Abstract
Background
We retrospectively investigated the prognostic significance of various clinicopathological factors and preoperative nutritional status to select patients with stage IV colorectal cancer (CRC) who will have a poor prognosis after palliative resection of the primary tumor.
Methods
A total of 100 stage IV CRC patients who underwent palliative resection were enrolled. Various clinicopathological factors and Onodera’s prognostic nutritional index (OPNI) were evaluated to identify any possible relationship with the prognosis.
Results
At the time of the analysis, 83 patients had died, and the median survival time was 21 months. Of the 100 patients, 24 had primary tumor-related symptoms such as obstruction or bleeding. No significant correlation was noted between the OPNI and various clinicopathological factors. The multivariate analysis of patients without primary tumor-related symptoms revealed that the OPNI was an independent prognostic factor. The overall survival of the low-OPNI group was significantly worse than that of the high-OPNI group.
Conclusions
This retrospective study suggested that patients with a low OPNI may not be candidates for palliative resection, because it provides no survival benefit to these patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite the increasing use of screening strategies, ~20 % of colorectal cancer (CRC) patients present with stage IV disease at the time of diagnosis [1]. In stage IV disease, complete surgical resection of the primary tumor and distant metastases remains the only potentially curative therapy [2]. For patients with unresectable metastatic disease, the treatment will be mostly palliative, with a goal of prolonging the survival with the best possible quality of life. The median survival time (MST) for the patients with unresectable metastatic disease is ~8 months when they are given optimal supportive care without chemotherapy [3]. Several studies have shown that palliative resection of the primary tumor leads to prolonged survival, with a reported MST of 11–26 months [4–7]. In contrast, the recent development of chemotherapeutic and molecular targeting agents markedly improved the MST to almost 24 months [8–11]. Therefore, the prognostic benefit of palliative resection of the primary tumor in patients with unresectable metastatic disease remains controversial [12–15].
It has previously been reported that the postoperative prognosis of patients with gastrointestinal malignancies could be reflected by their preoperative nutritional condition [16–19]. Onodera’s prognostic nutritional index (OPNI) is thought to be a simple and useful parameter to determine the nutritional and immunological status of patients [20]. Nozoe et al. [18, 21] reported that a low OPNI was significantly associated with a poor prognosis in patients with gastrointestinal cancers.
In the present study, to identify parameters that can be used to select patients who will have a poor prognosis after palliative resection, we retrospectively investigated the correlation between various clinicopathological factors, preoperative nutritional status, and prognosis in patients with stage IV CRC who underwent palliative resection of the primary tumor at our institution.
Patients and methods
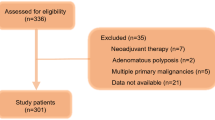
A total of 1,647 patients with CRC underwent surgical resection in the Department of Surgical Oncology, Osaka University Hospital, between 1 January 2002 and 31 December 2010. From these patients, we identified 129 patients with stage IV CRC. Of these, 25 patients who underwent R0–1 resection and four patients who underwent emergency surgery due to colonic perforation were excluded from the study. The other 100 patients who underwent palliative resection were enrolled in this study. Palliative resection was defined as resection of a primary lesion of the colon and/or rectum, along with regional lymphadenectomy, but no resection of incurable factors, such as peritoneal dissemination or hepatic and distant metastasis. The following parameters were evaluated: age, gender, preoperative performance status, primary tumor-related symptoms (such as constipation, ileus, melena, and anemia), tumor location, histological type, TNM factors, number of incurable factors, serum carcinoembryonic antigen (CEA) level (the cut-off level was 5.0 mg/ml), postoperative complications, peri-operative transfusion, postoperative hospital stay, postoperative chemotherapy, and molecular targeting therapy. Pathological diagnoses and classifications were made according to the 7th edition of the International Union Against Cancer (UICC) TNM Classification of Malignant Tumors [22]. None of the enrolled patients had inflammatory or infectious diseases before surgery. The patients who had received preoperative therapy were excluded from the analysis. All blood samples were collected 1 or 2 days before surgery.
The OPNI was calculated using the following formula: 10× serum albumin concentration (g/dl) + 0.005 × lymphocyte count (number/mm2) in the peripheral blood.
The cut-off value of the OPNI was determined to be 40, based on an original investigation by Onodera et al. [20]
Statistical analysis
The statistical analyses were performed using the JMP 10 software program (SAS Institute Japan, Tokyo, Japan). The χ 2 test was used to compare the data. Survival curves were created via the Kaplan–Meier method and analyzed with the log-rank test. The Cox proportional hazards model was used for the multivariate analysis to identify the independent prognostic factors. Values of p < 0.05 were considered to be statistically significant.
Results
Patient characteristics
The clinicopathological features of the 100 patients are summarized in Table 1. In total, 24 patients had symptoms associated with the primary tumor; 17 had symptoms associated with obstruction, including constipation, ileus, and abdominal fullness, six patients had symptoms related to tumor bleeding, including melena and anemia, and one patient had anal pain. Of the 24 patients with primary tumor-related symptoms, 23 (95.8 %) experienced relief of these symptoms after palliative surgery. A total of 58 patients had a single incurable factor, and 42 had multiple incurable factors. Postoperative complications were observed in 18 patients. Among these, superficial surgical site infections were observed in six patients, ileus was noted in six patients, anastomotic leakage in three patients, anastomotic bleeding in two patients, and pneumonia developed in one patient. However, all of the patients healed with conservative treatment without the need for surgical intervention. There were no postoperative deaths.
With regard to the administration of postoperative chemotherapy, nine patients refused to receive chemotherapy and six patients were judged to be contraindicated for chemotherapy because of a poor performance status and/or severe co-morbidities. The other 85 patients underwent chemotherapy. Among these 85 patients, 25 were administered a single fluoropyrimidine-based chemotherapy, such as infusional 5-fluorouracil (5FU) or an oral pro-drug, such as capecitabine. The other 60 patients were treated with a combination of 5FU plus irinotecan or oxaliplatin. Molecular targeting therapy with bevacizumab became available in 2007, and cetuximab was available in 2008 in our country. Therefore, only 32 patients received molecular targeting therapy.
At the time of the analysis, 83 (83 %) patients had died, with a median follow-up of 24.8 months after surgery. The MST was 21 months.
Correlation between the OPNI and clinicopathological factors
The preoperative values of the OPNI ranged from 29.5 to 65, with a mean value ± standard deviation of 44 ± 2.6.
The correlations between the OPNI and clinicopathological factors are shown in Table 2. No significant correlation was noted between the OPNI and any of the various clinicopathological factors examined. Only 11 patients had normal serum CEA levels; the other 89 patients had higher than normal serum CEA values. The serum CEA level was also not associated with the OPNI. According to the postoperative therapy, the administration of the chemotherapy and the targeting therapy tended to be more frequent in the high-OPNI group compared with the low-OPNI group, but statistical significance was not reached.
The correlations between prognosis and various clinicopathological factors were also investigated. In the univariate analysis, a significantly worse prognosis was associated with preoperative performance status, depth of tumor invasion, number of incurable factors, and OPNI (Table 3). A multivariate analysis using Cox’s model revealed that both the number of incurable factors and the OPNI were independent prognostic factors.
Because there is a possibility that the value of the OPNI may be lower in patients with primary tumor-related symptoms, such as obstructing or excessively bleeding tumors, we investigated the correlation between the OPNI and the prognosis of the 76 patients without primary tumor-related symptoms (Table 4). In the univariate analysis, a significantly worse prognosis was associated with peritoneal metastasis, liver metastasis, the depth of tumor invasion, number of incurable factors, and the OPNI. According to the multivariate analysis, the OPNI was the only independent prognostic factor.
The overall survival of the low-OPNI group was significantly (p = 0.0005) worse than that of the high-OPNI group (Fig. 1). The MST was 9.5 months for the low-OPNI group and 27 months for the high-OPNI group.
Discussion
Resection of the primary tumor may relieve symptoms and avoid potential complications, such as obstruction or bleeding, in patients with stage IV CRC. However, in the case of resection, the postoperative recovery period and hospital stay may worsen the quality of life for patients and delay the start of systemic chemotherapy, which may be related to a poorer prognosis. In the present study, the MST was 21 months, which was similar to that of patients treated with the current combination chemotherapy and molecular targeting agents [8–11]. Therefore, since the prognostic benefit of palliative resection for the patients with unresectable metastatic disease remains controversial, it is especially important to identify patients who will have a poor prognosis after palliative resection so that they can receive other treatments.
It is well known that the conventional clinicopathological factors and serum CEA level are important and useful when predicting the prognosis of patients with various gastrointestinal cancers [23]. However, because stage IV is regarded as the final stage of CRC, most of these factors are relatively ineffective for the evaluation of patients with such advanced disease. In fact, our study, using a multivariate analysis, revealed that only the number of incurable factors were associated with survival. Therefore, in stage IV CRC patients, another parameter should be identified to predict the survival of patients.
Although both clinicopathological factors and serum CEA level reflect tumor characteristics, tumor progression is not solely determined by the local characteristics of the tumor, but also by the host systemic immune/inflammatory responses [24]. Furthermore, the presence of an inflammatory response has been proposed to be pathogenic in the development of cancer-associated malnutrition [25]. Several studies have reported that patients with advanced gastrointestinal malignancies are often malnourished, and that preoperative nutrition status is associated with postoperative complications, tumor progression, and a poor clinical outcome [16–19]. Several assessment tools are applied for nutritional status, such as the Malnutrition Universal Screening Tool (MUST), the Nutritional Risk Scoring 2002 (NRS2002), and the mini nutritional assessment [26–28]. These tools are simple, well-validated, and cost-effective tools that are widely utilized to assess the nutritional status of cancer patients. The OPNI is one such tool and is also a simple index that is calculated using only two parameters: the serum albumin (Alb) and the total lymphocyte count (TLC) [20]. Alb is a main component of the plasma protein that preserves the colloid osmotic pressure, and it reflects nutritional status. The TLC has also been proposed as a useful indicator of nutritional status, as well as host inflammatory status. Both the Alb and the TLC are usually examined in daily clinical practice. Therefore, the OPNI, which reflects the immunonutritional status, is thought to be a useful and convenient index to predict tumor progression and survival in patients with malignancies. With regard to the correlation between the OPNI and clinicopathological factors, Watanabe et al. [17] and Nozoe et al. [18, 21] reported that the OPNI was significantly associated with the depth of tumor invasion. However, almost all of the patients (72 %) in our study had T4 tumors, therefore, we could not find any significant correlation between the OPNI and the depth of tumor invasion. According to prognosis, previous studies [17, 21] have reported that the OPNI was significantly correlated with prognosis of patients with gastrointestinal cancers. These studies examined not only stage IV patients, but also stage I, II, and III patients. Moreover, Nozoe et al. [21] reported that the OPNI was significantly lower in patients with intestinal obstruction, which was one of the primary tumor-related symptoms. Therefore, we investigated the 76 patients without primary tumor-related symptoms. As a result, it was revealed that a low OPNI was an independent predictor of worse prognosis even in the patients limited to stage IV CRC. The MST of patients with a low OPNI was 9.5 months, which was shorter than that reported for patients with stage IV CRC who underwent chemotherapy alone. Although the necessity of palliative resection for patients with asymptomatic primary tumor and unresectable metastatic disease remains controversial, the OPNI may be useful to select patients who will have a survival benefit associated with the palliative resection. Thus, patients with a low OPNI may not be candidates for palliative resection.
Although there were no significant differences in the administration of chemotherapy and molecular targeting therapy in the low-OPNI and the high-OPNI groups, the frequency of postoperative therapy tended to be lower in the low-OPNI group. It has been reported that malnutrition leads to a loss of lean body mass, impaired immune function, a reduced response rate to chemotherapy, and poor survival [29, 30]. Furthermore, it has been reported that nutritional interventions could improve the immunonutritional system, response to chemotherapy, and patient survival [31, 32]. Such nutritional interventions should be implemented to improve the survival in patients with low OPNI.
Conclusions
In this retrospective study, the number of incurable factors and the OPNI were found to be significantly associated with prognosis in patients with stage IV CRC who underwent palliative resection. The OPNI was a strong independent prognostic factor in patients without primary tumor-related symptoms. Therefore, patients with a low OPNI may not be candidates for palliative resection due to its lack of a survival benefit. This was a single-arm retrospective study; therefore, a prospective study is required to confirm the present findings by selecting patients who will have a poor survival after palliative resection for stage IV CRC.
References
Ries LA, Wingo PA, Millers DS et al (2000) The annual report to the nation on the status of cancer, 1973–1997, with a special section on colorectal cancer. Cancer 88:2398–2424
Morise Z, Sugioka A, Fujita J et al (2006) Does repeated surgery improve the prognosis of colorectal liver metastases? J Gastorointest Surg 10:6–11
Simmonds PC (2000) Palliative chemotherapy for advanced colorectal cancer: systemic review and meta-analysis. Colorectal Cancer Collaborative Group. Br Med J 321:531–535
Kemeny MM (2006) Surgery should be the primary treatment of synchronous colorectal metastasis in the asymptomatic patient. Ann Surg Oncol 13:140–141
Verbrene CJ, de Bock GH, Pijl MEJ et al (2011) Palliative resection of the primary tumor in stage IV rectal cancer. Colorectal Dis 14:314–319
Cook AD, Single R, McCahill LE (2005) Surgical resection of primary tumors in patients who present with stage IV colorectal cancer: an analysis of surveillance, epidemiology, and end results data, 1998–2000. Ann Surg Oncol 12:637–645
Konyalian VR, Rosing DK, Haukoos JS et al (2007) The role of primary tumor resection in patients with stage IV colorectal cancer. Colorectal Dis 9:430–437
Grothey A, Sargent D, Goldberg RM et al (2004) Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil–leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol 22:1209–1214
Saltz LB, Clarke S, Diaz-Rubio E et al (2008) Bevacizumab in combination with oxaliplatin-based chemotherapy as first line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 26:2013–2019
Van Custem E, Kohne CH, Hitre E et al (2009) Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 360:1408–1417
Chibaudel B, Tournigand C, Andre T et al (2012) Therapeutic strategy in unresectable metastatic colorectal cancer. Ther Adv Med Oncol 4:75–89
Damjanov N, Weiss J, Haller DG (2009) Resection of primary colorectal cancer is not necessary in nonobstructed patients with metastatic disease. Oncologist 14:963–969
Rahban NN, Lordick F, Fink C et al (2012) Resection of the primary tumour versus no resection prior to systemic therapy in patients with colon cancer and synchronous unresectable metastases (UICC stageIV): SYNCHRONOUS: a randomized controlled multicenter trial (ISRCTN30964555). BMC Cancer 12:142–151
Poultsides GA, Paty PB (2011) Reassessing the need for primary tumor surgery in unresectable metastatic colorectal cancer: overview and perspective. Ther Adv Med Oncol 3:35–42
Katoh H, Yamashita K, Kokuba Y et al (2008) Surgical resection of stage IV colorectal cancer. World J Surg 32:1130–1137. doi:10.1007/s00268-008-9535-7
Delmore G (1997) Assessment of nutritional status in cancer patients: widely neglected? Support Care Cancer 5:376–380
Watanabe M, Iwatsuki M, Iwagami S et al (2012) Prognostic nutritional index predicts outcomes of gastrectomy in the elderly. World J Surg 36:1632–1639. doi:10.1007/s00268-012-1526-z
Nozoe T, Kimura Y, Ishida M et al (2002) Correlation of pre-operative nutritional condition with post-operative complications in surgical treatment for esophageal carcinoma. Eur J Surg Oncol 28:396–400
Pinato DJ, North BV, Sharma R (2012) A novel, externally validated inflammation-based prognostic algorithm in hepatocellular carcinoma: the prognostic nutritional index (PNI). Br J Cancer 106:1439–1445
Onodera T, Goseki N, Kosaki G (1984) Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nippon Geka Gakkai Zasshi 85:1001–1005 in Japanese; English abstract
Nozoe T, Kohno M, Iguchi T et al (2012) The prognostic nutritional index can be a prognostic indicator in colorectal carcinoma. Surg Today 42:532–535
Sobin L, Gospodarowicz M, Wittekind C (2009) International Union Against Cancer. TNM classification of malignant tumors, 7th edn. Wiley-Blackwell, New York, pp 73–77
Chapman MA, Buckley D, Henson DB et al (1998) Preoperative carcinoembryonic antigen is related to tumor stage and long-term survival in colorectal cancer. Br J Cancer 78:1346–1349
Colotta F, Allavena P, Sica A et al (2009) Cancer-related inflammation, the seventh hallmark of cancer: link to genetic instability. Carcinogenesis 30:1073–1081
Argiles JM, Busquets S, Lopez-Soriano FJ (2003) Cytokines in the pathogenesis of cancer cachexia. Curr Opin Clin Nutr Metab Care 6:401–406
Beleo-Tome C, Monteiro-Grillo I, Camilo M et al (2012) Validation of the malnutrion universal screening tool (MUST) in cancer. Br J Nutr 108:343–348
Kondrup J, Allison SP, Elia M et al (2003) ESPEN guidelines for nutrition screening 2002. Clin Nutr 22:415–421
Burden ST, Hill J, Shaffer JL et al (2010) Nutritional status of preoperative colorectal cancer patients. J Hum Nutr Diet 23:402–407
Dewys WD, Begg C, Lavin PT et al (1980) Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med 69:491–497
Ross PJ, Ashley S, Norton A et al (2004) Do patients with weight loss have a worse outcome when undergoing chemotherapy for lung cancers? Br J Cancer 90:1905–1911
Murry DJ, Riva L, Poplack DG et al (1998) Impact of nutrition on pharmacokinetics of anti-neoplastic agents. Int J Cancer Suppl 11:48–51
Zhang Y, Gu Y, Guo T et al (2012) Perioperative immunonutrition for gastrointestinal cancer: a systematic review of randomized controlled trial. Surg Oncol 21:87–95
Conflict of interest
There is no financial support or relationship that may pose any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maeda, K., Shibutani, M., Otani, H. et al. Low Nutritional Prognostic Index Correlates with Poor Survival in Patients with Stage IV Colorectal Cancer Following Palliative Resection of the Primary Tumor. World J Surg 38, 1217–1222 (2014). https://doi.org/10.1007/s00268-013-2386-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-013-2386-x