Abstract
Background
We investigated the role of 18F-fluorodeoxyglucose positron emission tomography-computed tomography (PET-CT) in predicting the effect of induction therapy in patients with thymic epithelial tumors.
Methods
Fourteen patients with thymic epithelial tumors who underwent PET-CT before and after induction therapy were retrospectively analyzed. The relationship between the change in the maximum standardized uptake value (SUVmax) in PET-CT, the response evaluation criteria in solid tumors and the pathologic response (Ef0, no necrosis of tumor cells; Ef1, some necrosis of tumor cells with more than one-third of viable tumor cells; Ef2, less than one-third of tumor cells were viable; and Ef3, no tumor cells were viable) was analyzed.
Results
The study cohort consisted of 5 males and 9 females. Nine of the patients had thymoma, and 5 had thymic carcinoma. The induction therapy included chemotherapy in 9 cases, chemoradiation therapy in 4 cases and radiation therapy in 1 case. Among the 8 patients with a pathologic response of Ef0/1, 5 were clinically evaluated as having stable disease (SD), while 3 were found to have had a partial response (PR). The SUVmax was elevated in 2 cases, unchanged in 1 and decreased in 5. On the other hand, 3 of the 6 patients with a pathologic response of Ef2, 3 were classified as having SD, while the other 3 had a PR. The SUVmax decreased in all of the patients.
Conclusions
In comparison with CT, PET-CT seems to be useful for predicting the pathologic response to induction therapy in patients with thymic epithelial tumors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Thymic epithelial tumors, which include thymomas, thymic carcinomas and thymic neuroendocrine carcinomas, are the most common tumors in the mediastinum [1]. Surgical resection is the mainstay of treatment, and the complete resection of the tumor is considered to be associated with a favorable prognosis. In advance-staged cases, induction therapy [including chemotherapy, chemoradiation therapy (CRT) and radiation therapy] is often considered. A good response to induction therapy is essential to achieving a complete resection. Thus, the response to induction therapy is considered to be important.
Recently, positron emission tomography using 18F-fluorodeoxyglucose (FDG-PET) has started to play an important role in many oncological settings, not only in the pre-treatment diagnosis but also in the prediction of the treatment outcome [2, 3]. This imaging technique offers a holistic approach for the diagnosis of malignant tumors because it integrates the structural, functional and metabolic information of the tumors. The role of FDG-PET computed tomography (PET-CT) in predicting the pathologic response to induction therapy has been reported in several types of malignant tumors, including (but not limited to) breast cancer [4], pancreatic cancer [5] and rectal cancer [6]. PET-CT imaging is also utilized in response evaluation to neoadjuvant therapy in patients with thoracic malignancies such as primary lung cancer [7, 8], esophageal cancer [9] and malignant pleural mesothelioma [10]. However, there have been few reports on the role of PET-CT in evaluating induction therapy for thymic epithelial tumors [11, 12]. Furthermore, the relationship between the pathologic response to induction therapy and the change in PET-CT findings was not fully investigated.
In this study, we retrospectively analyzed 14 patients with thymic epithelial tumors who underwent a PET-CT examination before and after undergoing induction therapy. The relationships among the change in the maximum standardized uptake value (SUVmax) and SUV index in PET-CT, the response evaluation criteria in solid tumors (RECIST) [13] and the pathologic response were analyzed.
Patients and methods
From May 2007 to December 2014, 14 patients with invasive thymic epithelial tumors who underwent PET-CT before and after induction therapies at Nagoya University Hospital were enrolled in the present study. The definitive diagnoses of the tumors were obtained by examining resected or biopsied specimens. These specimens were reviewed by an experienced pathologist, and the tumors were classified by the World Health Organization (WHO) classification. All of the tumors were staged using the Masaoka-Koga staging system [14]. PET-CT imaging was performed using the same scanner and the same protocol as in our previous report [15]. In brief, the patients received an intravenous injection of 3.7–4.07 MBq/kg of FDG and then rested for 50–60 min before undergoing imaging. Image acquisition was performed using a PET/CT scanner (Biograph16; Siemens Medical Solutions, Erlangen, Germany). The emission PET images were reconstructed using iterative ordered subset expectation maximization with non-contrast CT. For the semiquantitative assessment, regions of interest (ROIs) were overlaid on FDG-avid tumors, and the SUVmax (the maximum ROI activity [MBq/g]/injected dose [MBq]/body weight [g]) of each tumor was measured. In order to normalize SUVmax, the mean SUV of the right liver lobe (liver SUVmean) for each patient was also calculated. The normalized SUV was defined as the SUVindex, which was calculated as the ratio of tumor SUVmax to liver SUVmean. The mean duration between PET-CT imaging and the initiation of induction therapy was 23 ± 13 days. The mean period from the last date of induction therapy to the second PET-CT imaging session was 28 ± 18 days. We classified the pathologic response to induction therapy into four groups (Ef0, no necrosis of tumor cells; Ef1, some necrosis of tumor cells with more than one-third of viable tumor cells; Ef2, less than one-third of tumor cells were viable; and Ef3, no tumor cells were viable) according to the criteria of the Japan Lung Cancer Society [16]. The relationships among the changes in the SUVmax in PET-CT, the RECIST score and the pathologic response were analyzed.
The Wilcoxon signed-rank test was used for the comparison of the SUVmax and SUVindex before and after induction therapy. p values of <0.05 were considered to indicate statistical significance. All of the statistical analyses were performed using the STATA Ver. 11 (College Station, TX, USA) software program. The Institutional Review Board of the Nagoya University Hospital approved this retrospective study (approval number: 2014-0100).
Results
The study cohort consisted of 5 males and 9 females who were from 25 to 71 years of age (median, 60 years). Pathological examinations of the resected or biopsied specimens revealed 9 thymomas, including 1 type AB tumor, 3 type B1 tumors, 4 type B2 tumors and 1 type B3 tumor. The remaining 5 tumors were all diagnosed as thymic carcinomas, including 4 squamous cell carcinomas and 1 large cell neuroendocrine carcinoma (Table 1). Three stage II tumors, 6 stage III tumors and 5 stage IV tumors were observed. There were no patients with distant metastasis. The induction therapies that were administered included chemotherapy (n = 9), chemoradiation therapy (n = 4) and radiation therapy (n = 1). Eight patients with thymoma underwent induction chemotherapy using a cisplatin, doxorubicin and methylprednisolone (CAMP) regimen [17]. The distribution of the patients’ pathologic responses was as follows: Ef0 (n = 1), Ef1 (n = 7) and Ef2 (n = 6). No patients showed a pathologically complete response (Ef3), after induction therapy.
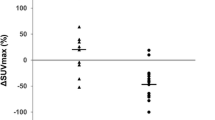
The change in the patients’ SUVmax from before to after induction therapy is shown in Table 1 and Fig. 1. Among the 8 patients with an Ef0/1 response, 5 (62.5%) were classified as having stable disease (SD) and 3 (37.5%) were classified as having a partial response (PR). The SUVmax of the tumor was elevated in 2 patients (25%), unchanged in 1 (12.5%) and decreased in 5 (62.5%) (reduction rate: 10–37%). No differences were observed in the SUVmax (p = 0.1824) and SUVindex (p = 0.125) before and after induction therapy. On the other hand, among the 6 patients with an Ef2 response, 3 were classified as having SD and 3 were classified as having a PR. The SUVmax decreased in all cases (reduction rate: 3–87%). The SUVmax (p = 0.0277) and SUVindex (p = 0.0277) after induction therapy were significantly lower than those before induction therapies.
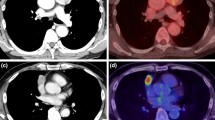
Figure 2 shows the CT and PET-CT images of a patient before (a, b) and after (c, d) induction therapy (case 14). This patient underwent induction CRT due to Masaoka-Koga stage III squamous cell carcinoma. The maximum tumor size changed from 7.3 to 6.3 cm (SD). The SUVmax dramatically decreased from 18.2 to 2.4 (−87%). A pathological examination of resected tumor showed that the response was nearly Ef3. In this case, PET-CT seems to have been more useful for predicting the pathological response to induction therapy than the CT findings.
Figure 3 demonstrates the receiver operating characteristic (ROC) curve for predicting good pathological responders (Ef2). The area under the curve (AUC) was 0.667 when the rate of the reduction in the tumor size on CT was used (Fig. 3a). The sensitivity and specificity were 88.3 and 62.5%, respectively, when the cutoff point was set at −27%. On the other hand, the AUC was 0.896 when the rate of the reduction in the SUVmax on PET-CT was used (Fig. 3b). The sensitivity and specificity were 88.3 and 100%, respectively, when the cutoff point was set at −55%. There was little change in AUC (0.8875) by normalizing SUVmax (Fig. 3c).
Receiver operating characteristic curve for predicting pathological good responders (Ef2). The area under the curve values using the rate of the reduction in the tumor size on CT (a), the rate of the reduction in the SUVmax value on PET-CT (b) and SUVindex (c) was 0.667, 0.896 and 0.8875, respectively
Discussion
The role of PET-CT in thymic epithelial tumors has been well reported. Most researchers have reported that PET-CT was useful in the differential diagnosis between thymomas and thymic carcinomas, because the SUVmax of thymic carcinoma was significantly higher than that of thymoma [15, 18, 19]. A few studies have been reported regarding the role of PET-CT in the evaluation of the treatment response in thymic epithelial tumors. Kaira et al. [11] reported the role of FDG-PET in patients with unresectable thymic tumors. They concluded that FDG-PET might be useful for monitoring the response and prognosis after treatment in such cases. Thomas et al. [12] evaluated the utility of PET-CT by analyzing 56 patients with unresectable Masaoka stage III or IV thymic epithelial tumors. They concluded that there was a close correlation between the change in the SUV in PET-CT and the subsequent best response using RECIST. The authors also reported that metabolic responders whose SUV decreased by ≥ 30% after treatment showed significantly better progression-free survival. However, both studies focused on the relationship between the change in the FDG uptake on PET-CT and the RECIST score in patients with unresectable thymic epithelial tumors. To the best of our knowledge, this is the first report regarding the role of PET-CT in the evaluation of the pathologic response to induction therapy in patients with resectable thymic epithelial tumors.
Several studies have documented a relationship between the change in the SUVmax on PET-CT and the pathologic response to induction therapy in patients with other thoracic malignancies, including non-small-cell lung cancer (NSCLC) and esophageal cancer. Cerfolio et al. [7] analyzed 56 NSCLC patients who underwent an FDG-PET scan before and after induction therapy and reported that repeat FDG-PET can predict the pathologic response to induction therapy. Out of 56 patients, 19 patients were complete pathologic responders (CPRs). The AUC of ROC curve for CPR was 0.935, which is considered to be quite high. Kukar et al. [9] investigated the role of repeat PET-CT in predicting the pathologic response following induction CRT for esophageal adenocarcinoma. Out of 77 esophageal adenocarcinoma patients who underwent PET-CT before and after induction CRT, 22 patients were CPRs and the rest were incomplete pathologic responders (IPRs). The authors reported that patients with a <45% decrease in their SUVmax were more likely to be IPRs, with a positive predictive value of 91.7%. Unfortunately, there were no CPRs in our study cohort. However, patients presenting a CPR might appear in a larger cohort or after new induction chemotherapy regimens are introduced.
Our retrospective analysis is associated with some limitations. First, the number of patients was small; however, to our knowledge, this is the first report to show the relationship between the change in the SUVmax on PET-CT and the pathologic response to induction therapy in patients with invasive thymic tumors. Second, the period from the last date of induction therapy to the second PET-CT imaging showed some range (mean 28 ± 18 days). Thirdly, the method for determining the SUVmax varies between institutions because they use different reconstruction algorithms and methods for drawing the ROI. One method of making the SUVmax universal would be to calculate the ratio of SUVmax of the tumors and the mean SUV of the mediastinum [19]. Correcting the SUVmax by the mean liver SUV is another method [20]. Even though some additional steps are required to calculate these adjusted SUVmax, it would be necessary to employ these methods to perform multi-institutional prospective studies. In our series, all of the patients underwent PET-CT imaging at our institution using the same machine and the same protocols, which is considered to be the strength of this study. Lastly, the variety of induction therapy (chemotherapy, radiation therapy and chemoradiation therapy) is also considered to be the limitation of this study.
Conclusions
In comparison with CT, PET-CT seems to be a useful modality for predicting the pathologic response of induction therapy in patients with invasive thymic epithelial tumors. Validations using larger study cohorts are needed to confirm our findings.
References
Masuda M, Kuwano H, Okumura M et al (2015) Thoracic and cardiovascular surgery in Japan during 2013: annual report by the Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 63:670–701
Fischer B, Lassen U, Mortensen J et al (2009) Preoperative staging of lung cancer with combined PET-CT. N Engl J Med 361:32–39
Liu J, Dong M, Sun X et al (2016) Prognostic value of 18F-FDG PET/CT in surgical non-small cell lung cancer: a meta-analysis. PLoS ONE 11:e0146195
Groheux D, Sanna A, Majdoub M et al (2015) Baseline tumor 18F-FDG uptake and modifications after 2 cycles of neoadjuvant chemotherapy are prognostic of outcome in ER +/HER2 − breast cancer. J Nucl Med 56:824–831
Kittaka H, Takahashi H, Ohigashi H et al (2013) Role of (18)F-fluorodeoxyglucose positron emission tomography/computed tomography in predicting the pathologic response to preoperative chemoradiation therapy in patients with resectable T3 pancreatic cancer. World J Surg 37:169–178. doi:10.1007/s00268-012-1775-x
Maffione AM, Marzola MC, Capirci C et al (2015) Value of 18F-FDG PET for predicting response to neoadjuvant therapy in rectal cancer: systematic review and meta-analysis. AJR Am Roentgenol 204:1261–1268
Cerfolio RJ, Bryant AS, Winokur TS et al (2004) Repeat FDG-PET after neoadjuvant therapy is a predictor of pathologic response in patients with non-small cell lung cancer. Ann Thorac Surg 78:1903–1909
Ozeki N, Kawaguchi K, Fukui T et al (2015) Which variables should be considered in patients with stage II and III non-small cell lung cancer after neoadjuvant therapy? Nagoya J Med Sci 77:475–480
Kukar M, Alnaji RM, Jabi F et al (2015) Role of repeat 18F-fluorodeoxyglucose positron emission tomography examination in predicting pathologic response following neoadjuvant chemoradiotherapy for esophageal adenocarcinoma. JAMA Surg 150:555–562
Cheng L, Tunairu N, Collins DJ et al (2015) Response evaluation in mesothelioma: beyond RECIST. Lung Cancer 90:433–441
Kaira K, Murakami H, Miura S et al (2011) 18F-FDG uptake on PET helps predict outcome and response after treatment in unresectable thymic epithelial tumors. Ann Nucl Med 25:247–253
Thomas A, Mena E, Kurdziel K et al (2013) 18F-fluorodeoxyglucose positron emission tomography in the management of patients with thymic epithelial tumors. Clin Cancer Res 19:1487–1493
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Masaoka A, Monden Y, Nakahara K et al (1981) Follow-up study of thymomas with special reference to their clinical stages. Cancer 48:2485–2492
Fukumoto K, Taniguchi T, Ishikawa Y et al (2012) The utility of [18F]-fluorodeoxyglucose positron emission tomography-computed tomography in thymic epithelial tumours. Eur J Cardiothorac Surg 42:e152–e156
Japan Lung Cancer Society (2003) General rule for clinical and pathological record of lung cancer, 7th edn. Kanehara, Tokyo, pp 175–177
Yokoi K, Matsuguma H, Nakahara R et al (2007) Multidisciplinary treatment for advanced invasive thymoma with cisplatin, doxorubicin, and methylprednisolone. J Thorac Oncol 2:73–78
Treglia G, Sadeghi R, Giovanella L et al (2014) Is 18F-FDG PET useful in predicting the WHO grade of malignancy in thymic epithelial tumors? A meta-analysis. Lung Cancer 86:5–13
Terzi A, Bertolaccini L, Rizzardi G et al (2011) Usefulness of 18F FDG PET/CT in the pre-treatment evaluation of thymic epithelial neoplasms. Lung Cancer 74:239–243
Shiono S, Abiko M, Okazaki T et al (2011) Positron emission tomography for predicting recurrence in stage I lung adenocarcinoma: standardized uptake value corrected by mean liver standardized uptake value. Eur J Cardiothorac Surg 40:1165–1169
Acknowledgements
We thank Hisashi Tateyama, MD, at the Department of Pathology, Clinical Laboratory, Kasugai Municipal Hospital, Kasugai, Japan, for providing the pathologic data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest in association with the present study.
Rights and permissions
About this article
Cite this article
Fukumoto, K., Fukui, T., Okasaka, T. et al. The Role of 18F-fluorodeoxyglucose Positron Emission Tomography-Computed Tomography for Predicting Pathologic Response After Induction Therapy for Thymic Epithelial Tumors. World J Surg 41, 1828–1833 (2017). https://doi.org/10.1007/s00268-017-3938-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-017-3938-2