Abstract
Background
Total mesorectal excision (TME) and preoperative chemoradiation therapy (PCRT) for rectal cancer are used sequentially in our center. The aim of this study was to evaluate survival of patients with stage II/III rectal cancer chronologically and to determine whether therapeutic advances associated with TME and PCRT have improved patient survival.
Methods
A retrospective review of 2,197 patients from July 1989 to December 2006 was conducted. The time period (P) for this study was divided into three groups: P1 (1989–1995), P2 (1996–2001) for TME, P3 (2002–2006) for PCRT. Cancer-specific survival (CSS), disease-free survival (DFS), and recurrences among the three periods were investigated.
Results
A total of 293 patients in P1, 836 patients in P2, and 1,068 patients in P3 were enrolled. The 5-year CSS in stages II and III was statistically different between P1/P2 and P3 (stage II, p = 0.008; stage III, p < 0.001). The 5-year DFS was significantly different between P1/P2 and P3 for stage III (p = 0.001). The local recurrence and systemic recurrence rates decreased during P3, but there was no significant difference between the three periods for stage II. For stage III, local recurrence was significantly different between the three periods (P1 vs. P2, p = 0.002; P1 vs. P3, p < 0.001; P2 vs. P3, p = 0.008).
Conclusions
We identified an improvement in survival for stage II/III rectal cancer and a decrease in local recurrence for stage III rectal cancer during P3, the most recent period. This may be due to frequent application of PCRT based on the TME.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Advances in the treatment of rectal cancer have contributed to marked improvements in patient outcomes over the past two to three decades. In the United States, overall survival has increased significantly from a 5-year relative survival of 49 % between 1975 and 1977 to 69 % between 1999 and 2005 [1]. The 5-year relative survival also increased in European countries over time between 1988–1990 and 2000–2002 [2]. Similarly, the 5-year relative survival for colorectal cancer in Korea has increased from 54.8 % between 1993 and 1995 to 71.3 % between 2001 and 2005 [3]. Although part of this improvement is attributable to early detection of disease by screening, the widespread adoption of optimal surgery techniques and increased utilization of both radiotherapy and chemotherapy have likely contributed to the improvement in patient outcomes [4, 5].
In the treatment of rectal cancer, adoption of total mesorectal excision (TME) and preoperative chemoradiation therapy (PCRT) were applicable to this improvement. The use of TME has become a standard operation for rectal cancer and has led to lower local recurrence rates and better survival [6–8]. The value of adding PCRT to surgery for the treatment of patients with resectable rectal cancer has been assessed in several trials [9–13]. Therefore, TME and PCRT are considered an optimal part of the multidisciplinary treatment of rectal cancer. TME and PCRT were used sequentially in our center since the establishment of TME during the mid-1990s and the beginning of PCRT with TME during the early 2000s. The purpose of this study was to evaluate the chronologic survival of patients with stage II/III rectal cancer and to determine whether therapeutic advances in the use of PCRT and TME have led to improved survival.
Patients and methods
Patients
A retrospective review was conducted on patients who underwent radical surgery for stage II or III rectal cancer at the Asan Medical Center, Seoul, Korea between July 1989 and December 2006. Inclusion criteria included tumors below the level of S1/S2 with an inferior tumor margin being ≤15 cm from the anal verge based on operative or endoscopic findings. Patients with hereditary nonpolyposis colorectal cancer, familial adenomatous polyposis, or other malignancy and patients with previous treatment of rectal cancer were excluded from this study. Of 2,251 patients, 53 patients were also excluded because of their unknown cause of death: six patients during period 1 (P1), 28 patients in P2, 19 patients in P3. Finally, 2,197 patients were included in the study.
Local recurrence was defined as any detectable local disease at follow-up, either alone or in conjunction with systemic disease. We used the 7th American Joint Committee on Cancer (AJCC) staging manual for cancer staging. Patients were followed-up until April 1, 2012. Patients with incomplete follow-up were censored at the date of their last follow-up.
Periods
Patients were classified into three groups depending on their year of operation: P1 (1989–1995), P2 (1996–2001), P3 (2002–2006). Our standard surgical protocol for rectal cancer was wide mesorectal excision. It was a tailored mesorectal excision during P1 and TME during P2. The change from postoperative to preoperative radiation therapy occurred progressively since the early 2000s. PCRT with TME was adopted gradually during P3 for rectal cancer located in the mid to low rectum. Autonomic nerve preservation was performed throughout the entire study period.
Surgery
Three experienced colorectal surgeons performed all of the operations uniformly. Two surgeons were involved throughout the whole study period and a third surgeon joined in the surgery during the early part of P2. We did not perform lateral lymph node dissection routinely, but we did perform limited lymph node sampling for enlarged lymph nodes. Surgery was performed 5–8 weeks after completion of the preoperative radiotherapy for patients who received PCRT.
Radiotherapy
Radiotherapy was delivered at a total 50.4 Gy within 6 weeks: 45 Gy in 25 fractions to the pelvis and 5.4 Gy in a three-fraction boost to the primary tumor. Preoperative radiation therapy was performed on patients with locally advanced (T3/T4 and/or node-positive on an imaging study) mid- or low-rectal cancer. Postoperative radiation therapy was performed on patients with biopsy-proven T3/T4 and/or node-positive mid or low rectal cancer and who underwent surgery with curative intent, agreed to this therapy, and were deemed able to tolerate it.
Chemotherapy
In the PCRT setting, capecitabine or fluorouracil-based chemotherapy started on day 1 of the first radiotherapy round. Capecitabine was administered orally at a dose of 1,650 mg/m2/day, in two doses, during the whole period of radiotherapy and without weekend breaks. Fluorouracil (375 mg/m2/day) and leucovorin (20 mg/m2/day) were administered by rapid intravenous injection for 3 days during the first and fifth weeks of radiotherapy. Adjuvant chemotherapy usually commenced 4 weeks after surgical resection in all of the PCRT patients and started on day 1 of the first radiotherapy session in the postoperative CRT group. For oral adjuvant chemotherapy, we used doxifluridine (800–1,200 mg/day), tegafur/uracil (tegafur 300–600 mg/day + uracil 672–1,344 mg/day), and capecitabine (1,650 mg/m2/day).
Patient follow-up
We regularly evaluated patients for disease recurrence over a follow-up period of 5 years. History taking and physical examination, complete blood counts, blood chemistry studies, carcinoembryonic antigen (CEA) testing, chest radiography, and computed tomography of the abdomen and pelvis were repeated every 6 months during the 5 years. Whenever reasonably possible, we biopsied suspected lesions to confirm metastatic or recurrent disease. If histologic or cytologic evidence was not available, sequential enlargement of a mass in radiology studies was accepted. Elevated CEA alone was not considered evidence of recurrence.
Statistical analysis
Categorized variables were compared using the χ2 test, and continuous variables were compared using the unpaired Student’s t test. Cancer-specific survival (CSS) and disease-free survival (DFS) curves over the three periods were plotted using the Kaplan–Meier method and compared using the log-rank test. The significance level was set at 5 % (p = 0.05) for all analyses. The analyses were performed using a SPSS software program (version 19; SPSS, Chicago, IL, USA)
Results
Characteristics of patients
A total of 2,197 patients (293 in P1, 836 in P2, 1,068 in P3) were included in this study. A definite increase in the number of cases and a slight rise in the average age of the patients were observed from P1 to P3 (p = 0.01). The mean follow-up period was longer toward P1 (p = 0.001). No statistical differences in patient sex or histologic differentiation of tumors were found between the three periods. Into the subgroups by stage, the results showed low IIA (30.0 %) and high IIIC (18.8 %) proportions during P1 and a high IIA (45.5 %) and low IIIC (9.8 %) proportions during P3. However, when we analyzed patients who undersent PCRT using clinical stage, the results showed the same proportions in P1 and altered proportions in P2 and P3 as follows: IIA (39.8 %), IIIB (36.7 %), and IIIC (15.0 %) during P2; and IIA (38.1 %), IIIB (40.5 %), and IIIC (14.1 %) during P3. The three periods differed in the distribution of clinical cancer stage, but the difference did not reach significance (p = 0.13). Also, there was a significant difference between groups in terms of the number of patients who underwent adjuvant radiation therapy: 63.1 % in P1, 65.3 % in P2, and 45.0 % in P3 (p < 0.001) (Table 1). Considering a combination of both preoperative and postoperative radiotherapy, there was no significant difference in the proportion of patients undergoing radiotherapy over the three periods: 63.8 % in P1, 67.5 % in P2, 63.5 % in P3 (p = 0.08). Table 2 shows other causes of death for each period, regardless of the colorectal cancer, during the 5-year follow-up.
Adjuvant chemotherapy
The proportion of patients who did not undergo adjuvant chemotherapy was lower in P1 than in P2 or P3. There was no significant difference in the proportion of patients who did not undergo adjuvant chemotherapy for stage II and III disease (p = 0.07 and p = 0.14, respectively). The proportion of patients who underwent oral adjuvant chemotherapy (including doxifluridine or tegafur/uracil), except capecitabine, was lower during P3 than during P1/P2. By contrast, the use of capecitabine was markedly increased during P3 than during P1/P2 (Table 3).
Survival
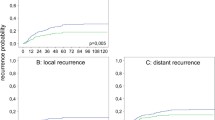
The 5-year CSS for all patients with stage II/III rectal cancer increased from 70.0 % in P1 to 84.3 % in P3 (P1/P2 vs. P3: p < 0.001, irrespective of stage). The 5-year DFS also increased, from 64.3 % in P1 to 69.8 % in P2 to 76.8 % in P3 (P1 vs. P2: p = 0.002; P1 vs. P3: p < 0.001; P2 vs. P3: p < 0.001, irrespective of stage). When an analysis of rectal cancer was performed according to tumor stage, the 5-year CSS in both stage II and III was statistically different between P1/P2 and P3 (stage II: P1 vs. P3, p = 0.008; P2 vs. P3, p = 0.001; stage III: P1/P2 vs. P3, p = 0.001) (Fig. 1). The 5-year DFS was slightly different between P1 and P3 for stage II (p = 0.044). For stage III, the 5-year DFS was significantly different between P1/P2 and P3 (P1 vs. P3: p < 0.001; P2 vs. P3: p = 0.001) (Fig. 1)
Five-year cancer-specific survival for stage II a and stage III b rectal cancer during the three study periods (P1, P2, P3). P1: 85.1 ± 3.5 % in stage II and 62.0 ± 3.5 % in stage III; P2: 88.2 ± 1.7 and 67.7 ± 2.1 %, respectively; P3: 91.3 ± 1.3 and 78.0 ± 1.7 %, respectively. Five-year disease-free survival for stage II c and stage III d rectal cancer during the three periods. P1: 83.7 ± 3.7 % in stage II and 53.7 ± 3.6 % in stage III; P2: 84.4 ± 1.9 and 58.7 ± 2.3 %, respectively; P3: 87.5 ± 1.5 and 66.9 ± 2.0 %, respectively
Recurrence
The number of local and systemic recurrences showed a decreasing trend toward the later period, but the difference was not significant for stage II rectal cancer. For stage III rectal cancer, local recurrence—but not systemic recurrence—was significantly different for the three periods (P1 vs. P2: p = 0.002; P1 vs. P3: p < 0.001; P2 vs. P3: p = 0.008) (Table 4).
Discussion
This chronological study showed improvements in survival and local control of rectal cancer in patients undergoing surgery during the most recent period (2002–2006). Similar survival results were found in The Netherlands [14, 15] and by a Swedish group [16], but their data were based on population registries, unlike our study with a uniform treatment environment. Although pathologic stages for the three periods showed significantly different proportions in the subgroups, these differences might be attributed to pathologic down-staging after PCRT. TME and PCRT were applied sequentially at our center. Therefore, the current study indirectly investigated the effects of TME and PCRT separately under uniform patient and treatment environments.
In the current study, we aimed to determine the effect of TME by comparing survival and recurrence between the P1 and P2 time periods. Although the 5-year DFS irrespective of stage was statistically different between P1 and P2, the 5-year CSS and DFS for stage II and III patients were not statistically different between the P1 and P2 time periods when considered separately. Also, there was no statistical difference between P1 and P2 regarding systemic recurrence, but there was a significant difference in terms of local recurrence (with and/or without systemic recurrence) for stage III disease (p = 0.002). These results imply that the outcome for wide mesorectal excision performed during P1 was similar to that for TME performed during P2, except for the local recurrence rate in stage III patients.
Zaheer et al. [17] stated that “tumor-specific” mesorectal excision (TSME) when the tumor was high in the rectum appeared to achieve a low rate of local recurrence and good long-term survival, similar to those for TME. Law and Chu et al. [18] also stated that partial mesorectal excision (PME) for cancer in the upper rectum yielded similar results when compared with TME for mid and distal rectal cancer. Therefore, the outcomes from wide mesorectal excision at our center during P1 may be similar to those from TSME or PME. In terms of the prognostic impact of TME, concerns about inter-surgeon and inter-institution variability have been described in several studies [19–21]. In our study, data were collected and analyzed from a single center. Also, three experienced colorectal surgeons performed all of the operations uniformly, thereby minimizing potential inter-surgeon and inter-institution variability.
The current study showed that the 5-year CSS for stage II/III and the 5-year DFS for stage III rectal cancer during P3 was statistically different from that during P1/P2. The main difference in treatment between P2 and P3 in this study was the introduction of PCRT during P3. Considering the change of treatment modality between P2 and P3 and the pathologic down-staging after PCRT, the survival improvement in P3 may be due to the indirect effect of PCRT. Also, there was a statistically significant difference in the rate of local recurrence between P2 and P3 in stage III patients and an insignificant but steady decrease in the local recurrence rate from P1 to P3 for stage II patients.
These outcomes are in line with those of previous randomized controlled studies. The Swedish Rectal Cancer trial [22] and the NSABP R-03 (National Surgical Adjuvant Breast and Bowel Project R-03) trial [13] showed that preoperative radiotherapy reduced local recurrence rates and improved survival. In addition, the German trial [23], the European Organization for Research and Treatment of Cancer (EORTC) [24], and the Dutch Colorectal Cancer Group [12] reported that PCRT with TME contributed to improved local control despite having no effect on survival. Although this study was not a prospective randomized trial, these data suggest that PCRT was indirectly involved in the improvement of survival of patients with stage II or III rectal cancer and in lowering local recurrence of stage III rectal cancer. For this reason, the current standard of treatment at our institution is PCRT for patients with locally advanced (T3/T4 and/or node-positive on imaging study) mid or low rectal cancer.
Another factor that influences survival and recurrence is the use of chemotherapy. In this study, there was a significant difference in the adjuvant chemotherapy regimen for the three periods. Oral chemotherapy (except capecitabine) was usually used during P1, but its use gradually decreased over the years between P1 and P3. Although there is a lack of consensus on the utility of oral FU, a meta-analysis suggested a statistically significant benefit in terms of survival with the use of oral fluoropyrimidines [25]. A recent randomized Phase III trial in Germany confirmed noninferiority for overall survival when infusional 5-FU was replaced by oral capecitabine during radiotherapy and adjuvant chemotherapy [26]. Newer-generation chemotherapeutics, such as oxaliplatin and irinotecan, were seldom used as the primary regimen of adjuvant chemotherapy throughout the entire study period. Although the proportion of patients who did not undergo chemotherapy was the lowest in P1 and the highest in P2, there was no significant difference for stage II cancer (p = 0.128). There was, however, a significant difference for stage III (p = 0.02) rectal cancer. Hence, in this study there was a difference in the chemotherapy regimens, but it did not appear to explain the different outcomes for the three periods. Furthermore, there was no difference in systemic recurrence among the three periods. Thus, the results suggested that adjuvant chemotherapy was not sufficient to achieve better long-term survival rates.
The present study has a number of limitations that should be considered when interpreting the results. As in all single-institution retrospective observational cohort studies, there is potential for both referral and selection bias. The clarifying division of each period was difficult, and inter-stage analysis may not be obvious because of the stage change due to PCRT in 18.5 % of the patients in P3. In addition, the bias due to the drastic increase in the number of patients in P3 and the associated change toward lower cancer stage (although without significance) would have an impact on survival results. The analyses in this study were not direct comparisons between the effect of the TME and PCRT but, rather, indirect comparisons between each of the periods that implemented TME and/or PCRT. Despite these limitations, the study suggests that globally used treatment, such as TME/PCRT, can be beneficial for patients with rectal cancer.
Conclusions
We identified an improvement in survival for patients with stage II/III rectal cancer and a reduction in local recurrence for those with stage III rectal cancer during the most recent period (P3: 2002–2006). These improved parameters may be due to the introduction of routine PCRT, based on TME. During P2, when TME became routine, the local recurrence rate for stage III rectal cancer decreased—although there was no significant improvement in survival compared with that for P1. The outcome of wide mesorectal excision performed during P1 may be similar to that of TME and TSME. We conclude that therapeutic advances have improved the survival of patients, but new agents for adjuvant chemotherapy and multidisciplinary treatment are still needed to further improve survival rates in patients with rectal cancer.
References
Jemal A, Siegel R, Xu J et al (2010) Cancer statistics, 2010. CA Cancer J Clin 60:277–300
Brenner H, Bouvier AM, Foschi R et al (2012) Progress in colorectal cancer survival in Europe from the late 1980s to the early 21st century: the EUROCARE study. Int J Cancer 131:1649–1658
Korean NCIC (2012) Available at: http://www.cancer.go.kr/ncic/cics_f/03/032/index.html. Accessed April 2012 (in Korean)
Van Gijn W, Krijnen P, Lemmens VE et al (2010) Quality assurance in rectal cancer treatment in The Netherlands: a catch up compared to colon cancer treatment. Eur J Surg Oncol 36:340–344
Chang KH, Smith MJ, McAnena OJ et al (2012) Increased use of multidisciplinary treatment modalities adds little to the outcome of rectal cancer treated by optimal total mesorectal excision. Int J Colorectal Dis 27:1275–1283
Heald RJ, Husband EM, Ryall RD (1982) The mesorectum in rectal cancer surgery: The clue to pelvic recurrence? Br J Surg 69:613–616
Heald RJ, Ryall RD (1986) Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1:1479–1482
Quirke P, Durdey P, Dixon MF et al (1986) Local recurrence of rectal adenocarcinoma due to inadequate surgical resection: histopathological study of lateral tumour spread and surgical excision. Lancet 2:996–999
Sauer R, Becker H, Hohenberger W et al (2004) Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 351:1731–1740
Folkesson J, Birgisson H, Pahlman L et al (2005) Swedish Rectal Cancer Trial: long lasting benefits from radiotherapy on survival and local recurrence rate. J Clin Oncol 23:5644–5650
Bosset JF, Collette L, Calais G et al (2006) Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 355:1114–1123
Kapiteijn E, Marijnen CA, Nagtegaal ID et al (2001) Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 345:638–646
Roh MS, Colangelo LH, O’Connell MJ et al (2009) Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol 27:5124–5130
Elferink MA, van Steenbergen LN, Krijnen P et al (2010) Marked improvements in survival of patients with rectal cancer in The Netherlands following changes in therapy, 1989–2006. Eur J Cancer 46:1421–1429
Martijn H, Voogd AC, van de Poll-Franse LV et al (2003) Improved survival of patients with rectal cancer since 1980: a population-based study. Eur J Cancer 39:2073–2079
Dahlberg M, Pahlman L, Bergstrom R et al (1998) Improved survival in patients with rectal cancer: a population-based register study. Br J Surg 85:515–520
Zaheer S, Pemberton JH, Farouk R et al (1998) Surgical treatment of adenocarcinoma of the rectum. Ann Surg 227:800–811
Law WL, Chu KW (2004) Anterior resection for rectal cancer with mesorectal excision: a prospective evaluation of 622 patients. Ann Surg 240:260–268
Wexner SD, Rotholtz NA (2000) Surgeon influenced variables in resectional rectal cancer surgery. Dis Colon Rectum 43:1606–1627
Harmon JW, Tang DG, Gordon TA et al (1999) Hospital volume can serve as a surrogate for surgeon volume for achieving excellent outcomes in colorectal resection. Ann Surg 230:404–411 (discussion 411–413)
Blomqvist P, Ekbom A, Nyren O et al (1999) Survival after rectal cancer: differences between hospital catchment areas—a nationwide study in Sweden. Gut 45:39–44
Anonymous (1997) Improved survival with preoperative radiotherapy in resectable rectal cancer: Swedish Rectal Cancer Trial. N Engl J Med 336:980–987
Sauer R, Liersch T, Merkel S et al (2012) Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol 30:1926–1933
Bonnetain F, Bosset JF, Gerard JP et al (2012) What is the clinical benefit of preoperative chemoradiotherapy with 5FU/leucovorin for T3-4 rectal cancer in a pooled analysis of EORTC 22921 and FFCD 9203 trials: Surrogacy in question? Eur J Cancer 48:1781–1790
Sakamoto J, Ohashi Y, Hamada C et al (2004) Efficacy of oral adjuvant therapy after resection of colorectal cancer: 5-year results from three randomized trials. J Clin Oncol 22:484–492
Hofheinz RD, Wenz F, Post S et al (2012) Chemoradiotherapy with capecitabine versus fluorouracil for locally advanced rectal cancer: a randomised, multicentre, non-inferiority, phase 3 trial. Lancet Oncol 13:579–588
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, J.L., Yu, C.S., Kim, C.W. et al. Chronological Improvement in Survival Following Rectal Cancer Surgery: A Large-Scale, Single-Center Study. World J Surg 37, 2693–2699 (2013). https://doi.org/10.1007/s00268-013-2168-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-013-2168-5