Abstract
Background
Colorectal cancer (CRC) harbors accumulated genetic alterations with cancer progression, which results in uncontrollable disease. To regulate the most malignant CRC, we have to know the most dismal phenotype of stage IV disease.
Methods
A retrospective review of the Kitasato University Hospital was performed (from 1990 to 2001) to extract the 162 resected stage IV CRC. Clinical variables were tested for their relationship to survival in a multivariate prognostic analysis and revealed the interaction of the prognostic factors.
Results
In stage IV CRC with noncurable resection, the most robust univariate predictors for poor prognosis were preoperative high value of CA19-9, peritoneal dissemination, depth of invasion, age, extent of liver metastases, pathologic lymph node metastasis status, and gender as tumor factors, and postoperative therapy, perioperative transfusion, and lymph node dissection extent as treatment factors. Among these factors, postoperative therapy (p < 0.0001), perioperative transfusion (0.0002), CA19-9 (0.001), extent of liver metastases (0.004), and peritoneal dissemination (0.02) were identified as independent prognostic factors by multivariate analysis. Interestingly, among the independent prognostic factors, treatment factors did not depend upon tumor factors and the combination of the three tumor factors (CA19-9, extent of liver metastases, and peritoneal dissemination) can clearly classify the patients into the definite prognostic groups.
Conclusion
Our results suggested that the most dismal CRC harbors three definite vectors that may represent the strongest phenotype of putative systemic immune (CA19-9), distant metastasis (extent of liver metastases), and local progression (peritoneal dissemination).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is the second most prevalent cancer and the fourth leading cause of cancer death worldwide, causing about 530,000 deaths every year [1]. Complete surgical resection of primary CRC and metastases remains the only potentially curative therapy [2]. On the other hand, for stage IV CRC involving synchronous distant metastases or peritoneal dissemination, the prognostic impact of primary tumor resection is not well documented so that both biological behavior and optimal treatment strategy have not been well established so far.
CRC is a genetic disease and accumulated genetic alterations result in cancer progression, leading to uncontrollable disease [3]. Therefore, the identification of genetic alterations in the most dismal prognostic phenotype of CRC would be beneficial for the development of novel diagnostic and treatment options.
Stage IV disease is curatively uncontrollable by any treatment modality, and previous studies suggested that patients with stage IV CRC comprise heterogeneous groups with clinicopathologic predictors that identify subgroups with significantly different prognoses such as lymph node metastasis [4, 5], peritoneal dissemination [4, 5], extent of liver metastases [5–8], depth of primary tumor invasion [5], age [8], preoperative CA19-9 [9], and preoperative CEA [8, 10].
In this study, we simultaneously validated all such promising clinical parameters reported so far and extracted the excellent combination of prognostic predictors as the most dismal phenotype of CRC. Then we suggested that the three definite vectors may represent the strongest phenotype of putative systemic immune, distant metastasis, and local progression. These prognostic indicators would be the clue for the therapeutic target of CRC.
Patients and methods
Registration and characteristics of patients with stage IV CRC after resection of primary cancer
A total of 1101 patients underwent surgical resection of primary CRC at the Kitasato University Hospital from January 1, 1990, to March 31, 2001, and all were entered into a retrospective database. From this patient source, we identified 946 patients with sporadic CRC, among which we identified 183 patients with stage IV CRC disease (19%) using the JCCC (Japanese Classification of Colorectal Cancer) [11] staging system. Exclusion criteria included operative or other disease-related death, extracolorectal or intracolorectal multiple cancers with previous histologic documentation, and insufficient clinical data such as preoperative tumor marker. The remaining 174 stage IV CRC patients were divided into two groups according to whether they underwent curable operation (n = 12) or not (n = 162), because as curably resected liver metastasis has been reported as having excellent outcome, hepatic resection has gained wide acceptance as safe and successful treatment [12] (Fig. 1).
The clinical parameters of the remaining 162 patients are depicted as noncurable stage IV CRC in Table 1. D2/D3 lymph node dissection was performed in 135 patients (83%) as the standard therapy, and the remaining 27 patients were restricted to D0/D1 lymph node dissection because of the severe condition of the heart, lung, kidney, or liver. Forty-five operators participated. The average survival was 14.9 months and the 5-year survival rate was 2.6%. All patients were informative for prognosis, namely, death within 5 years or alive at 5 years, and there were four censored cases. These censored cases were four patients who died from organ complications including one from ischemic heart disease, one from chronic renal failure, one from cerebrovascular disease, and one from postoperative complication (pneumonia).
In both the JCCC and the UICC (Unio Internationalis Contra Cancrum) staging system, stage IV CRC includes those patients with distant metastasis and extraregional lymph node metastasis (M in TNM classification). Moreover, in the JCCC staging system, metastasis contains those with peritoneal dissemination, so M is broken down into hepatic metastasis (H), extraregional lymph node metastasis, and peritoneal dissemination (P). For patients with hepatic metastases, hepatic tumor burden was defined according to the JCCC staging system: H1 (up to 4 hepatic metastases and ≤5 cm in maximum tumor size), H2 (neither H1 nor H3), and H3 (≥5 hepatic metastases and >5 cm in maximum tumor size). Patients without hepatic metastases were defined as H0 (Table 2). This classification of the H factor was based on the grading of liver metastases that consisted of metastatic tumor size and number in terms of prognostic difference [13]. Because T and N factors are the usual terms used in the TNM classification, we used T and N factors in the Japanese classification, which is the same as the TNM classification except for detailed subdivisions in N. N values were scored by pathologic reports (pN).
Patient demographics, tumor characteristics, and postoperative course were recorded and analyzed. Perioperative transfusion (POT) was defined as allogeneic blood transfusion during the operation or during the first two postoperative days [14]. POT was performed at the discretion of the treating surgeons and anesthesiologists. Postoperative therapy (PTx) included chemo-immunotherapy and radiation therapy; chemo-immunotherapy consisted of various 5-FU-based chemotherapies (e.g., 5-FU alone, 5-FU/mitomycin-C, or 5-FU/leucovorin). In this period, we could not use irinotecan or oxaliplatin. Tumor stage and grade were classified according to the 7th edition (the latest version) of JCCC staging system [11].
Statistical analysis
Statistical computations were performed using SAS StatView version 5.0 (SAS Institute, Cary, NC). A result was considered statistically significant when p < 5% (p < 0.05). The time of follow-up was calculated from the date of the first operation. Disease-specific survival (DSS) was estimated according to the Kaplan-Meier method and compared using the log-rank test [15]. A Cox’s proportional hazard model was built using the variables that had prognostic potential suggested by univariate analysis (p < 0.1) [16]. On the other hand, multivariate logistic regression analyses were performed for the significant univariate prognostic predictors of tumor factors and treatment factors to reveal the interaction.
Results
Univariate prognostic analysis in 162 noncurable stage IV CRCs
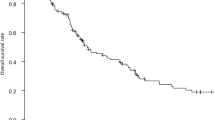
Table 1 shows the univariate prognostic factors on disease-specific-survival (DSS). Preoperative CA19-9 (p < 0.0001), H factor (H0, 1 or H2, 3) (0.03), P factor (0.002), T factor (0.002), age (0.01), gender (0.04), pathologic N (pN) factor (0.04), lymph node dissection extent (LNDE) (<0.0001), perioperative transfusion (POT) (0.0005), and postoperative therapy (PTx) (<0.0001) were associated with a poor outcome in noncurable stage IV CRC, and Kaplan-Meier curves are shown in Fig. 2 (A: CA19-9, B: H factor, C: P factor). On the other hand, preoperative CEA did not show significant association with prognosis (0.09).
Multivariate characterization of prognostic factors
The attempt at building a multivariate model for DSS was done using Cox’s proportional hazard model analysis. Included were all factors that had prognostic potential as suggested by the univariate analysis (p < 0.1). The model defined preoperative CA19-9 (p = 0.001), H factor (0.004), and P factor (0.02) as independent prognostic tumor factors, and postoperative therapy (PTx) (<0.0001) and perioperative transfusion (POT) (0.0002) as independent prognostic treatment factors (Table 3). Preoperative CEA, pN factor, T factor, and gender were eliminated after multivariate analysis.
To reveal the interaction between both independent and dependent prognostic factors, multivariate logistic regression analyses were also performed. For example, the results of multivariate logistic regression analyses of tumor factors (preoperative CA19-9, H factor, and P factor) and treatment factors (PTx, POT, and LNDE) are given in Tables 4 and 5, respectively. H factor was significantly related to preoperative CA19-9 and P factor. Preoperative CEA was a predictor for preoperative CA19-9 and H factor. Similarly, pN factor, over 20% of lymph node metastasis density (ND20), T factor, and gender were involved in P factor. PTx and LNDE were significantly correlated with each other and both were associated with age. Unexpectedly, LNDE was related to preoperative CA19-9. Multivariate logistic regression analysis revealed the interrelationship of the univariate prognostic factors as shown in Fig. 3. Among tumor factors, three independent prognostic factors were CA19-9, H factor, and P factor, where CA19-9 and H factor are interrelated and H factor and P factor are also mutually associated. Intriguingly, P factor was related to T factor, pN factor, and ND20, suggesting that it represents the most dismal phenotype of local progression. As treatment factors, PTx and POT remained independent prognostic factors, but these could not be associated with the three independent prognostic tumor factors (Fig. 3), suggesting that treatment factors were not affected by the independent tumor prognostic factors.
Combination of the independent prognostic tumor factors and prognosis in noncurable stage IV CRC
We believe that tumor factors are constant and treatment factor could be affected by a physician’s judgment. We then focused on remnant-independent prognostic tumor factors (CA19-9, H factor, and P factor) and examined whether a combination of them could actually predict the definite patient prognosis at staging (Fig. 4). We assigned positivity of 0 factor (n = 23), 1 or 2 factors (n = 122), and 3 factors (n = 17) among the three independent prognostic tumor factors to the staging group A, B, and C, respectively. Group A showed the best prognosis (average survival = 25.1 months), followed by group B (14.4 months) and the most dismal group C (5.4 months) (A vs B, p = 0.002; B vs C, p < 0.0001).
Combination of independent prognostic tumor factors extracted from multivariate analysis. Preoperative CA19-9, H factor, and P factor were combined to predict patient survival of noncurable stage IV CRC. Definitions of groups A, B, and C were given in the Results section. The statistical difference was found between groups A and B (p = 0.002), and between groups B and C (p < 0.0001)
Discussion
Our primary goal was to identify tumor factors reflecting the most dismal phenotype of CRC in order to know the therapeutic target. Our multivariate analysis revealed high-hazard risks for preoperative CA19-9 (1.93), H factor (1.78), and P factor (1.69), which were identified as the most potent independent prognostic factors in noncurable stage IV CRC among tumor factors (Table 3), and the combination was proven to be an excellent predictor of definite survival (Fig. 4). Interestingly, these three tumor factors are independent of treatment factors (Fig. 3). Previous multivariate analysis of stage IV CRC revealed that the independent prognostic tumor factor was preoperative CA19-9, where H factor was classified as solitary or not and eliminated by multivariate analysis; moreover, P factor was not included in the patient’s characteristics [9]. Hotta et al. [4] also reported that lymph node metastasis and peritoneal dissemination were final remnant prognostic factors, where H factor (classified as solitary or not) was eliminated and CA19-9 was not informative for the analysis. The present study is the first multivariate analysis that examined all the possible tumor factors to predict stage IV CRC patient outcome.
In this study, when patients were divided into H0, 1 group and H2, 3 group, H factor (H0, 1 or H2, 3) was the independent prognostic tumor factor in noncurable stage IV CRC (Table 3), whereas H factor would not be univariate prognostic factor when divided into H0 or not (Table 1). Using this criterion, we could extract H factor as the independent prognostic factor, which is different from the literature [4, 9]. Classification of H0, 1 vs. H2, 3 may represent multiple metastasis-prone phenotype (H2, 3) and other phenotypes (H0, 1). There have been numerous reports about identification of the molecules that differentiated liver metastasis from localized primary CRC by means of the microarray method [17] or SAGE analysis [18]. However, there have not been any reports that compared the two phenotypes H0, 1 and H2, 3 in the JCCC staging system. Hereafter, we plan to identify oncogenes and tumor suppressor genes that explain rigorous growth of multiple liver metastasis [19, 20].
As to P factor, T factor, ND, gender, and pN factor were predictors in our multivariate logistic regression analysis (Table 4, Fig. 3). It is quite intriguing that these predictors for P factor represent local progression and were eliminated after multivariate analysis. Finally, we may insist that P factor could be one of the most robust phenotypes of local progression in CRC. Tanaka et al. [21] reported a correlation between extranodal invasion of lymph nodes and peritoneal dissemination in gastric cancer. In the present study, lymphatic progression significantly correlated with P factor, supporting this hypothesis in CRC, too.
It is surprising that only one glycoform, serum CA19-9, predicts prognosis more strongly than the powerful prognostic phenotypes of H factor (H2, 3) and P factor. In our multivariate logistic regression analysis, H factor was significantly correlated with preoperative CA19-9 (Table 4), but both were involved in prognosis independently. These findings could support the intriguing hypothesis that CA19-9 makes the oncogenic disease more systemic through something different than tumor-bearing metastatic ability, and its inhibition is promising as a metastatic controller. Matsumoto et al. [22, 23] showed that blocking CA19-9 by cimetidine is beneficial to CRC patient outcome. This could also allow for the classical hypothesis that CA19-9 enhances extravasation and metastasis by interaction with E-selectin expressed on endothelium [24].
From the earlier publications, we may propose the following three mechanisms of CA19-9 involving systemic dissemination of cancer cells: (1) There was a report that described tumor-produced mucins expressing CA19-9 (sialyl Lea) lead the immune status to Th2 dominance [25]. For tumor rejection by the immune system, appropriate Th1 dominance is believed to be more critical [26, 27]. (2) Mucins expressing CA19-9 were associated with the induction of inflammatory molecules in human cancers [25, 28], suggesting chronic stimulation of E-selectin expression on systemic endothelial cells. Such preparation for metastasis may be associated with systemic metastasis. (3) Finally, P-selectin, another specific ligand for CA19-9, may be involved in promoting tumor aggregation with platelet [29], leading to cancer cell metastasis [30]. These studies suggest that CA19-9 could facilitate systemic metastasis not only by the mechanisms of selectin-related progression but also by immune evasion, which may be the reason why only one glycoform, CA19-9, predicted prognosis more significantly than the phenotypes of H factor and P factor.
Preoperative CEA was not an independent prognostic factor in this study (Table 3), which is different from that found in other reports [8, 10]. Lack of predictive effect of CEA might be affected by the fact that not all patients would be CEA producers. However, we would rather consider that the reason was because preoperative CEA was strongly correlated with preoperative CA19-9, and preoperative CA19-9 was a more robust prognostic predictor than preoperative CEA (Table 4). When using a cutoff level for preoperative CEA at 100 ng/ml, CEA was in fact the most robust independent prognostic factor instead of preoperative CA19-9 (hazard ratio = 1.99, p = 0.001). The cutoff level of CEA (100 ng/ml) may be of clinical and biological importance; however, use of this cutoff level is unusual in practical medicine, so we did not use it in the present model. In our study, CEA was the predictor for H factor, because published articles reported an association of CEA with liver metastasis [31, 32], and preoperative CEA did not predict P factor (Fig. 3, Table 4).
In this study, PTx and POT were the most robust independent treatment prognostic indicators (Table 3). Nevertheless, we do not conclude that treatment factors are significant effectors against stage IV CRC. Because such factors contained a variety of therapies and were affected by surgeon intuition, they could not be constant, which is different than tumor factors. We would need further prospective study under unified eligibility, on condition of the same treatment, in order to validate the prognostic effect of tumor factors which we identified in this study.
In conclusion, we propose the hypothesis that the most dismal phenotypes of noncurable CRC may be determined by three definite vectors: putative immunologic indicator, CA19-9; metastatic indicator, H factor; and local progression indicator, P factor. However, these three factors are collinear in the present study, with the exception of the interaction of CA19-9 and P factor, although they are independent prognostic predictors. Therefore, in the near future we plan to assess these three tumor factors by prospective validation to see whether they are bona fide prognostic determinants in a multivariate analysis adjusted for the collinear factors.
References
Parkin DM, Bray F, Ferlay J et al (2005) Global cancer statistics, 2002. CA Cancer J Clin 55(2):74–108
Morise Z, Sugioka A, Fujita J et al (2006) Does repeated surgery improve the prognosis of colorectal liver metastases? J Gastrointest Surg 10(1):6–11
Vogelstein B, Kinzler KW (2004) Cancer genes and the pathways they control. Nat Med 10(8):789–99
Hotta T, Takifuji K, Arii K et al (2006) Potential predictors of long-term survival after surgery for patients with stage IV colorectal cancer. Anticancer Res 26(2B):1377–1383
Kato H, Yoshimatsu K, Ishibashi K et al (2005) A new staging system for colorectal carcinoma with liver metastasis. Anticancer Res 25(2B):1251–1255
Tocchi A, Mazzoni G, Brozzetti S et al (2004) Hepatic resection in stage IV colorectal cancer: prognostic predictors of outcome. Int J Colorectal Dis 19(6):580–585
Ruo L, Gougoutas C, Paty PB et al (2003) Elective bowel resection for incurable stage IV colorectal cancer: prognostic variables for asymptomatic patients. J Am Coll Surg 196(5):722–728
Kuo LJ, Leu SY, Liu MC et al (2003) How aggressive should we be in patients with stage IV colorectal cancer? Dis Colon Rectum 46(12):1646–1652
Wang WS, Lin JK, Chiou TJ et al (2002) CA19-9 as the most significant prognostic indicator of metastatic colorectal cancer. Hepatogastroenterology 49(43):160–164
Dixon MR, Haukoos JS, Udani SM et al (2003) Carcinoembryonic antigen and albumin predict survival in patients with advanced colon and rectal cancer. Arch Surg 138(9):962–966
Japanese Society for Cancer of the Colon, Rectum (2006) General rules for clinical and pathological studies on cancer of the colon, rectum and anus, 7th ed. Kanehara & Co. Ltd., Tokyo
Choti MA, Sitzmann JV, Tiburi MF et al (2002) Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg 235(6):759–766
Katoh T, Yasui K, Hirai T (2004) Study of liver metastases from colorectal cancer. In: Mutoh T (ed) Colorectal disease now 2004. Japan Medical Center, Tokyo, pp 89–104
Weitz J, D’Angelica M, Gonen M et al (2003) Interaction of splenectomy and perioperative blood transfusions on prognosis of patients with proximal gastric and gastroesophageal junction cancer. J Clin Oncol 21(24):4597–603
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. Am Stat Assoc 53:457–481
Cox D (1972) Regression models and life-tables. J R Stat Soc (B) 34:187–220
Nakagawa H, Liyanarachchi S, Davuluri RV et al (2004) Role of cancer-associated stromal fibroblasts in metastatic colon cancer to the liver and their expression profiles. Oncogene 23(44):7366–7377
Parle-McDermott A, McWilliam P, Tighe O et al (2000) Serial analysis of gene expression identifies putative metastasis-associated transcripts in colon tumour cell lines. Br J Cancer 83(6):725–728
Yamashita K, Upadhyay S, Osada M et al (2002) Pharmacologic unmasking of epigenetically silenced tumor suppressor genes in esophageal squamous cell carcinoma. Cancer Cell 2(6):485–495
Kim MS, Yamashita K, Baek JH et al (2006) N-methyl-D-aspartate receptor type 2B is epigenetically inactivated and exhibits tumor-suppressive activity in human esophageal cancer. Cancer Res 66(7):3409–3418
Tanaka T, Kumagai K, Shimizu K et al (2000) Peritoneal metastasis in gastric cancer with particular reference to lymphatic advancement; extranodal invasion is a significant risk factor for peritoneal metastasis. J Surg Oncol 75(3):165–171
Matsumoto S (1995) Cimetidine and survival with colorectal cancer. Lancet 346(8967):115
Matsumoto S, Imaeda Y, Umemoto S et al (2002) Cimetidine increases survival of colorectal cancer patients with high levels of sialyl Lewis-X and sialyl Lewis-A epitope expression on tumour cells. Br J Cancer 86(2):161–167
Magnani JL (2004) The discovery, biology, and drug development of sialyl Lea and sialyl Lex. Arch Biochem Biophys 426(2):122–131
Yokoigawa N, Takeuchi N, Toda M et al (2005) Enhanced production of interleukin 6 in peripheral blood monocytes stimulated with mucins secreted into the bloodstream. Clin Cancer Res 11(17):6127–6132
Check JH, Nazari P, Goldberg J et al (2001) A model for potential tumor immunotherapy based on knowledge of immune mechanisms responsible for spontaneous abortion. Med Hypotheses 57(3):337–343
Harada N, Kodama N, Nanba H (2003) Relationship between dendritic cells and the D-fraction-induced Th-1 dominant response in BALB/c tumor-bearing mice. Cancer Lett 192(2):181–187
Yokoigawa N, Takeuchi N, Toda M et al (2007) Overproduction of PGE2 in peripheral blood monocytes of gastrointestinal cancer patients with mucins in their bloodstream. Cancer Lett 245(1–2):149–155
Kannagi R, Izawa M, Koike T et al (2004) Carbohydrate-mediated cell adhesion in cancer metastasis and angiogenesis. Cancer Sci 95(5):377–384
Honn KV, Tang DG, Crissman JD (1992) Platelets and cancer metastasis: a causal relationship? Cancer Metastasis Rev 11(3–4):325–351
Pietra N, Sarli L, Costi R et al (1998) Role of follow-up in management of local recurrences of colorectal cancer: a prospective, randomized study. Dis Colon Rectum 41(9):1127–1133
Graham RA, Wang S, Catalano PJ et al (1998) Postsurgical surveillance of colon cancer: preliminary cost analysis of physician examination, carcinoembryonic antigen testing, chest x-ray, and colonoscopy. Ann Surg 228(1):59–63
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Katoh, H., Yamashita, K., Kokuba, Y. et al. Surgical Resection of Stage IV Colorectal Cancer and Prognosis. World J Surg 32, 1130–1137 (2008). https://doi.org/10.1007/s00268-008-9535-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-008-9535-7