Abstract
Aim
Laparoscopic total mesorectal excision (LaTME) following preoperative chemoradiotherapy (PCRT) in locally advanced rectal cancer (LARC) is technically demanding. The present study is intended to evaluate predictive factors of surgical difficulty of LaTME following PCRT by using pelvimetric and nutritional factors.
Method
Consecutive LARC patients receiving LaTME after PCRT were included. Surgical difficulty was classified based upon intraoperative (operation time, blood loss, and conversion) and postoperative outcomes (postoperative hospital stay and morbidities). Pelvimetry was performed using preoperative T2-weighted MRI. Nutritional factors such as albumin-to-globulin ratio (AGR) and prognostic nutritional index (PNI) were calculated. Multivariable logistic analysis was used to identify predictors of high surgical difficulty. A predictive nomogram was developed and validated internally.
Results
Among 294 patients included, 36 (12.4%) patients were graded as high surgical difficulty. Logistic regression analysis demonstrated that previous abdominal surgery (OR = 6.080, P = 0.001), tumor diameter (OR = 1.732, P = 0.003), intersphincteric resection (vs. low anterior resection, OR = 13.241, P < 0.001), interspinous distance (OR = 0.505, P = 0.009), and preoperative AGR (OR = 0.041, P = 0.024) were independently predictive of high surgical difficulty of LaTME after PCRT. Then, a predictive nomogram was built (C-index = 0.867).
Conclusion
Besides previous abdominal surgery, type of surgery (intersphincteric resection), tumor diameter, and interspinous distance, we found that preoperative AGR could be useful for the prediction of surgical difficulty of LaTME after PCRT. A predictive nomogram for surgical difficulty may aid in planning an appropriate approach for rectal cancer surgery after PCRT.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Laparoscopic total mesorectal excision (LaTME) for rectal cancer has been widely accepted due to well-established advantages over open surgery, such as reduced postoperative pain, rapid recovery, and shorter hospital stay [1,2,3,4]. However, LaTME could be technically challenging in mid/low rectal cancers, especially within a deep and narrow pelvis. Recently, novel minimally invasive techniques, including robotic and transanal TME, may help to overcome difficulties encountered during LaTME [5]. Preoperative chemoradiotherapy (PCRT) is an essential part of the multimodality treatment of locally advanced rectal cancer (LARC) [6, 7]. It can induce tumor downsizing and thus facilitate surgical exposure in the pelvis, whereas PCRT-induced tissue edema and fibrosis may hamper dissection around the mesorectum. Therefore, it is advisable to predict surgical difficulty in LaTME after PCRT to preoperatively plan the appropriate surgical approach (e.g., open, laparoscopic, robotic, or transanal) [5].
Surgical difficulty of LaTME is affected by surgical skills as well as the patient’s clinical factors, such as male sex, a high body mass index (BMI), previous abdominal surgery, low rectal cancer, and advanced-stage tumors [8, 9]. Difficult pelvic structures could also add to surgical difficulty of LaTME. Recently, MRI-based pelvimetry, radiologic measurement of pelvic dimensions, has been proposed as a usefull tool for prediction of surgical difficulty of LaTME [10, 11]. A recent meta-analysis[12] has demonstrated that bony pelvic measurements based on MRI pelvimetry may predict surgical difficulty of TME. The influence of pelvic anatomy on surgical difficulty of LaTME is expected to be more pronounced due to radiation-induced tissue edema or fibrosis. However, the evidence is sparse regarding the prediction of surgical difficulty of LaTME following PCRT [13, 14].
Nutritional status is also associated with postoperative morbidity and length of hospital stay in the treatment for gastrointestinal cancers [15, 16]. Several hematological nutritional indexes have been utilized to reflect the patient’s nutritional status, such as serum albumin, albumin-to-globulin ratio (AGR), and prognostic nutritional index (PNI). Recent studies have demonstrated the predictive value of these nutritional indexes on postoperative complications in gastric [17] and colorectal [18] cancer surgery. In rectal cancers, PCRT could impair the patients’ nutritional status and possibly influences surgical and oncological outcome [19]. We hypothesized that these nutritional indexes may be useful in predicting surgical difficulty of LaTME. Herein, we attempted to predict surgical difficulty of LaTME after PCRT by combining clinical, pelvimetric, and nutritional factors and to develop a predictive nomogram for surgical difficulty.
Patients and methods
Patients
Between 2014 and 2016, LARC patients who underwent PCRT followed by laparoscopic surgery were identified from our colorectal cancer database. Patients with pathologically proven mid/low rectal adenocarcinoma (within 10 cm above the anal verge) and T3/4 and/or N + disease (staged by MRI) were included. Patients were excluded if: underwent abdominoperineal resection (APR) or other surgeries (e.g., Hartmann’s procedure, emergency surgery, palliative surgery, pelvic exenteration, multivisceral resection, para-aortic lymph node dissection, or lateral pelvic lymph node dissection) and incompletion of preoperative MRI. This study was reported in accordance with the The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (https://www.equator-network.org/reporting-guidelines/strobe/), as shown in Appendix.
Treatment
Preoperative radiation was delivered at a dose of 45 Gy to the pelvis, followed by a 5.4 Gy boost to the tumor over a 5–6 week period. Concomitant chemotherapy was administered using CapeOX or FOLFOX regimen. Radical resection was planned at 8 weeks after the end of radiation. LaTME was performed via an abdominal ‘‘up-to-down’’ TME approach as previously described [20, 21].
Definition of surgical difficulty
Since many differences exist between Eastern and Western countries patients, such as weight, BMI, pelvis, medical insurance policy, and strategy of enhanced recovery after surgery, we have made appropriate modifications of the criteria of surgical difficulty modified from that previously proposed by Escal et al. [10]: duration of surgery >300 min (3 points), estimated blood loss >200 ml (1 point), conversion to open procedure (3 points), Clavien–Dindo classifications [22] grade II and III postoperative morbidities (1 point), need for transanal dissection (2 points), and postoperative hospital stay >7 days (2). After calculating the total score, we categorized patients into low (0–2 points) and high (≥3 points) surgical difficulty groups.
MRI-based pelvimetry
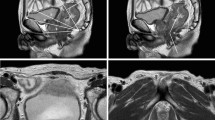
MRI images were centrally evaluated by one radiologist (CJH). Pelvimetry parameters and angles were measured using mid-sagittal and axial planes as described previously [23], as shown in Fig. 1. The definitions of pelvimetric parameters were listed in Supplementary Table 1.
MRI-based pelvimetry. a Sagittal T2-weighted image showing the pelvic inlet (a), pubic tubercle height (b), pelvic outlet length (c), sacral length (d), sacral depth (e), and pelvic depth (f). b Sagittal T2-weighted image illustrating the pelvic angles (α, β, δ and ε). c Axial T2-weighted image showing the interspinous distance. d Axial T2-weighted image showing the manual tracing of the circumference of the rectum (a) that represented the rectal area; axial T2-weighted image showing the manual tracing of the circumference of the mesorectum (b) that represented the mesorectal area
Hematological nutritional indexes
Blood samples were obtained within 1–2 weeks before surgical resection. Hematological nutritional indexes were calculated as follows: AGR = albumin/(total serum protein—albumin) and PNI = serum albumin (g/L) + 5 × total lymphocytes count (109/L).
Statistical analysis
SPSS 20.0 software (IBM SPSS INC., Chicago, USA) was used for statistical analyses. Variables were compared using χ2 test or Student t test, as appropriate. Predictive factors for surgical difficulty were identified by using Logistic regression analysis. A predictive nomogram was constructed by R version 3.5.1. The nomogram was internally validated and evaluated by C-index. P <0.05 was considered of statistical significance.
Results
Patient characteristics
Totally, 294 patients were eligible for the analysis, including 203 men and 91 women. The median BMI was 22.7 kg/m2, and the average distance from the anal verge was 6.8 cm, as seen in Table 1. An overview of the bony pelvis and soft tissue measurement based on MRI pelvimetry was shown in Table 2. The mean interspinous distance was 9.0 ± 1.0 cm. The mean mesorectal and rectal area was 26.6 ± 6.1 cm2 and 7.6 ± 3.2 cm2, respectively, and the mean mesorectal fat area was 19.1 ± 5.5 cm2. As shown in Table 2, the average preoperative PNI was 46.0 ± 6.4, and the average preoperative AGR was 1.3 ± 0.2.
Surgical outcomes
The duration of surgery was 224.8 ± 69.7 min, and the estimated blood loss was 67.0 ± 69.7 ml. A total of four (1.4%) patients were converted to open procedure; 13 (4.4%) patients experienced the use of transanal dissection. The postoperative hospital stay was 8.0 ± 5.1 days, and 42 (14.2%) patients developed postoperative morbidity (Clavien–Dindo classification grade I n = 26, and Clavien–Dindo classification grade II/III n = 16). Accordingly, surgical difficulty was classified as low in 258 (87.6%) patients and high in 36 (12.4%) patients, as seen in Table 3.
Predictors of high surgical difficulty of LaTME after PCRT
In the univariate analysis, higher BMI (P = 0.038), shorter tumor distance from the anal verge (P = 0.006), previous abdominal surgery (P <0.001), larger tumor diameter (P <0.001), type of surgery (P <0.001), defunctioning ileostomy (P = 0.015), shorter interspinous distance (P = 0.026), larger mesorectal area (P = 0.017), larger mesorectal fat area (P = 0.018), larger angle δ (P = 0.012), smaller angle ε (P = 0.024), lower serum albumin level (P = 0.004), lower preoperative AGR (P = 0.002), and lower preoperative PNI (P = 0.010) were significantly associated with high surgical difficulty of LaTME following PCRT (Table 4). Logistic regression analysis demonstrated that previous abdominal surgery (OR = 6.080, 95% CI 2.150–17.190, P = 0.001), tumor diameter (OR = 1.732, P = 0.003), type of surgery (ISR vs. LAR, OR = 13.241, P <0.001), interspinous distance (OR = 0.505, P = 0.009), and preoperative AGR (OR = 0.041, P = 0.024) were independently predictive of high surgical difficulty of LaTME following PCRT (Table 4).
A predictive nomogram for high surgical difficulty of LaTME following PCRT
A predictive nomogram for high surgical difficulty of LaTME after PCRT was then developed (Fig. 2A). A higher total score indicated a higher likelihood of high surgical difficulty of LaTME after PCRT. The AUC of the predictive nomogram was 0.867 (Fig. 2B). The nomogram was validated internally (C-index: 0.867, 95%CI 0.838–0.896). The calibration curve demonstrated good accordance of the predicted and observed probability of high surgical difficulty of LaTME after PCRT (Fig. 2C).
A nomogram for predicting the probability of high surgical difficulty of LaTME for LARC following PCRT. a A predictive nomogram for high surgical difficulty of LaTME for LARC following PCRT; b ROC analysis of the nomogram; c calibration curves for the nomogram with internal validation. LaTME laparoscopic total mesorectal excision, LARC locally advanced rectal cancer, PCRT preoperative chemoradiotherapy, AGR albumin-to-globulin ratio, ROC receiver-operating characteristics curve
Discussion
The present study demonstrated that besides previous abdominal surgery, type of surgery (ISR), tumor diameter, and interspinous distance, preoperative AGR could be useful in the prediction of surgical difficulty of LaTME after PCRT. We further developed a predictive nomogram for surgical difficulty of LaTME following PCRT to help select a proper surgical approach preoperatively.
There are several indicators used to estimate surgical difficulty for TME, including operative time, blood loss, postoperative morbidity, and so on [24,25,26]. To make a more complete outcome definition for surgical difficulty, we employed both intraoperative and postoperative parameters that were previously proposed by Escal et al. [10] and made a slight modification. It is meaningful to include both operative and postoperative parameters because impaired surgical quality and an eventful postoperative course might increase local recurrence and impair survival [27].
During LaTME after PCRT, the mesorectum dissection was hampered by edema and extensive mist and exudates caused by PCRT, as seen in Supplementary Figure S1. Surgical difficulty may depend on surgical expertise; our surgeries were performed by a high-volume surgical team with more experience in LaTME (Supplementary Figure S2). Herein, LaTME after PCRT was performed with a low rate of conversion rate (1.4%) and postoperative morbidity (14.2%). Thus, learning curve factors or surgical skills were not major contributors to surgical difficulty in the present study. Herein, 12.4% of patients were classified as high surgical difficulty of LaTME after PCRT, similar to that (12.8%) of Escal et al. [10].
Pelvic anatomical factors are important in affecting the surgical difficulty of rectal cancer surgery. A narrow pelvis, a prominent sacral promontory, and a shallow sacral angle could impede vision, access, and working space in the operation field during LaTME for rectal cancer [13]. Many efforts have been made in utilization of pelvimetry to predict surgical difficulty of transabdominal TME. Many pelvimetric parameters have been utilized to predict surgical difficulty for LaTME, including both pelvic dimensions and pelvic angles [9, 24,25,26]. As demonstrated in a recent meta-analysis [12], although the role of MRI pelvimetry in predicting surgical difficulty in rectal cancer surgery has been well demonstrated, it is still controversial that which pelvis parameter weighes heaviest in influencing surgical difficulty. Herein, we employed seven dimensions, four angles, and three areas of the pelvis based on MRI images. Univariate analysis demonstrated that shorter interspinous distance, larger mesorectal area, larger MFA, larger angle δ, and smaller angle ε were associated with high surgical difficulty of LaTME after PCRT. After adjusting for confounding factors, shorter interspinous distance remained to be independently associated with high surgical difficulty. Consistent with previous findings [10, 14], our result reaffirmed that shorter interspinous distance could represent an anatomical bottleneck of the deep pelvis that hinders laparoscopic resection maneuvers during LaTME. Besides, a larger tumor within the bony pelvis is associated with surgical difficulty [28]. The present study demonstrated that tumor diameter was independently associated with surgical difficulty of LaTME after PCRT.
A greater mesorectal volume could restrict the pelvic working space for the TME procedure. Two recent studies have investigated the predictive value of the mesorectal fat area on surgical difficulty of laparoscopic [10] and robotic [23] TME. In the present study, we found that both the mesorectal area and the mesorectal fat area were associated with high surgical difficulty of LaTME by univariate analysis; however, none of them were independent predictors of surgical difficulty after adjustment for confounders. The lack of statistical significance might be explained that our patients had a much lower BMI and the mesorectal fat area as compared with that of patients in Western countries [10, 23], thus reducing the influence on surgical difficulty.
Nutritional status is a well-established factor associated with postoperative complications for gastrointestinal tumors [15, 16], such as surgical site infection and anastomotic leakage. Interestingly, lower preoperative AGR was independently associated with high surgical difficulty of LaTME after PCRT. It has been reported that PCRT could impair the patients’ nutritional status of rectal cancer patients and possibly increase the risk of postoperative complications [19]. In our experience, patients with malnutrition are more likely to encounter tissue edema, a large amount of mist and exudates caused by PCRT, which hinders the dissection of the tissue. Unfortunately, whether nutritional status (as indicated by albumin or AGR) contributes to different tissue reactions to CRT among patients is unclear. In addition, the mechanism underlying the predictive value of AGR on surgical difficulty remains to be further explored.
Prior abdominal surgery may increase tissue adhesion and fibrosis, and our study reaffirmed it as an independent predictor of surgical difficulty of LaTME, consistent with previous studies [13, 29]. ISR for low-lying tumors is a technically demanding procedure, in which a distal dissection, transection, and anastomosis proceeding into the sphincter complex structures of the long “tube within a tube,” and even more challenging in patients with bulky tumors in a narrow pelvis [21, 30]. Not surprisingly, we herein demonstrated that laparoscopic ISR was independently correlated with high surgical difficulty of LaTME after PCRT. Contrary with previous findings, BMI was not associated with surgical difficulty of LaTME, probably because the average BMI of our patient cohort was much lower than that of Western patients.
Several scoring systems have been proposed for predicting the difficulty of LaTME for rectal cancer [8, 10, 11]. The present study, for the first time, constructed a predictive nomogram for surgical difficulty of LaTME after PCRT. The AUC of the nomogram was 0.867, indicating a good discriminative power. Surgical trainees or early-career surgeons could select appropriate cases during their learning curve to improve surgical quality and to minimize adverse outcomes caused by lack of experience. The present nomogram can help improve doctor–patient communication by informing patients of possible perioperative risks and complications. Finally yet importantly, this nomogram might help to select the appropriate surgical approach (e.g., open, laparoscopic, robotic, or transanal) for LARC patients after PCRT.
There are several limitations. Firstly, the present study was a retrospective single-center analysis. Secondly, we made a modification of the Escal et al. score for surgical difficulty, which may potentially hamper direct comparison with previous similar works. Thirdly, Nicola de'Angelis et al. [31] have demonstrated that pelvimetry and restaging MRI play an important role in predicting surgical difficulties in LARC management. Since some data concerning restaging MRI were missing, we did not include it in the score and nomogram. Fourthly, operations in our patient cohort were performed by highly experienced laparoscopists; thus, the results may not be extrapolated to less-experienced surgeons. Fifthly, the completeness of TME could not be fully assessed in the present study. Finally, our predictive nomogram required further validation in other independent patient cohorts.
In conclusion, besides previous abdominal surgery, type of surgery (ISR), tumor diameter, and interspinous distance, preoperative AGR could also help predict surgical difficulty of LaTME after PCRT.
References
Green BL, Marshall HC, Collinson F et al (2013) Long-term follow-up of the Medical Research Council CLASICC trial of conventional versus laparoscopically assisted resection in colorectal cancer. Br J Surg 100:75–82
van der Pas MH, Haglind E, Cuesta MA et al (2013) Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol 14:210–218
Fleshman J, Branda ME, Sargent DJ et al (2019) Disease-free survival and local recurrence for laparoscopic resection compared with open resection of stage II to III rectal cancer: follow-up results of the ACOSOG Z6051 randomized controlled trial. Ann Surg 269:589–595
Stevenson A, Solomon MJ, Brown C et al (2019) Disease-free survival and local recurrence after laparoscopic-assisted resection or open resection for rectal cancer: the Australasian laparoscopic cancer of the rectum randomized clinical trial. Ann Surg 269:596–602
Dayal S, Battersby N, Cecil T (2017) Evolution of surgical treatment for rectal cancer: a review. J Gastrointest Surg 21:1166–1173
van Gijn W, Marijnen CA, Nagtegaal ID et al (2011) Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol 12:575–582
Bosset JF, Calais G, Mineur L et al (2014) Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: long-term results of the EORTC 22921 randomised study. Lancet Oncol 15:184–190
Veenhof AA, Engel AF, van der Peet DL et al (2008) Technical difficulty grade score for the laparoscopic approach of rectal cancer: a single institution pilot study. Int J Colorectal Dis 23:469–475
Akiyoshi T, Kuroyanagi H, Oya M et al (2009) Factors affecting the difficulty of laparoscopic total mesorectal excision with double stapling technique anastomosis for low rectal cancer. Surgery 146:483–489
Escal L, Nougaret S, Guiu B et al (2018) MRI-based score to predict surgical difficulty in patients with rectal cancer. Br J Surg 105:140–146
De’Angelis N, Pigneur F, Martinez-Perez A et al (2019) Assessing surgical difficulty in locally advanced mid-low rectal cancer: the accuracy of two MRI-based predictive scores. Colorectal Dis 21:277–286
Hong JS, Brown K, Waller J et al (2020) The role of MRI pelvimetry in predicting technical difficulty and outcomes of open and minimally invasive total mesorectal excision: a systematic review. Tech Coloproctol 24:991–1000
Ishihara S, Watanabe T, Fukushima Y et al (2014) Safety and factors contributing to the difficulty of laparoscopic surgery for rectal cancer treated with preoperative chemoradiotherapy. Tech Coloproctol 18:247–255
De’Angelis N, Pigneur F, Martinez-Perez A et al (2018) Predictors of surgical outcomes and survival in rectal cancer patients undergoing laparoscopic total mesorectal excision after neoadjuvant chemoradiation therapy: the interest of pelvimetry and restaging magnetic resonance imaging studies. Oncotarget 9:25315–25331
Mosquera C, Koutlas NJ, Edwards KC et al (2016) Impact of malnutrition on gastrointestinal surgical patients. J Surg Res 205:95–101
Lee H, Cho YS, Jung S et al (2013) Effect of nutritional risk at admission on the length of hospital stay and mortality in gastrointestinal cancer patients. Clin Nutr Res 2:12–18
Jiang N, Deng JY, Ding XW et al (2014) Prognostic nutritional index predicts postoperative complications and long-term outcomes of gastric cancer. World J Gastroenterol 20:10537–10544
Tokunaga R, Sakamoto Y, Nakagawa S et al (2015) Prognostic nutritional index predicts severe complications, recurrence, and poor prognosis in patients with colorectal cancer undergoing primary tumor resection. Dis Colon Rectum 58:1048–1057
Lee YJ, Kim WR, Han J et al (2016) Prognostic impact of immunonutritional status changes during preoperative chemoradiation in patients with rectal cancer. Ann Coloproctol 32:208–214
Li S, Chi P, Lin H et al (2011) Long-term outcomes of laparoscopic surgery versus open resection for middle and lower rectal cancer: an NTCLES study. Surg Endosc 25:3175–3182
Chi P, Huang SH, Lin HM et al (2015) Laparoscopic transabdominal approach partial intersphincteric resection for low rectal cancer: surgical feasibility and intermediate-term outcome. Ann Surg Oncol 22:944–951
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Yamaoka Y, Yamaguchi T, Kinugasa Y et al (2019) Mesorectal fat area as a useful predictor of the difficulty of robotic-assisted laparoscopic total mesorectal excision for rectal cancer. Surg Endosc 33:557–566
Atasoy G, Arslan NC, Elibol FD et al (2018) Magnetic resonance-based pelvimetry and tumor volumetry can predict surgical difficulty and oncologic outcome in locally advanced mid-low rectal cancer. Surg Today 48:1040–1051
Killeen T, Banerjee S, Vijay V et al (2010) Magnetic resonance (MR) pelvimetry as a predictor of difficulty in laparoscopic operations for rectal cancer. Surg Endosc 24:2974–2979
Chen W, Li Q, Fan Y et al (2016) Factors predicting difficulty of laparoscopic low anterior resection for rectal cancer with total mesorectal excision and double stapling technique. PLoS ONE 11:e151773
Sprenger T, Beissbarth T, Sauer R et al (2018) Long-term prognostic impact of surgical complications in the German Rectal Cancer Trial CAO/ARO/AIO-94. Br J Surg 105:1510–1518
Zhou XC, Su M, Hu KQ et al (2016) CT pelvimetry and clinicopathological parameters in evaluation of the technical difficulties in performing open rectal surgery for mid-low rectal cancer. Oncol Lett 11:31–38
Akagi T, Inomata M, Etoh T et al (2012) Multivariate evaluation of the technical difficulties in performing laparoscopic anterior resection for rectal cancer. Surg Laparosc Endosc Percutan Tech 22:52–57
Huang S, Huang Y, Chi P et al (2019) Completely abdominal approach laparoscopic partial intersphincteric resection after neoadjuvant chemoradiation for initial cT3 juxta-anal rectal cancer. J Laparoendosc Adv Surg Tech A 29:809–816
De’Angelis N, Pigneur F, Martínez-Pérez A et al (2018) Predictors of surgical outcomes and survival in rectal cancer patients undergoing laparoscopic total mesorectal excision after preoperative chemoradiation therapy: the interest of pelvimetry and restaging magnetic resonance imaging studies. Oncotarget 9:25315–25331
Acknowledgements
The authors thank all the staff in Department of colorectal surgery, Fujian Medical University Union Hospital, Fuzhou, Fujian Province, People’s Republic of China.
Funding
This study was supported by Construction Project of Fujian Province Minimally Invasive Medical Center (Grant Number: [2017]171), Startup Fund for Scientific Research, Fujian Medical University (Grant Number: 2017XQ1028), Joint Funds for the Innovation of Science and Technology, Fujian province (Grant Number: 2018Y9030), and Young and Middle-aged Backbone Training Project in the Health System of Fujian province (Grant Number: 2019-ZQN-45).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
None declared.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sun, Y., Chen, J., Ye, C. et al. Pelvimetric and Nutritional Factors Predicting Surgical Difficulty in Laparoscopic Resection for Rectal Cancer Following Preoperative Chemoradiotherapy. World J Surg 45, 2261–2269 (2021). https://doi.org/10.1007/s00268-021-06080-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-021-06080-w