Abstract
Purpose
The aim of this study was to evaluate the ability of fluorine-18-fluorodeoxyglucose positron emission tomography coupled with computed tomography (18F-FDG-PET/CT) to predict the WHO malignancy grade, initial staging, and invasive potential of thymic epithelial tumors.
Methods
We retrospectively reviewed the medical records of 56 patients with thymic epithelial tumors who were evaluated by PET/CT before surgery and underwent surgical resection. We analyzed the relationship of the maximum standardized uptake value (SUVmax) with the WHO histological classification, tumor invasion, TNM classification, and the Masaoka–Koga classification.
Results
There were differences of SUVmax of the FDG-PET between thymic carcinoma (9.09 ± 3.34) and thymoma (4.86 ± 2.45; p < 0.01), thymic carcinoma (9.09 ± 3.34) and high-grade thymoma (6.01 ± 2.78; p < 0.01), and high-grade thymoma (6.01 ± 2.78) and low-grade thymoma (4.06 ± 1.86; p < 0.01). The cut-off value for the SUVmax was 7.40 and 5.40, and the sensitivity/specificity for predicting the histologic subtype of each group was 0.72/0.79 and 0.61/0.85, respectively. According to T classification, SUVmax was significantly higher in T3 (8.31 ± 2.57) than in T1a (4.45 ± 2.06; p < 0.01). Regarding Masaoka–Koga classification and WHO histological classification, a significantly higher SUVmax was detected in patients with stage III and IV disease than in those with stage I and II diseases (p < 0.01). The cut-off value for SUVmax was 5.40 in Masaoka–Koga stage and 5.60 in the WHO classification; the sensitivity/specificity for predicting the histologic subtype was 0.85/0.80 and 0.89/0.78, respectively.
Conclusions
FDG-PET is a useful tool to predict aggressiveness of thymic epithelial tumors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Thymic epithelial tumors are the most common neoplasms arising from the anterior mediastinum [1]. Surgery is the mainstay of treatment for early stage thymic epithelial tumors, and complete resection is considered the radical cure [2]. Complete resection can even improve the prognosis of patients with advanced disease. It is important to ascertain the WHO-based classification of malignancy grade and the invasive potential of thymic epithelial tumors. Surgeons can preoperatively diagnose the anterior mediastinal tumors using a computed tomography (CT) scan. However, it is difficult to preoperatively predict the WHO malignancy grade and the invasive potential of thymic epithelial tumors. Irrespective of size, a tumor may be considered high-grade and could invade into the surrounding organs. Histopathology can be used for the diagnosis of thymic epithelial tumors and for selecting the optimal therapeutic strategy for patient management. Neoadjuvant therapy may be useful for patients with locally advanced thymic epithelial tumors. Moreover, following neoadjuvant therapy, patients with thymic carcinoma may not be required to undergo minimally invasive surgery, such as robot-assisted surgery or video-assisted thoracic surgery (VATS) [3, 4]. Recently, a correlation between radio-metabolic findings and the WHO histological classification was shown in patients with thymic epithelial tumors. Thus, fluorine-18-fluorodeoxyglucose (18F-FDG) and the WHO histological classification may be relevant [5,6,7,8]. Several imaging studies have used a simplified staging system that is subdivided into low-grade thymoma (type A, AB, B1), high-grade thymoma (type B2, B3), and thymic carcinoma [9,10,11]. Furthermore, it is possible that SUVmax correlates with T factor in accordance with Union for International Cancer Control (UICC) TNM Classification of Malignant Tumors 8th edition, and SUVmax might be useful in predicting T classification for the thymic epithelial tumor [12]. 18F-FDG-positron emission tomography (PET) may predict the histological stage of thymic epithelial tumors according to the WHO classification. The aim of this study was to evaluate the ability of 18F-FDG-PET/CT to predict the WHO malignancy grade, initial staging, and invasive potential of thymic epithelial tumors.
Methods
Patients
We retrospectively reviewed the medical records of 142 patients with thymic epithelial tumors who underwent surgical resection at Chiba University Hospital, Chiba, Japan, between 2009 and 2016. Of these, 86 patients were excluded, because a preoperative PET/CT was not performed. FDG-PET was not popular in the first half of the study periods, and we have not undergone FDG-PET before surgery in all cases for the thymic epithelial tumor. Surgeon in charge of the patients has decided whether or not to perform FDG-PET at his discretion. Finally, the data of 56 patients were included in this study. A definitive diagnosis was made by histological examination of surgical specimens obtained after thymectomy. The patients were staged according to the WHO classification, UICC TNM Classification of Malignant Tumors 8th edition, and the Masaoka–Koga classification. The tumors were divided into six subtypes based on the WHO classification: type A, type AB, type B1, type B2, type B3, and thymic carcinoma; these subtypes were further categorized into three groups: low-grade thymoma (types A, AB, B1), high-grade thymoma (types B2, B3), and thymic carcinoma. We analyzed the relationship of the maximum standardized uptake value (SUVmax) with the WHO histological classification, tumor invasion, TNM classification, and the Masaoka–Koga classification.
18F-FDG-PET/CT
Whole-body 18F-FDG-PET/CT was performed using a dedicated PET/CT scanner (Discovery PET/CT, GE Medical System, Tokyo, Japan or Aquiduo, Toshiba Medical Systems, Tochigi, Japan). Patients fasted ≥ 6 h before the intravenous injection of FDG was given. The intravenous 18F-FDG dose was calculated based on the body weight of the patient. PET images were obtained at 1 h after 18F-FDG administration. We used a 16-slice CT scanner. Together with the PET system, the CT scanner was used both for attenuation correction of PET data and for localization of FDG uptake in PET images. The PET/CT scans were reviewed by two experienced chest radiologists. Imaging findings were recorded for each patient. A clinical CT stage was assigned, and FDG uptake was recorded to determine the SUVmax. Figure 1 shows representative CT and PET/CT images of patients with thymoma and thymic carcinoma (Fig. 1a–d).
Positron emission tomography/computed tomography (CT) and CT images of type AB thymoma. Axial enhanced CT demonstrated an anterior mediastinal mass (a). FDG-PET image shows intense FDG uptake within the mass with SUVmax of 3.0 (b). PET/CT and CT images of thymic cancer. Seventy-one-year-old man with thymic carcinoma. Axial enhanced CT demonstrated an anterior mediastinal mass (c). FDG-PET image shows intense FDG uptake within the mass with SUVmax of 9.5(d)
Statistical analysis
Pairwise comparisons were performed using Student’s t test or Wilcoxon two-sample test. A p value < 0.05 was considered statistically significant. We used the R statistical package (www.r-project.org) for analyses. In the null hypothesis (no difference between the groups), 95% of all risk differences over time will lie within the confidence interval.
Results
Patient characteristics
The clinical and histopathological characteristics of patients are shown in Table 1. There were 32 men (57.1%) and 24 women (42.9%). The median age was 61.3 years (range 27–80 years). Forty-four (78.6%) patients had a histologically diagnosed thymoma, and twelve (21.4%) had thymic carcinoma. When staged according to the WHO histological classification, there were two patients with type A thymoma, 14: with type AB thymoma, 10 with type B1 thymoma, eight with type B2 thymoma, 10 with type B3 thymoma, and 12 with thymic carcinoma. Subsequently, 26 patients (46.4%) were categorized into the low-grade thymoma group, 18 (32.2%) into the high-grade thymoma group, and 12 (21.4%) into the thymic carcinoma group. Among the 12 patients with thymic carcinoma, there were five patients (41.6%) with stage II, two (16.6%) with stage III, one (8.4%) with stage IVA, and four (33.4%) with stage IVB disease. A new TNM classification for thymic epithelial tumors was proposed by the International Association for the Study of Lung Cancer (IASLC) and the International Thymic Malignancy Interest Group (ITMIG); based on this staging system patient were categorized as follows: stage I, n = 36 (64.3%); stage II, n = 3(5.3%); stage IIIA, n = 6 (10.7%); stage IIIB, n = 0 (0%); stage IVA, n = 9 (16.1%); and stage IVB, n = 2 (3.6%). When staged according to the Masaoka–Koga system, there were 19 patients (34.0%) with stage I, 16 (28.6%) with stage II, 11 (19.5%) with stage III, 6 (10.7%) with stage IVa, and 4 (7.2%) with stage IVb disease.
18F-FDG-PET/CT and tumor classification
The SUVmax of the thymic epithelial tumor in each group is shown in Fig. 2. We found that the SUVmax was significantly higher in the thymic carcinoma group (9.09 ± 3.34) than in the thymoma group (4.86 ± 2.45; p < 0.01). The cut-off value for the SUVmax was 7.40; the sensitivity and specificity for predicting the histologic subtype was 0.84 and 0.73, respectively (Fig. 2a). The SUVmax was significantly higher in the high-grade thymoma group (6.01 ± 2.78) than in the low-grade thymoma group (4.06 ± 1.86; p < 0.01). The cut-off value for SUVmax was 5.40; the sensitivity and specificity for predicting the histologic subtype was 0.61 and 0.85, respectively (Fig. 2b). The SUVmax was significantly higher in the thymic carcinoma group (9.09 ± 3.34) than in the high-grade thymoma group (6.01 ± 2.78; p < 0.01). The cut-off value for the SUVmax was 7.40; the sensitivity and specificity for predicting the histologic subtype were 0.72 and 0.79, respectively (Fig. 2b).
18F-FDG-PET/CT and tumor invasion
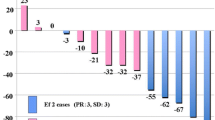
The SUVmax of the thymic epithelial tumor in each group is shown in Fig. 3. The mean SUVmax ± SD of thymic epithelial tumors that did not invade the pericardium was 5.41 ± 2.85, and of those that invaded the pericardium was 8.17 ± 3.06 (p < 0.01; Fig. 3a). The mean SUVmax ± SD of thymic epithelial tumors that did not invade the lungs was 5.54 ± 3.14, and of those that invaded the lungs was 7.96 ± 2.21 (p = 0.02; Fig. 3b). The mean SUVmax ± SD of thymic epithelial tumors that did not invade the brachiocephalic vein was 5.56 ± 2.92, and of those that invaded the brachiocephalic vein was 8.48 ± 3.02 (p = 0.01; Fig. 3c).
18F-FDG-PET/CT and TNM classification
The distributions of SUVmax for thymic epithelial tumors according to T classification are shown in Fig. 4. The SUVmax was 4.45 ± 2.06 in T1a, 4.9 ± 0 in T1b, 7.12 ± 2.69 in T2, 8.31 ± 2.57 in T3, and 9.79 ± 7.48 in T4. Although there was no significant difference among the five groups, the SUVmax was significantly higher in T3 thymic epithelial tumors (8.31 ± 2.57) than in T1a thymic epithelial tumors (4.45 ± 2.06; p < 0.01; Fig. 4). The SUVmax of thymic epithelial tumors that have metastasized to the lymph nodes (7.58 ± 1.76) is not significantly different from those that have not metastasized to the lymph nodes (5.69 ± 3.0). The SUVmax of thymic epithelial tumors that have distantly metastasized (7.97 ± 3.45) is not significantly different from those that have not distantly metastasized (5.43 ± 2.74).
18F-FDG-PET/CT and tumor staging
The SUVmax of the thymic epithelial tumor in each group is shown in Fig. 5. When staged according to the Masaoka–Koga classification, the mean SUVmax ± SD of patients with stage I and II diseases was 4.52 ± 2.66, and of those with III and IV was 7.73 ± 2.83. The SUVmax detected in patients with stage III and IV disease was significantly higher than in those with stage I and II diseases (p < 0.01). The cut-off value for the SUVmax was 5.40; the sensitivity and specificity for predicting the histologic subtype were 0.85 and 0.80, respectively (Fig. 5a). When staged based on the newly proposed TNM staging system, the mean SUVmax ± SD of patients with stage I and II disease was 4.56 ± 2.66, and of those with stage III and IV disease was 8.18 ± 2.77. The SUVmax detected in patients with stage III and IV diseases was significantly higher than in those with stage I and II diseases (p < 0.01). The cut-off value for the SUVmax was 5.60; the sensitivity and specificity for predicting the histologic subtype were 0.89 and 0.78, respectively (Fig. 5b).
Discussion
For the standard surgical treatment of patients with thymic epithelial tumors, the trans-sternal approach and minimally invasive approach (VATS, radius surgical system, and the da Vinci surgical system) are commonly used [13]. A preoperative diagnosis is required to determine the optimal surgical approach. It has been shown that patients with thymic carcinoma have poor survival and a higher tumor recurrence rate than those with thymoma [14]. Additionally, the outcomes of surgery for patients with stage III thymoma were found to be favorable unless chest wall invasion was present. Chest wall invasion is the only independent adverse predictor for disease-free survival [15].
We have reported cases of synchronous multiple thymoma [16]. PET is a useful tool, and may provide additional radiological findings and a more accurate preoperative diagnosis of synchronous multiple thymoma [17]. The detection of mediastinal fat infiltration on the CT images is important predictors of carcinoma [11]. The contact length between the tumor contour and the lung on a CT scan was found to be associated with pleural recurrence after complete resection of thymoma. The contact length between the tumor contour and the lung can be evaluated before surgery to predict pleural recurrence [18]. A magnetic resonance imaging (MRI) scan also may help to accurate staging for the IASLC/ITMIG thymic epithelial tumor [19].
Thymic epithelial tumors are the most common adult anterior mediastinal tumors [20, 21]. Thymoma accounts for 37.3%, thymic carcinoma accounts for 7.1%, and thymic carcinoid accounts for 0.8% of mediastinal tumors. Surgery is the standard treatment for patients with thymic epithelial tumors [21]. Thymomas are present in 10% of the patients with myasthenia gravis [22]. A recent study showed that thymectomy could improve clinical outcomes over a 3-year period in patients with nonthymomatous myasthenia gravis [23].
Timely preoperative diagnosis of thymic epithelial tumors is important, because patients in an advanced stage receive neoadjuvant chemotherapy that enables effective resection. PET findings provided useful information for the differential diagnosis of anterior mediastinal tumors [12, 24]. Recently, several studies have reported that the SUVmax helps to distinguish thymic epithelial tumors according to the histological classification. We found a significant difference in SUVmax between patients with thymoma and those with thymic carcinoma [5, 9]. A refined and simplified histological classification of thymic epithelial tumors has been proposed by WHO: low-grade thymoma (type A, AB, B1), high-grade thymoma (type B2, B3), and thymic carcinoma [10]. The previous studies suggested that the SUVmax might be useful for discriminating low-grade thymoma from high-grade thymoma [28]. Moreover, recently, some studies have showed that the relationship is observed between SUVmax, tumor size, and histological WHO classification of thymic epithelial tumors [6, 7]. In addition, SUVmax may be the parameter for predicting the recurrence of anterior mediastinal tumors [8].
In the present study, we retrospectively analyzed the relationship between the SUVmax and the WHO malignancy grade in patients with thymic epithelial tumors using 18F-FDG-PET/CT in the initial disease staging. As reflected by the SUVmax, 18F-FDG uptake was significantly higher in the thymic carcinoma group than in the thymoma group. Moreover, 18F-FDG uptake was significantly higher in the thymic carcinoma group than in the high-grade thymoma group. Similarly, 18F-FDG uptake detected in the high-grade thymoma group was significantly higher than that in the low-grade thymoma group.
The Masaoka–Koga staging system has been widely used for staging patients with thymic epithelial tumors [26, 27]. As reflected by the SUVmax, a significantly higher 18F-FDG uptake was detected in patients with stage III and IV thymoma than those with stage I and II thymoma. Recently, the IASLC and the ITMIG proposed a new TNM classification for thymic epithelial tumors. When staged according to this TNM classification, a significantly higher 18F-FDG uptake, as reflected by the SUVmax, was detected in patients with stage III and IV thymic epithelial tumors than those with stage I and II thymic epithelial tumors. SUVmax correlate with T factor and may be useful in predicting T classification for the thymic epithelial tumor. As our study indicates, Watanabe et al. reported that the SUVmax may reflect the invasiveness of thymic epithelial tumor [12].
In contrast to this study, Nakagawa et al. reported that 18F-FDG-PET is useful for differentiating from thymic cancer, but not for predicting the grade of malignancy of thymoma [28]. 18F-FDG-PET could not differentiate between low-risk and high-risk thymomas, these parameters might be able to distinguish between benign and malignant mediastinal tumors noninvasively, as well as thymomas and thymic carcinomas [29].
A preoperative identification of the WHO malignancy grade and the Masaoka–Koga stage of thymic epithelial tumors helps surgeons to decide the management strategy for the patients. FDG-PET-based preoperative diagnosis of advanced-stage disease, high-grade thymoma, or thymic carcinoma helps to evaluate the need for preoperative chemotherapy [30]. Biopsy is considered the gold standard for diagnosing a suspected thymoma. However, this may not confirm the diagnosis. Fine-needle aspiration and core needle biopsy are used; however, biopsy procedures sometimes cause tumor implantation along the needle track after aspiration. Therefore, non-invasive imaging studies that help to determine the histologic subtype of thymic epithelial tumors may be useful.
Our study has several limitations. First, the study had a retrospective design, which might have introduced a selection bias. Second, the sample size was small. Third, the use of different FDG-PET might have influenced the evaluation of some features of the thymic epithelial tumors. Additional prospective and large multicenter clinical trials using a similar FDG-PET/CT protocol are required to confirm the findings of our study.
Conclusion
In this study, we showed that the SUVmax determined by 18F-FDG-PET/CT could be useful for diagnosing patients with thymic carcinoma and thymoma. Furthermore, we demonstrated a statistically significant difference in the SUVmax between low-grade thymoma and high-grade thymoma, between thymic carcinoma and high-grade thymoma, and between early and advanced stages of the disease. In addition, we found that 18F-FDG-PET/CT could predict tumor invasion into the surrounding tissues. Further investigation will be required to confirm our conclusions.
References
Ruffini E, Filosso PL, Mossetti C, Bruna MC, Novero D, Lista P, et al. Thymoma: inter-relationships among World Health Organization histology, Masaoka staging and myasthenia gravis and their independent prognostic significance: a single-centre experience. Eur J Cardiothorac Surg. 2011;40:146–53.
Regnard JF, Magdeleinat P, Dromer C, Dulmet E, de Montpreville V, Levi JF, et al. Prognostic factors and long-term results after thymoma resection: a series of 307 patients. J Thorac Cardiovasc Surg. 1996;112:376–84.
Falkson CB, Bezjak A, Darling G, Gregg R, Malthaner R, Maziak DE, et al. The management of thymoma: a systematic review and practice guideline. J Thorac Oncol. 2009;4:911–9.
Seong YW, Kang CH, Choi JW, Kim HS, Jeon JH, Park IK, et al. Early clinical outcomes of robot-assisted surgery for anterior mediastinal mass: its superiority over a conventional sternotomy approach evaluated by propensity score matching. Eur J Cardiothorac Surg. 2014;45:e68–73; discussion e.
Lococo F, Cesario A, Okami J, Cardillo G, Cavuto S, Tokunaga T, et al. Role of combined 18F-FDG-PET/CT for predicting the WHO malignancy grade of thymic epithelial tumors: a multicenter analysis. Lung Cancer. 2013;82:245–51.
Shinya T, Tanaka T, Soh J, Matsushita T, Sato S, Toyooka S, et al. Diagnostic Value of Dual-time-point F-18 FDG PET/CT and Chest CT for the Prediction of Thymic Epithelial Neoplasms. Acta Med Okayama. 2017;71:105–12.
Zhao J, Wang H, Li Q. Value of 18F-FDG PET/computed tomography in predicting the simplified WHO grade of malignancy in thymic epithelial tumors. Nucl Med Commun. 2020;41:405–10.
Yajima T, Mogi A, Shimizu K, Kosaka T, Ohtaki Y, Obayashi K, et al. Quantitative analysis of metabolic parameters at (18)F-fluorodeoxyglucose positron emission tomography in predicting malignant potential of anterior mediastinal tumors. Oncol Lett. 2020;19:1865–71.
Benveniste MF, Moran CA, Mawlawi O, Fox PS, Swisher SG, Munden RF, et al. FDG PET-CT aids in the preoperative assessment of patients with newly diagnosed thymic epithelial malignancies. J Thorac Oncol. 2013;8:502–10.
Treglia G, Sadeghi R, Giovanella L, Cafarotti S, Filosso P, Lococo F. Is (18)F-FDG PET useful in predicting the WHO grade of malignancy in thymic epithelial tumors? A meta-analysis. Lung Cancer. 2014;86:5–13.
Tomita M, Ayabe T, Tsuchiya K, Nakamura K. Fluorodeoxyglucose positron emission tomography can provide useful information for differentiating thymic epithelial tumors. Thorac Cardiovasc Surg. 2018;66:345–9.
Watanabe T, Shimomura H, Mutoh T, Saito R, Goto R, Yamada T, et al. Positron emission tomography/computed tomography as a clinical diagnostic tool for anterior mediastinal tumors. Surg Today. 2019;49:143–9.
Yoshida S, Yoshino I, Moriya Y, Hoshino H, Okamoto T, Suzuki M, et al. Video-assisted thoracoscopic surgery extended thymectomy for myasthenia gravis using manual manipulators: the radius surgical system. Ann Thorac Surg. 2011;92:2246–8.
Tagawa T, Suzuki H, Nakajima T, Iwata T, Mizobuchi T, Yoshida S, et al. Long-term outcomes of surgery for thymic carcinoma: experience of 25 cases at a single institution. Thorac Cardiovasc Surg. 2015;63:212–6.
Yamada Y, Yoshino I, Nakajima J, Miyoshi S, Ohnuki T, Suzuki M, et al. Surgical outcomes of patients with stage III thymoma in the Japanese nationwide database. Ann Thorac Surg. 2015;100:961–7.
Suzuki H, Yoshida S, Hiroshima K, Nakatani Y, Yoshino I. Synchronous multiple thymoma: report of three cases. Surg Today. 2010;40:456–9.
Yamatani C, Maniwa T, Takahashi S, Isaka M, Ohde Y, Endo M, et al. Variation in (18)F-FDG PET findings in a patient with synchronous multiple thymoma. Gen Thorac Cardiovasc Surg. 2015;63:586–9.
Kato T, Iwano S, Taniguchi T, Kawaguchi K, Fukui T, Ishiguro F, et al. The contact length between the tumor contour and the lung on computed tomography is a risk factor for pleural recurrence after complete resection of thymoma. Gen Thorac Cardiovasc Surg. 2015;63:343–8.
Ohno Y, Kishida Y, Seki S, Koyama H, Yui M, Aoyagi K, et al. Comparison of Interobserver Agreement and Diagnostic Accuracy for IASLC/ITMIG Thymic Epithelial Tumor Staging Among Co-registered FDG-PET/MRI, Whole-body MRI, Integrated FDG-PET/CT, and Conventional Imaging Examination with and without Contrast Media Administrations. Acad Radiol. 2018.
Duwe BV, Sterman DH, Musani AI. Tumors of the mediastinum. Chest. 2005;128:2893–909.
Shimizu H, Okada M, Tangoku A, Doki Y, Endo S, Fukuda H, et al. Thoracic and cardiovascular surgeries in Japan during 2017: Annual report by the Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg. 2020;68:414–49.
Drachman DB. Myasthenia gravis. N Engl J Med. 1994;330:1797–810.
Wolfe GI, Kaminski HJ, Aban IB, Minisman G, Kuo HC, Marx A, et al. randomized trial of thymectomy in myasthenia gravis. N Engl J Med. 2016;375:511–22.
Proli C, De Sousa P. A diagnostic cohort study on the accuracy of 18-fluorodeoxyglucose ((18)FDG) positron emission tomography (PET)-CT for evaluation of malignancy in anterior mediastinal lesions: the DECiMaL study. 2018;8:e019471.
Scagliori E, Evangelista L, Panunzio A, Calabrese F, Nannini N, Polverosi R, et al. Conflicting or complementary role of computed tomography (CT) and positron emission tomography (PET)/CT in the assessment of thymic cancer and thymoma: our experience and literature review. Thorac Cancer. 2015;6:433–42.
Masaoka A, Monden Y, Nakahara K, Tanioka T. Follow-up study of thymomas with special reference to their clinical stages. Cancer. 1981;48:2485–92.
Koga K, Matsuno Y, Noguchi M, Mukai K, Asamura H, Goya T, et al. A review of 79 thymomas: modification of staging system and reappraisal of conventional division into invasive and non-invasive thymoma. Pathol Int. 1994;44:359–67.
Nakagawa K, Takahashi S, Endo M, Ohde Y, Kurihara H, Terauchi T. Can 18F-FDG PET predict the grade of malignancy in thymic epithelial tumors? An evaluation of only resected tumors. Cancer Manag Res. 2017;9:761–8.
Morita T, Tatsumi M, Ishibashi M, Isohashi K, Kato H, Honda O, et al. Assessment of mediastinal tumors using SUV(max) and volumetric parameters on FDG-PET/CT. Asia Ocean J Nucl Med Biol. 2017;5:22–9.
Detterbeck FC. Clinical value of the WHO classification system of thymoma. Ann Thorac Surg. 2006;81:2328–34.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ito, T., Suzuki, H., Sakairi, Y. et al. 18F-FDG-PET/CT predicts grade of malignancy and invasive potential of thymic epithelial tumors. Gen Thorac Cardiovasc Surg 69, 274–281 (2021). https://doi.org/10.1007/s11748-020-01439-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11748-020-01439-7