Abstract
Background
The difficulty of performing total mesorectal excision (TME) for rectal cancer partly relies on the surgeon’s subjective assessment of the individual patient’s pelvic anatomy and tumour characteristics, which generally influences the choice of platform used (open, laparoscopic, robotic or trans-anal surgery). Recent studies have found associations between several anatomical pelvic measurements and surgical difficulty. The aim of this study was to systematically review existing data reporting the use of magnetic resonance imaging (MRI)-based pelvic measurements to predict technical difficulty and outcomes of TME, and determine whether pelvimetry could optimise patient-specific selection of a particular surgical approach.
Methods
MEDLINE, Embase and Cochrane Library databases were systematically searched for studies reporting MRI-based pelvic measurements in patients undergoing surgery for rectal cancer, and the effect of these measurements on surgical difficulty.
Results
Eleven studies reporting the association between MRI-pelvimetry measurements and rectal cancer surgical outcomes were included. Indicators for surgical difficulty used in the included studies were involved circumferential resection margin, longer operative time, incomplete TME, higher blood loss, anastomotic leak, conversion to open surgery and overall complications. Bony pelvic measurements which were associated with increased surgical difficulty in more than one study were a smaller interspinous distance, a smaller intertubercle distance, a smaller pelvic inlet and larger pubic tubercle height. Two studies identified larger mesorectal fat area as a predictor of surgical difficulty.
Conclusions
Bony pelvic measurements may predict surgical difficulty during TME, however, use of different indicators of difficulty limit comparison between studies. Early data suggest MRI soft tissue measurements may predict surgical difficulty and warrants further investigation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Both completeness of the TME (i.e. an intact fascia propria) and the circumferential resection margin (CRM) are well established as important predictors of local and distant recurrence after operation for rectal cancer [1,2,3]. The colorectal surgeon has the choice of four platforms (open, laparoscopic, robotic or transanal surgery) which can be used in isolation or combination to achieve the same oncological aim—a complete TME. One platform may be better than another in particular situations, depending on patient, surgeon and tumour characteristics.
The anatomical confines of the rectum in the bony pelvis have a direct impact on surgical access and the ability to achieve precise mesorectal dissection. Certain patient factors may further affect surgical access to the pelvis and thereby make rectal cancer surgery even more difficult (the “difficult pelvis”). For example, on average, the true male pelvis is anatomically narrower than that of a female, while patients with a higher body mass index (BMI) have a greater volume of visceral fat, limiting the ability to retract pelvic viscera and, therefore, access to the rectum. There is no widely accepted objective definition of the “difficult pelvis”, and current practice relies on the surgeon’s subjective clinical assessment. This intuition often drives the surgeon’s choice between a minimally invasive or open approach in an individual patient.
Pelvimetry refers to radiologically measured pelvic dimensions and, was originally used to assess the likelihood of successful vaginal delivery [4]. More recently, it has been used to predict the difficulty of rectal resection. Several studies have found associations between particular pelvic measurements (bony and soft tissue) and surgical difficulty for both open and minimally invasive approaches, where a number of parameters have been used to reflect operative difficulty (involved resection margins, conversion to open surgery in the case of minimally invasive surgery, operating time or blood loss) [5,6,7,8,9,10,11,12,13,14,15]. The aim of this study was to systematically review existing data reporting the use of MRI-based bony and soft tissue pelvic measurements, to predict the technical difficulty and oncological outcomes of rectal cancer surgery using open and minimally invasive approaches. This was with a view to defining the ‘difficult pelvis’ surgically, and determining whether pelvimetry could optimise patient-specific selection of a particular surgical approach.
Materials and methods
Search strategy
The systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. Electronic searches of MEDLINE/PubMed, EMBASE and the Cochrane Central Register of Controlled Trials were conducted. MeSH term pelvimetry and related text words (including MeSH term ‘pelvimetry’, or text words pelvic measurements, dimensions, anatomy or volume) were combined with the MeSH terms ‘Magnetic resonance imaging’ and ‘Rectal neoplasms’ or related text words using the Boolean operator AND. No search limits were set. The final search was performed on 10th September 2019.
Inclusion and exclusion criteria
All studies reporting the use of MRI to measure pelvic bony or soft tissue measurements, dimensions or volumes, and the effect of these measurements on rectal cancer surgical outcomes were included. The primary outcome of interest was surgical difficulty, which may be defined using pathological parameters (e.g. involved resection margins), conversion to open surgery in the case of minimally invasive surgery, operating time, blood loss, or a combination of such factors. Studies were excluded if an imaging modality other than MRI was used or not reported separately, the outcomes of surgery were not reported or pelvic measurements were not reported. The article types of reviews, technical notes, comments, conference abstracts and letters were not included. Only studies in the English language were included. Where a single institution had published multiple reports with accumulating patient cohorts, the largest or most informative study was included. Citation lists were manually searched for studies not identified in the initial search.
Study selection
After removal of duplicate search results, the title and abstract of all retrieved citations was reviewed by a single author for potential eligibility. Two authors (KGMB and JW) independently assessed those abstracts identified as potentially eligible in full text and according to the inclusion and exclusion criteria. Differences of opinion between reviewers were discussed with a senior investigator (JH).
Appraisal of internal validity or risk of bias using a standardised approach was not feasible in this systematic review as the included studies were case series with no controlled intervention studies.
Results
Eleven eligible studies including a total of 1270 patients were identified during the search and included in this review (Fig. 1). Included studies are summarized in Table 1 and all were published within the last 15 years (from 2005 to 2019). Four studies included patients who had laparoscopic TME [9, 10, 13, 14], while three studies included patients who had robotic TME [11, 13, 15]. Eight studies categorised patients according to low, mid or high rectal tumours, in which 65–100% of patients had a low or mid rectal tumour (Table 1). The majority of patients in all included studies had TME with primary rectal anastomosis (53–100%), while 0–33% and 0–14% had abdominoperineal resection and a Hartmann’s procedure, respectively.
Seven studies investigated pelvic measurements based on bony landmarks alone (Table 2), while three investigated both soft tissue and bony dimensions, and one investigated soft tissue dimensions only (Table 3). Indicators for surgical difficulty used in the included studies were involvement CRM [six studies], longer operative time [five studies], incomplete TME (one study), higher blood loss (one study), anastomotic leak (one study), conversion to open surgery (one study), overall complications (one study). One study used a composite scoring system which incorporated multiple parameters to reflect surgical difficulty [13].
Bone measurements
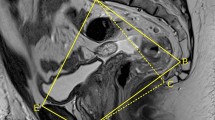
A smaller interspinous distance (IS, Fig. 2) was associated with increasing surgical difficulty in three studies (Tables 2, 3). Boyle and colleagues showed that in 25 female patients who were predicted to have a negative CRM prior to open rectal cancer surgery, a smaller IS was associated with pathological CRM positivity (97.3 mm vs 110.4 mm, p = 0.031) during open surgery [5]. Baik et al. demonstrated that on multivariate analysis, a smaller IS was associated with both incomplete TME (RR 0.502; 95% CI 0.269–0.936) and an involved circumferential resection margin (RR 0.388; 95% CI 0.195–0.770) [7]. In a retrospective analysis of the European MRI and Rectal Cancer Surgery (EuMaRCS) study, which included 170 patients undergoing laparoscopic TME, de’Angelis et al. demonstrated a relationship between a smaller IS and conversion to open surgery (odds ratio 0.85; 95% CI 0.74–0.97; p = 0.018) [14].
Three studies demonstrated an association between a smaller intertubercle distance (IT, Fig. 3) and surgical difficulty (Tables 2, 3). de’Angelis et al. showed that patients with a larger IT were less likely to develop postoperative complications after laparoscopic TME (odds ratio 0.85; 95% CI 0.74–0.97; p = 0.018) [14], while Kim and colleagues identified a smaller IT (less than 8.9 cm) as an independent predictor of longer laparoscopic pelvic dissection time (p = 0.034) [10]. Escal et al. demonstrated that an IT > 10.1 cm was associated with increased surgical difficulty according to their composite score described above (OR 0.35; 95% CI 0.12–0.94; p = 0.041) [13].
Three studies found an association between size of the pelvic inlet and surgical difficulty [5, 7, 12]. Boyle and colleagues showed that in the subgroup of female patients who were predicted to have a negative CRM, a smaller pelvic inlet (measured as the anterior–posterior distance) was associated with CRM involvement (107 mm vs 116.1 mm, p = 0.017) [5]. Baik et al. demonstrated that a smaller obstetric conjugate (distance from the sacral promontory to the top of the pubic symphysis) is associated with a higher risk of incomplete TME on multivariate analysis (RR 0.472; 95% CI 0.248–0.897) [7]. Atasoy and colleagues identified a smaller pelvic inlet as an independent predictor of blood loss (p = 0.004) [12].
Pubic tubercle height was found to be associated with surgical difficulty in two studies [6, 14]. The pubic tubercle height was not described, but was taken to be defined as the superior aspect of the pubic tubercle to the inferior aspect of the pubic symphysis. de’Angelis et al. showed that larger pubic tubercle height was associated with conversion to open during laparoscopic TME (odds ratio 1.28; 95% CI 1.01–1.61; p = 0.042) [14], while Salerno et al. showed an association between larger pubic tubercle height and CRM involvement during open surgery (mean tubercle height 2.8 vs. 2.38 cm; p = 0.046) [6].
Soft tissue measurements
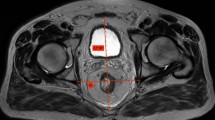
Of four studies which investigated soft tissue pelvic measurements, two identified mesenteric fat area (MFA) as a predictor of surgical difficulty [13, 15]. Yamaoka demonstrated that a on multivariate analysis of 98 patients undergoing robotic TME, a larger MFA area (≥ 26.0 cm2) was the only independent predictor of increased pelvic dissection time (p = 0.009) [15]. The MFA was calculated by manually tracing the mesorectal and rectal contours on axial MRI images, at the level of the 5th sacral vertebrae, giving the mesorectal area from which the rectal area was subtracted. Escal et al. showed that a larger MFA (> 20.7 cm2) was associated with increased surgical difficulty according to their composite score described below (OR 2.69; 95% CI 1.00–7.25; p = 0.051) [13]. The MFA was calculated using the same technique, but based on axial images at the level of the ischial spine (Fig. 4).
Surgical difficulty scoring systems
Escal and colleagues used an ‘in house’ composite score for surgical difficulty ranging from 0 (no difficulty) to 12 (high difficulty), incorporating six parameters: operating time > 300 min, conversion to open surgery, use of transanal dissection (performed to avoid conversion to open), postoperative hospital stay > 15 days, blood loss > 200 ml, and morbidity (Clavien Dindo grade II and III)[13]. Four factors (two pelvimetric) were found to be associated with higher surgical difficulty (difficulty score > 6): BMI > 30 kg/m2 (p = 0.021), need for coloanal anastomosis (p = 0.034), IT distance < 10.1 cm (p = 0⋅041) and MFA > 20⋅7 cm2 (p = 0.051). The authors proposed a surgical difficulty score that could be used preoperatively to predict surgical difficulty, ranging from 0 to 4, based on the presence or absence of the above factors.
Kim and colleagues categorised patients having laparoscopic TME as easy, moderate or difficult based on the presence of risk factors for longer pelvic dissection time [10]. Their study found that longer sacrum length, shallow sacral depth (Fig. 5), shorter IT distance, and tumor size were independent predictors of pelvic dissection time (p = 0.015, p < 0.001, p = 0.032, p = 0.028, respectively). The lower or upper quartile of each of these variables were defined as risk factors for surgical difficulty, and patients were categorised in easy (no risk factors), moderate [1–2 riskfactors] and difficult groups (> 3 risk factors). The authors found that patients in the difficult group had a longer mean pelvic dissection time (p < 0.001), high intraoperative transfusion requirement (P = 0.032) and were more likely to have an incomplete TME (p = 0.032). A subsequent study by the same unit found no relationship between any pelvimetric parameters and operating time in patients undergoing robotic TME, and when patients were grouped based on the same risk factors as identified in the previous study, categorised patients, there was no difference between the groups in terms of operative and pathologic outcomes, including operation time [11].
Discussion
This systematic review of 11 studies included 1270 patients who had open or minimally invasive TME for rectal cancer, where pelvimetry data based on preoperative MRI was used to identify predictors of surgical difficulty. These studies used a number of parameters alone or in combination to define surgical difficulty, including involved circumferential resection margins, TME specimen quality, conversion to open surgery from laparoscopic, use of transanal dissection (to avoid conversion to open surgery), operating time, complications and blood loss. Pelvimetric parameters shown to be associated with surgical difficulty in more than one study were a smaller IT or IS distance, a smaller pelvic inlet, a larger pubic tubercle height, and a larger MFA. Several other angles and distances are associated with difficult rectal surgery in individual studies, but were not replicated in subsequent studies.
The majority of studies in this review used bony measurements to assess the surgically difficult pelvis. The advantage of measurements of bony structures is that they are highly reproducible, as the high-density bone is easily identified on cross-sectional imaging. Bony landmarks can also act as markers of depth within the pelvis. Several studies included in this review demonstrated that a narrow IS and/or IT distance is associated with surgical difficulty. These parameters were derived from the traditional use of pelvimetry—assessing the bony anatomic constraints for vaginal delivery and the likelihood of a successful delivery by this method. These distances reflect the width of the pelvis at the mid rectum (IS plane) and lower rectum (IT plane), so can act as markers for access to the pelvis in rectal surgery. Arguably, deeper locations in the pelvis present the greatest challenge for the surgeon, independent of the technique selected.
While pelvimetry based on bony landmarks is easily identified and reproducible, the measurements are probably not a complete representation of rectal surgery, where, in addition to operating within the bony confines of the pelvis, the surgeon must also retract soft tissue to permit dissection. Unlike bone, soft tissue can be manipulated, but both the volume of the rectum and the surrounding tissues may limit compression of the soft tissues and thus surgical exposure. For example, increased soft tissue volume due to a bulky prostate or high volume of adipose tissue may increase the difficulty of adequate retraction in the pelvis. Thus, single distances on the cross-section are useful indicators but are likely to be simplistic assessments of the multiple variables, which can limit pelvic access, making surgery more difficult and affecting oncological outcomes. Interestingly, the 3 most recent studies included in this review all investigated soft tissue measurements. Two of them identified a large MFA as an independent predictor of surgical difficulty [13,14,15]. This is likely to be secondary to a narrower space between the fascia propria of the mesorectum and the surrounding pelvic fasica, where the mesorectum itself is bulkier. Based on these findings, further investigation of pelvimetric parameters using the mesorectum, the tumour dimensions itself and their relationship to other pelvic soft tissue structures (i.e. adipose tissue, muscles, genitourinary organs and neurovascular structures) is needed, and may assist in developing criteria by which surgeons can define the difficult pelvis and select the surgical technique which may increase the chance of optimal oncological outcomes.
This review identified two scoring systems used for surgical difficulty incorporating MRI-pelvimetry. Kim and colleagues categorized patients into easy, moderate and difficult groups based on the presence of risk factors for longer pelvic dissection time in patients undergoing laparoscopic TME [10]. The same group subsequently analysed these MRI-based criteria in patients undergoing robotic TME and found that there was no difference in operating time between easy, moderate and difficult groups [11]. Based on these findings, the authors concluded that robotic surgery may be able to overcome the anatomical difficulty in these patients. Escal et al. proposed a score to predictor surgical difficulty, based on the presence of both pelvimetric (IT distance < 10.1 cm, MFA > 20.7 cm2), patient (BMI > 30) and technical factors (coloanal anastomosis) [13]. Unfortunately, the external validity of this scoring system was not demonstrated when applied by de’Angelis and colleagues to an independent population (patients in the EuMaRCS study, where it had low predictive value [16].
There are several limitations of this systematic review and the existing literature in the area of MRI-pelvimetry. Current data are relatively heterogenous with respect to tumour location, treatment algorithms (with or without chemoradiotherapy), imaging protocols (pre- vs. post chemoradiotherapy, timing before surgery) and surgical technique. Moreover the majority of included studies did not include information about rectal tumour dimensions. Most importantly, the pelvic measurements investigated and the definitions of surgical difficulty varied significantly between studies, which limits any ability to make comparisons or draw meaningful conclusions. The subjectivity of how surgeons define a ‘difficult pelvis’ remains largely unaddressed. These issues and the fact that non-English articles were excluded from the review likely lead to publication bias and make any association between pelvic measurements and surgical outcomes difficult to interpret.
Conclusions
There are limited data reporting the use of MRI-based pelvic measurements to predict technical difficulty and outcomes of rectal cancer surgery. There is some data to suggest that a smaller IS or IT distance, a smaller pelvic inlet and a larger pubic tubercle height may be associated with increased surgical difficulty. Two more recent studies identified a larger MFA as a predictor of surgical difficulty, and should encourage future investigation into pelvic soft tissue measurements. Developing an accepted definition of the ‘difficult pelvis’ is likely to require more sophisticated methods of integrating multiple pelvimetric variables, tumour characteristics and surgeon factors (such as experience and training).
References
Wibe A, Rendedal PR, Svensson E, Norstein J, Eide TJ, Myrvold HE et al (2002) Prognostic significance of the circumferential resection margin following total mesorectal excision for rectal cancer. Br J Surg 89(3):327–334
Kitz J, Fokas E, Beissbarth T, Strobel P, Wittekind C, Hartmann A et al (2018) Association of plane of total mesorectal excision with prognosis of rectal cancer: secondary analysis of the CAO/ARO/AIO-04 phase 3 randomized clinical trial. JAMA Surg 153(8):e181607
Taylor FG, Quirke P, Heald RJ, Moran BJ, Blomqvist L, Swift IR et al (2014) Preoperative magnetic resonance imaging assessment of circumferential resection margin predicts disease-free survival and local recurrence: 5-year follow-up results of the MERCURY study. J Clin Oncol 32(1):34–43
Lenhard M, Johnson T, Weckbach S, Nikolaou K, Friese K, Hasbargen U (2009) Three-dimensional pelvimetry by computed tomography. Radiol Med 114(5):827–834
Boyle KM, Petty D, Chalmers AG, Quirke P, Cairns A, Finan PJ et al (2005) MRI assessment of the bony pelvis may help predict resectability of rectal cancer. Colorectal Dis 7(3):232–240
Salerno G, Daniels IR, Brown G, Norman AR, Moran BJ, Heald RJ (2007) Variations in pelvic dimensions do not predict the risk of circumferential resection margin (CRM) involvement in rectal cancer. World J Surg 31(6):1313–1320
Baik SH, Kim NK, Lee KY, Sohn SK, Cho CH, Kim MJ et al (2008) Factors influencing pathologic results after total mesorectal excision for rectal cancer: analysis of consecutive 100 cases. Ann Surg Oncol 15(3):721–728
Boyle KM, Chalmers AG, Finan PJ, Sagar PM, Burke D (2009) Morphology of the mesorectum in patients with primary rectal cancer. Dis Colon Rectum 52(6):1122–1129
Killeen T, Banerjee S, Vijay V, Al-Dabbagh Z, Francis D, Warren S (2010) Magnetic resonance (MR) pelvimetry as a predictor of difficulty in laparoscopic operations for rectal cancer. Surg Endosc 24(12):2974–2979
Kim JY, Kim YW, Kim NK, Hur H, Lee K, Min BS et al (2011) Pelvic anatomy as a factor in laparoscopic rectal surgery: a prospective study. Surg Laparosc Endosc Percutan Tech 21(5):334–339
Baek SJ, Kim CH, Cho MS, Bae SU, Hur H, Min BS et al (2015) Robotic surgery for rectal cancer can overcome difficulties associated with pelvic anatomy. Surg Endosc 29(6):1419–1424
Atasoy G, Arslan NC, Elibol FD, Sagol O, Obuz F, Sokmen S (2018) Magnetic resonance-based pelvimetry and tumor volumetry can predict surgical difficulty and oncologic outcome in locally advanced mid-low rectal cancer. Surg Today 48(12):1040–1051
Escal L, Nougaret S, Guiu B, Bertrand MM, de Forges H, Tetreau R et al (2018) MRI-based score to predict surgical difficulty in patients with rectal cancer. Br J Surg 105(1):140–146
de’Angelis N, Pigneur F, Martinez-Perez A, Vitali GC, Landi F, Torres-Sanchez T, et al. (2018) Predictors of surgical outcomes and survival in rectal cancer patients undergoing laparoscopic total mesorectal excision after neoadjuvant chemoradiation therapy: the interest of pelvimetry and restaging magnetic resonance imaging studies. Oncotarget. 9(38):25315-31
Yamaoka Y, Yamaguchi T, Kinugasa Y, Shiomi A, Kagawa H, Yamakawa Y et al (2019) Mesorectal fat area as a useful predictor of the difficulty of robotic-assisted laparoscopic total mesorectal excision for rectal cancer. Surg Endosc 33(2):557–566
de’Angelis N, Pigneur F, Martinez-Perez A, Vitali GC, Landi F, Gomez-Abril SA, et al. (2019) Assessing surgical difficulty in locally advanced mid-low rectal cancer: the accuracy of two MRI-based predictive scores. Colorectal Dis. 21(3):277–86.
Funding
Jonathan Hong is the recipient of the Mitchell J Notaras Fellowship in Colorectal Surgery.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix S1–Medline (OVID: 1946-present)
Appendix S1–Medline (OVID: 1946-present)
-
1.
(MRI or MR* or magnetic resonance).tw
-
2.
Magnetic Resonance Imaging/
-
3.
((pelvis or pelvic) adj3 (measure* or anatom* or distan* or imag* or volume* or area*)).tw
-
4.
Pelvimetry/
-
5.
Rectal Neoplasma/
-
6.
(rect* adj3 (cancer* or malign* or excis* or resect* or neoplas* or surg*)).tw
-
7.
(mesorect* adj3 (excision* or resect* or surg* or dissect*)).tw
-
8.
5 OR 6 OR 7
-
9.
3 OR 4
-
1.
1 OR 2
-
11.
8 AND 9 AND 10
Rights and permissions
About this article
Cite this article
Hong, J.SY., Brown, K.G.M., Waller, J. et al. The role of MRI pelvimetry in predicting technical difficulty and outcomes of open and minimally invasive total mesorectal excision: a systematic review. Tech Coloproctol 24, 991–1000 (2020). https://doi.org/10.1007/s10151-020-02274-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10151-020-02274-x