the TARE group. The results may therefore not be completely generalizable to all forms of yttrium-90 microsphere treatment.
Conclusion
In conclusion, TARE had a similar treatment response, overall survival, and side effect profile compared with TACE. TARE is promising for the treatment of unresectable HCC and warrants evaluation in randomized controlled trials.
References
Everhart JE, Ruhl CE (2009) Burden of digestive diseases in the United States part II: lower gastrointestinal diseases. Gastroenterology 136(3):741–754
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127(12):2893–2917
Schwartz M, Roayaie S, Konstadoulakis M (2007) Strategies for the management of hepatocellular carcinoma. Nat Clin Pract Oncol 4(7):424–432
Yang JD, Roberts LR (2010) Hepatocellular carcinoma: a global view. Nat Rev Gastroenterol Hepatol 7(8):448–458
Bruix J, Sherman M, Llovet JM et al (2001) Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona 2000 EASL conference. European Association for the Study of the Liver. J Hepatol 35(3):421–430
Geschwind JF, Salem R, Carr BI et al (2004) Yttrium-90 microspheres for the treatment of hepatocellular carcinoma. Gastroenterology 127(5 Suppl 1):S194–S205
Bruix J, Sherman M (2005) Management of hepatocellular carcinoma. Hepatology 42(5):1208–1236
Llovet JM, Real MI, Montana X et al (2002) Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 359(9319):1734–1739
Lo CM, Ngan H, Tso WK et al (2002) Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 35(5):1164–1171
Llovet JM, Bruix J (2003) Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology 37(2):429–442
Carr BI (2004) Hepatic arterial 90Yttrium glass microspheres (Therasphere) for unresectable hepatocellular carcinoma: Interim safety and survival data on 65 patients. Liver Transpl 10(2 Suppl 1):S107–S110
Lewandowski RJ, Kulik LM, Riaz A et al (2009) A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolization versus radioembolization. Am J Transplant 9(8):1920–1928
Hilgard P, Hamami M, Fouly AE et al (2010) Radioembolization with yttrium-90 glass microspheres in hepatocellular carcinoma: European experience on safety and long-term survival. Hepatology 52(5):1741–1749
Kulik LM, Carr BI, Mulcahy MF et al (2008) Safety and efficacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology 47(1):71–81
Salem R, Lewandowski RJ, Mulcahy MF et al (2010) Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology 138(1):52–64
EASL-EORTC (2012) Clinical practice guidelines Management of hepatocellular carcinoma. J Hepatol 56(4):908–943
Carr BI, Kondragunta V, Buch SC, Branch RA (2010) Therapeutic equivalence in survival for hepatic arterial chemoembolization and yttrium 90 microsphere treatments in unresectable hepatocellular carcinoma: a two-cohort study. Cancer 116(5):1305–1314
D’Avola D, Lnarrairaegui M, Bilbao JI et al (2009) A retrospective comparative analysis of the effect of Y90-radioembolization on the survival of patients with unresectable hepatocellular carcinoma. Hepatogastroenterology 56(96):1683–1688
Kooby DA, Egnatashvili V, Srinivasan S et al (2010) Comparison of yttrium-90 radioembolization and transcatheter arterial chemoembolization for the treatment of unresectable hepatocellular carcinoma. J Vasc Interv Radiol 21(2):224–230
Lambert B, Sturm E, Mertens J et al (2011) Intra-arterial treatment with 90Y microspheres for hepatocellular carcinoma: 4 years experience at the Ghent University Hospital. Eur J Nucl Med Mol Imaging 38(12):2117–2124
Lance C, McLennan G, Obuchowski N et al (2011) Comparative analysis of the safety and efficacy of transcatheter arterial chemoembolization and yttrium-90 radioembolization in patients with unresectable hepatocellular carcinoma. J Vasc Interv Radiol 22(12):1697–1705
Salem R, Lewandowski RJ, Kulik L, et al. (2011) Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology 140(2):497–507, e492
Sangro B, Carpanese L, Cianni R et al (2011) Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona Clinic liver cancer stages: a European evaluation. Hepatology 54(3):868–878
Lencioni R, Llovet JM (2010) Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 30(1):52–60
Bruix J, Sherman M (2011) Management of hepatocellular carcinoma: an update. Hepatology 53(3):1020–1022
Llovet JM, Di Bisceglie AM, Bruix J et al (2008) Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst 100(10):698–711
Sangro B, Gil-Alzugaray B, Rodriguez J et al (2008) Liver disease induced by radioembolization of liver tumors: description and possible risk factors. Cancer 112(7):1538–1546
Wong CY, Qing F, Savin M et al (2005) Reduction of metastatic load to liver after intraarterial hepatic yttrium-90 radioembolization as evaluated by [18F]fluorodeoxyglucose positron emission tomographic imaging. J Vasc Interv Radiol 16(8):1101–1106
Acknowledgments
This work was supported by Grants from the National Institutes of Health (Grants No. CA100882, CA128633 and CA165076) and Nordion to L. R. R.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Moreno-Luna, L.E., Yang, J.D., Sanchez, W. et al. Efficacy and Safety of Transarterial Radioembolization Versus Chemoembolization in Patients With Hepatocellular Carcinoma. Cardiovasc Intervent Radiol 36, 714–723 (2013). https://doi.org/10.1007/s00270-012-0481-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-012-0481-2