Abstract
Purpose
Total mesorectal excision (TME) is the standard surgical treatment for rectal cancer. The roles of chemotherapy and radiotherapy have become more defined, accompanied by improvements in preoperative staging and histopathological assessment. We analyse our ongoing results in the light of changing patterns of treatment over consecutive time periods.
Methods
In total, 151 consecutive patients underwent potentially curative rectal excision for cancer in a single institution. Management and outcomes were compared between 1993–1999 and 2000–2007 which corresponded with the restructuring of the regional oncological services.
Results
We found an increase in patients treated with neoadjuvant chemoradiotherapy after 1999 (20/89 vs 1/62, p < 0.001). There was an increase in the mean number of lymph nodes examined (11.9 vs 9.4, p = 0.037). The locoregional recurrence rate was 5.3%. The rates were not significantly different between the two study periods [4/89 (4.5%) 1999–2007 vs 4/62 (6.5%) 1993–1999, p = 0.597]. There was no statistical difference in overall or disease-free survival in the time periods examined.
Conclusions
Increasing use of neoadjuvant therapy and concomitant improvement in lymph node assessment did not translate into a concurrent reduction in the local recurrence, disease-free and overall survival rates. Our results demonstrate the enduring benefit of specialist training in TME in the outcome of rectal cancer surgery. This observational study suggests that low local recurrence rates are surrogate markers for improved overall and disease-free survival. Multidisciplinary team practice should be examined and made cost effective according to the individual unit’s local recurrence rate in the light of this and other reports.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Total mesorectal excision (TME) has become the standard surgical treatment in rectal cancer [1, 2]. It has led to durable reductions in local recurrence and overall survival in populations where it has been introduced [3–5]. Resection of rectal cancer by those skilled in TME may have a larger impact on disease control than either case volume, neoadjuvant or adjuvant treatment [6–9].
In parallel to the advancement of TME training, there has been an evolution in neoadjuvant and adjuvant treatment of rectal cancer, with preoperative radiotherapy becoming accepted practice for appropriate cases, with reported improved local control and a less morbid side effect profile compared with postoperative radiotherapy [10]. This has been aided by improvements in imaging, in particular development of magnetic resonance imaging (MRI), and helical computerised tomography (CT) [11, 12]. Histopathological assessment of resected tumours has improved, with recognition of the importance of lymph node assessment and the circumferential resection margin [13]. The benefit of adjuvant chemotherapy, after optimal resection of colonic cancer has been confirmed, with a small but definite survival benefit, even in those with stage II disease [14]. Novel targeted therapies in conjunction with conventional chemotherapy have established efficacy in advanced disease, and their utility as first line adjuvant therapy is being investigated [15]. The universal application of adjuvant chemotherapy in rectal cancer may lead to overtreatment of a significant number of patients, and may not be necessary in subgroups with favourable disease (e.g. ypT0-2N0) [16, 17]. Increasing complexity of multidisciplinary management has led to the widespread adoption of the multidisciplinary team meeting (MDT) as a forum to direct treatment and improve quality of care [1, 18].
These changes have been reflected in our practice. Prior to 1999, ready access to neoadjuvant/adjuvant therapy was not available locally. At the time of our previous study, rectal cancer treatment was primarily, and almost exclusively, surgical [7]. Changes in access to radiation and chemotherapy services in our geographical area, allied with improved adjuvant protocols have allowed us to analyse two time periods of rectal cancer treatment. The first (1993–2000) was previously analysed and published [7] .From 1999 to present, medical and radiation oncology services have been formally added to the surgical treatment of rectal cancer as per international protocols. This study assesses the impact of neoadjuvant and adjuvant treatments on the outcomes of rectal cancer in this geographical area.
Methods
Patients
Patients who underwent rectal excision in a single institution between September 1993 and June 2007 were identified. Patient and tumour parameters and survival data were retrospectively collected. Management and outcomes were compared between 1993–June 1999 (first study period) and July 1999–2007 (second study period) which corresponded with the restructuring of regional oncological services.
Operative technique
The rectal dissection for TME was performed, by, or under the supervision of a single surgeon, in a single institution, as previously described [7]. Sharp dissection in the appropriate anatomical plane was performed, and the splenic flexure and transverse colon were mobilized in all patients. At anterior resection colonic continuity was re-established by colorectal or coloanal anastomosis using a circular stapling device (Covidien, Norwalk, Connecticut, USA). The bowel edges excised by the circular stapler (“doughnuts”) were inspected, and the neorectum was tested for anastomotic air leak. A suction drain was placed in the pelvis and a diverting stoma was performed if the patient was aged 75 or over, in case of faecal spillage, if the resection “doughnuts” were not intact, if there was leak on testing, neoadjuvant radiotherapy, or where there was any concern regarding a satisfactory blood supply. Laparoscopic assisted resection was performed in four patients with intracorporeal anastomosis.
Patients undergoing abdominoperineal resection (APR) had dissection down to the levator ani muscles, completed during the abdominal component of the procedure. Only mobilization and dissection of the anal canal and sphincter complex was performed through the perineal incision. Following surgery, patients attended the outpatient clinic every 3 months for 2 years, 6 monthly up to 5 years and yearly thereafter. Liver ultrasonography or CT was performed yearly and colonoscopy was performed at 6 months, 1 year and 3 years post-surgery, then three yearly.
Radiotherapy and chemotherapy
In the time period from 1999 to 2007, patients with predicted MRI diagnosis of T3 or T4, or node positive rectal tumours, with no evidence of distant spread on CT and MRI, received neoadjuvant chemoradiotherapy (the German regime) [19]. Preoperatively 45–50 Gy of radiation was delivered in 25–28 fractions to the pelvis. During the first and fifth weeks of radiotherapy, concurrent 5-FU was administered as a 120-h continuous infusion at a dose of 1,000 mg/m2/day. Surgery was performed 4–6 weeks after the completion of therapy. Four weeks following surgery, bolus injections of 5-FU were given at a dose of 500 mg/m2/day for 5 consecutive days repeated every 4 weeks for a total of 4 cycles as tolerated [19].
Post resection of the rectum, patients with Stage II or Stage III disease received adjuvant therapy as per O’Connell et al. as tolerated [20]. Patients underwent an initial 9-week cycle of systemic chemotherapy, followed by radiation and concomitant fluorouracil, followed in turn by a second cycle of systemic chemotherapy. Radiation therapy consisted of 45 Gray, delivered in 1.8 Gy fractions over 5 weeks. During irradiation therapy, fluorouracil was administered by protracted venous infusion at a rate of 225 mg/m2/day. During systemic chemotherapy phase of the treatment, patients received fluorouracil by bolus injection at a dose of 500 mg/m2 on days 1 to 5 and days 36 to 40; and 450 mg/m2 on days 134 to 138 and days 169 to 173 [20].
Statistical analysis
Data were compared with Chi-square, Fisher’s exact test, Student’s t test and Mann–Whitney U test as appropriate. Survival was estimated using the Kaplan–Meier method and compared using the log-rank test. Cox proportional hazard regression was performed using the forward conditional method. All analyses were performed with SPSS version 16.0 (SPSS, Illinois, USA), and Microsoft Excel 2007 (Microsoft, Washington, USA).
Results
Patients
We identified 151 patients, out of 222 consecutive patients with rectal cancer (tumours located within 15 cm of the anal verge), who underwent potentially curative total mesorectal excision between September 1993 and June 2007 (62 during the first study period, 89 during the second study period). Of the 222 patients identified, 71 were excluded from the study group. The indication for rectal surgery amongst those excluded from subsequent analysis included tubulovillous adenomas with various levels of dysplasia (15) and patients with established metastatic disease or a macroscopic R2 resection at the time of treatment. Patient and tumour characteristics during the two time periods examined are summarized in Table 1. The mean number of curative resections per year was 10.8.
Operative procedures
There was no difference in the ratio of APR to sphincter-preserving surgery in the two time periods examined. In the first study period, there were 54 anterior resections [24 low anterior resections (LAR)], 8 APR (APR rate 12.9%). In the second, there were 77 anterior resections (27 LAR) and 12 abdominoperineal resections (APR rate 13.5%). There were two emergency rectal resections for obstructing rectal tumours and both were in the first study period. Overall, covering stomas were formed in 45 of 131 anterior resections (34%), including 13 of 17 patients receiving neoadjuvant radiotherapy. There were significantly more covering stomas formed in LAR, when compared to high anterior resections (24 of 27 vs 21 of 59, p < 0.001). There were less covering stomas in the first study period but this is not statistically significant (22 covering stomas in 52 anterior resections compared to 32 covering stomas in 54 anterior resections in the second study period).
With regard to complications, the majority occurred in the first study period. There were two radiologically confirmed anastomotic leaks and two peri-operative mortalities (within 30 days of the procedure), all of which occurred in the first study period. The mortalities were attributed to pulmonary embolism and Gram negative septicemia. There were four cases requiring major peri-operative transfusion (defined as replacement of greater than 50% of the blood volume, or >5 units of packed cells, in 24 h), three in the first study period and one in the second. Three post-operative rectal strictures were noted during the first study period and two cases of radiation enteritis occurred in the second, all occurring in patients who received adjuvant radiotherapy. There have been no rectal strictures or radiation enteritis since the shift in practice from adjuvant to neoadjuvant radiotherapy. There was one rectovaginal fistula in the first study period, in a patient who did not undergo radiotherapy.
Radiotherapy and chemotherapy
There was a significant increase in patients who received peri-operative (adjuvant or neoadjuvant) chemotherapy (15/62 vs 36/82, p = 0.014) in the second study period, which was accounted for by increased neoadjuvant treatment. There was a shift from adjuvant to neoadjuvant radiotherapy in the time periods studied, with one receiving neoadjuvant and 15 receiving adjuvant therapy in the first study period, compared with 20 and 9 receiving neoadjuvant (p < 0.001) and adjuvant radiotherapy (p = 0.035), respectively in the second study period. There was a higher proportion of stage II (22/62 vs 40/89, p = 0.320) and less stage III disease (29/62 vs 35/89, p = 0.457) in the second study period, probably as a result of downstaging neoadjuvant therapy.
The distribution of tumour stage in the preoperative group receiving neoadjuvant chemoradiotherapy was altered compared to the postoperative post-therapy tumour stage, with 13 of 20 downstaged (complete pathological response n = 1, ypT1 n = 1, ypT2 n = 12, ypT3 n = 7). Neoadjuvant radiotherapy did not lead to a statistically significant change in the number of patients receiving sphincter sparing surgery (p = 0.155).
Histopathological assessment
There was no significant difference in the tumour stage at histopathological examination between the two time periods studied (p = 0.666). There were seven R1 resections identified in total, with 6 occurring in the second study period, probably reflecting improved assessment of the circumferential resection margin. There was an overall increase in the mean number of lymph nodes examined over time, with a mean of 11.9 in the second study period, compared with 9.4 in the first (p = 0.037). There was no significant difference in the number of lymph nodes involved (p = 0.418) or assessed (p = 0.725) in those who received neoadjuvant radiotherapy. There was no significant increase in the number of N1 and N2 cases (N1 = 22 and N2 = 6 in the first study period; N1 = 34, N2 = 13 in the second, p = 0.564).
Outcomes
In total there were eight local recurrences, representing 5.3% of the total cases, without a significant difference between the two time periods [4/58 (6.5%) vs 4/89 (4.5%), p = 0.597]. There were 13 (8.6%) patients who developed distant metastases after resection, without a significant difference in the incidence between the two time periods [7/62 (11.3%) vs 6/89 (6.8%), p = 0.327]. Three had synchronous local and distant recurrence, all of which occurred in the second study period.
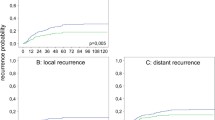
The mean overall survival in the first and second study periods were 11.2 and 6.4 years respectively (Fig. 1a). The mean disease-free survival rates were 10.1 and 5.7 years, respectively (Fig. 1b). The 5-year overall survival in the first and second study periods were 86% and 78%, respectively (p = 0.227, Fig. 2). The 5-year disease-free survival rates were 85% and 72%, respectively (p = 0.245, Fig. 3). Table 2 compares our local recurrence and survival rates with international literature.
Comparison of overall and disease-free survival for patients undergoing curative resection of rectal cancer in the first and second study periods. Kaplan–Meier survival curves for: a overall survival, b disease-free survival for the entire cohort before (first study period) and after June 1999 (second study period). There was no significant difference between the groups using the log-rank test
Discussion
In our cohort of patients with early stage rectal cancer treated with TME, there was a marked increase in the number of patients treated with radiotherapy and adjuvant chemotherapy over the two time periods, without a concurrent reduction in local disease recurrence. The most notable shift in management was from adjuvant to neoadjuvant radiotherapy. There was no case of rectal strictures and radiation enteritis in the second study period. Similarly, there was a significant increase in the use of adjuvant chemotherapy, although we found no difference in the disease-free or overall survival between the two study periods (1993–June 1999 and July 1999–2007). This may reflect the small numbers of recurrences involved in both time periods.
Our observations suggest that the most important factor in achieving good outcomes in localized rectal cancer remains technical proficiency in rectal cancer surgery. The time periods we studied are of interest, as locally and internationally, they span the transition from rectal cancer surgery treatment occurring as part of a general surgical practice, to a more subspecialised practice. From 1999 to present, the international shift to multidisciplinary management of rectal cancer has been mirrored in our institution. Local changes have included the establishment of a regional radiation oncology centre, improved radiological diagnostics (MRI and CT), appointment of medical oncologists, with a special interest in colorectal cancer, and the creation of a regional MDT meeting. Our cohort has been previously studied, and this data represents an opportunity for comparison of the contemporary MDT model [7]. While our results demonstrate improved access to chemotherapy and radiotherapy, and suggest improvements in the quality of multidisciplinary care in line with best practice, evidenced by increased mean lymph nodes assessed post-resection, reduced metastatic nodes after neoadjuvant treatment and increased identification of R1 resection, the overall and disease-free survival has not altered [13].
We report a satisfactory overall survival (67% actuarial overall survival after a median follow-up of 4.6 years), with a relatively low APR to anterior resection ratio (overall APR rate 13.2%). The shift to neoadjuvant treatment led to an apparent downstaging of tumours with increased number of stage II and reduced amount of stage III disease in the second study period. However, increased use of chemoradiotherapy has not translated into improvements in oncological outcome over and above that conferred by TME. There was no significant difference in overall and disease-free survival between the two study periods. Survival rates in the first study period appeared to be better, albeit not statistically significant. This may have been due to the inclusion of more advanced tumours in the second study period that had been downstaged following neoadjuvant therapy and underwent “curative” resections. The heterogeneity and relatively small size of our sample may obscure some of the benefit accruing from changes in management, in particular changes in chemoradiotherapy practice. It has been demonstrated that although benefit for adjuvant chemotherapy after curative resection may be real, that benefit may be small (e.g. absolute benefit ranging from 3.6% to 5.4%) [14]. The majority of the benefit may be conferred to younger patients, hence our sample may be underpowered to detect a difference, and the older age profile of our cohort may not achieve optimal benefit from adjuvant treatment. It could also be argued, that if a TME is performed adequately, in selected cases it may be difficult to justify the additive risk of morbidity associated with chemoradiotherapy.
A flaw with studies using volume as an indicator of rectal cancer resection quality is that pooling of outcome data from several surgeons may obscure or dilute the influence of specialist training on cancer outcomes. In the first study period, our case volume could be considered medium to low, however on current volumes, our institution would be considered a high volume centre (more than 30 cases of rectal cancer/year) and is increasing with the appointment of four additional gastrointestinal surgeons [21, 22]. One of the benefits of our study is that the operator was specifically trained in TME, and this allows a more accurate longitudinal assessment of outcomes and the influence of changes in multidisciplinary rectal cancer management.
Our local recurrence rate of 5.3% compares favourably to published data (Table 2) [21]. In low volume hospitals, where there is a proficiency in TME, results do not deviate significantly from the international average [22]. Although volume is often used as a surrogate for quality, recent literature suggests that training may be a more important factor in cancer, and specifically rectal cancer, outcomes [6, 23–25]. Smith et al. demonstrated that although case volume was associated with beneficial outcomes, there was a stronger association with specialist training and specialisation [6]. A recent analysis showed that individual surgeon volume had no effect on outcomes, if surgeons were adequately credentialed [26]. It has also become recognized that misclassification of data may bias the volume/outcome assumptions made on SEER-Medicare data [27].
From a health policy perspective, a treatment strategy should not only be effective, but also cost-effective. The clinical benefits of neoadjuvant chemoradiotherapy should be weighed against its societal costs and quality-adjusted life expectancy. Van den Brink et al. performed a cost-utility analysis of neoadjuvant radiotherapy in patients with rectal cancer undergoing TME in the Dutch trial and concluded that preoperative radiotherapy was both effective and cost-effective [28]. Similarly, Dahlberg et al. found that short course preoperative neoadjuvant radiotherapy in the context of the Swedish Rectal Cancer Trial to be a cost effective intervention [29]. These findings have been debated however, as the main economic benefit accruing from these trials may be related to a reduction in local recurrence, attributable to radiotherapy, with the local recurrence rates in the non-irradiated arms of these trials ranging from 8.2% to 27%. This local recurrence rate is higher than with current standards of TME and hence may limit general application of neoadjuvant radiotherapy, particularly in the context of low contemporary rates of local recurrence rates (for example our rate of 5.3%) with satisfactory TME [30]. It does appear that the forum of MDT meetings and multidisciplinary management of colorectal cancer are valued by clinicians, and may improve accuracy of staging and clinical outcomes, but the cost-effectiveness of the paradigm remains uncertain [31–33]. In our geographically peripheral unit, capital investment in radiation and chemotherapy services to attain international standards of rectal cancer treatment did not appear to translate into better outcomes.
In conclusion, the evolution in the management of rectal cancer in our centre reflects international best practice and has allowed us to examine the effect of multidisciplinary management of rectal cancer. In particular, we found an increased use of chemoradiotherapy and a shift from adjuvant to neoadjuvant treatment, without a concurrent reduction in the disease recurrence rates. Our results reaffirm the enduring benefit of specialist training in TME in the outcome of rectal cancer, and suggest an incremental benefit from improvements in the multidisciplinary management. Whilst this is a retrospective observational study, additional therapy has not impacted on local recurrence, overall and disease-free survival rates. The quality of TME surgery remains the mainstay of rectal cancer treatment.
References
Jessop J, Beagley C, Heald RJ (2006) The Pelican Cancer Foundation and the English National MDT-TME Development Programme. Colorectal Dis 8(Suppl 3):1–2. doi:10.1111/j.1463-1318.2006.01060.x
MacFarlane JK, Ryall RD, Heald RJ (1993) Mesorectal excision for rectal cancer. Lancet 341(8843):457–460. doi:0140-6736(93)90207-W
Martling A, Holm T, Rutqvist LE, Johansson H, Moran BJ, Heald RJ, Cedermark B (2005) Impact of a surgical training programme on rectal cancer outcomes in Stockholm. Br J Surg 92(2):225–229. doi:10.1002/bjs.4834
den Dulk M, Krijnen P, Marijnen CA, Rutten HJ, van de Poll-Franse LV, Putter H, Meershoek-Klein Kranenbarg E, Jansen-Landheer ML, Coebergh JW, van de Velde CJ (2008) Improved overall survival for patients with rectal cancer since 1990: the effects of TME surgery and pre-operative radiotherapy. Eur J Cancer 44(12):1710–1716. doi:10.1016/j.ejca.2008.05.004
Martling AL, Holm T, Rutqvist LE, Moran BJ, Heald RJ, Cedemark B (2000) Effect of a surgical training programme on outcome of rectal cancer in the County of Stockholm. Stockholm Colorectal Cancer Study Group, Basingstoke Bowel Cancer Research Project. Lancet 356(9224):93–96
Smith JA, King PM, Lane RH, Thompson MR (2003) Evidence of the effect of ‘specialization’ on the management, surgical outcome and survival from colorectal cancer in Wessex. Br J Surg 90(5):583–592. doi:10.1002/bjs.4085
Dowdall JF, Maguire D, McAnena OJ (2002) Experience of surgery for rectal cancer with total mesorectal excision in a general surgical practice. Br J Surg 89(8):1014–1019. doi:10.1046/j.1365-2168.2002.02158.x
Heald B (2006) Better surgery and better selection for adjuvants—still the key to improving outcomes in rectal cancer. Acta Chir Iugosl 53(2):35–37
Moore E, Heald RJ, Cecil TD, Sharpe GD, Sexton R, Moran BJ (2005) Almost all five year disease free survivors are cured following rectal cancer surgery, but longer term follow-up detects some late local and systemic recurrences. Colorectal Dis 7(4):403–405. doi:10.1111/j.1463-1318.2005.00791.x
Wong RK, Tandan V, De Silva S, Figueredo A (2007) Pre-operative radiotherapy and curative surgery for the management of localized rectal carcinoma. Cochrane Database Syst Rev (2):CD002102. doi:10.1002/14651858.CD002102.pub2
Strassburg J, Junginger T, Trinh T, Puttcher O, Oberholzer K, Heald RJ, Hermanek P (2008) Magnetic resonance imaging (MRI)-based indication for neoadjuvant treatment of rectal carcinoma and the surrogate endpoint CRM status. Int J Colorectal Dis 23(11):1099–1107. doi:10.1007/s00384-008-0531-z
Moran B, Brown G, Cunningham D, Daniels I, Heald R, Quirke P, Sebag-Montefiore D (2008) Clarifying the TNM staging of rectal cancer in the context of modern imaging and neo-adjuvant treatment: ‘y’‘u’ and ‘p’ need ‘mr’ and ‘ct’. Colorectal Dis 10(3):242–243. doi:10.1111/j.1463-1318.2007.01260.x
Smith AJ, Driman DK, Spithoff K, Hunter A, McLeod RS, Simunovic M, Langer B Guideline for optimization of colorectal cancer surgery and pathology. J Surg Oncol 101(1):5–12. doi:10.1002/jso.21395
Quasar Collaborative G, Gray R, Barnwell J, McConkey C, Hills RK, Williams NS, Kerr DJ (2007) Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet 370(9604):2020–2029. doi:10.1016/S0140-6736(07)61866-2
Benson AB 3rd (2007) New approaches to assessing and treating early-stage colon and rectal cancers: cooperative group strategies for assessing optimal approaches in early-stage disease. Clin Cancer Res 13(22 Pt 2):6913s–6920s. doi:10.1158/1078-0432.CCR-07-1188
Govindarajan A, Reidy D, Weiser MR, Paty PB, Temple LK, Guillem JG, Saltz LB, Wong WD, Nash GM (2011) Recurrence rates and prognostic factors in ypN0 rectal cancer after neoadjuvant chemoradiation and total mesorectal excision. Ann Surg Oncol 18(13):3666–3672. doi:10.1245/s10434-011-1788-y
Gunderson LL, Callister M, Marschke R, Young-Fadok T, Heppell J, Efron J (2008) Stratification of rectal cancer stage for selection of postoperative chemoradiotherapy: current status. Gastrointest Cancer Res 2(1):25–33
Burton S, Brown G, Daniels IR, Norman AR, Mason B, Cunningham D (2006) MRI directed multidisciplinary team preoperative treatment strategy: the way to eliminate positive circumferential margins? Br J Cancer 94(3):351–357. doi:10.1038/sj.bjc.6602947
Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, Karstens JH, Liersch T, Schmidberger H, Raab R (2004) Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 351(17):1731–1740. doi:10.1056/NEJMoa040694
O’Connell MJ, Martenson JA, Wieand HS, Krook JE, Macdonald JS, Haller DG, Mayer RJ, Gunderson LL, Rich TA (1994) Improving adjuvant therapy for rectal cancer by combining protracted-infusion fluorouracil with radiation therapy after curative surgery. N Engl J Med 331(8):502–507
Wibe A, Eriksen MT, Syse A, Tretli S, Myrvold HE, Soreide O (2005) Effect of hospital caseload on long-term outcome after standardization of rectal cancer surgery at a national level. Br J Surg 92(2):217–224. doi:10.1002/bjs.4821
Debes AJ, Storkson RH, Jacobsen MB (2008) Curative rectal cancer surgery in a low-volume hospital: a quality assessment. Eur J Surg Oncol 34(4):382–389. doi:10.1016/j.ejso.2007.06.007
Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL (2003) Surgeon volume and operative mortality in the United States. N Engl J Med 349(22):2117–2127. doi:10.1056/NEJMsa035205
Goodney PP, Stukel TA, Lucas FL, Finlayson EV, Birkmeyer JD (2003) Hospital volume, length of stay, and readmission rates in high-risk surgery. Ann Surg 238(2):161–167. doi:10.1097/01.SLA.0000081094.66659.c3
Birkmeyer JD (2000) Relation of surgical volume to outcome. Ann Surg 232(5):724–725
Larson DW, Marcello PW, Larach SW, Wexner SD, Park A, Marks J, Senagore AJ, Thorson AG, Young-Fadok TM, Green E, Sargent DJ, Nelson H (2008) Surgeon volume does not predict outcomes in the setting of technical credentialing: results from a randomized trial in colon cancer. Ann Surg 248(5):746–750. doi:10.1097/SLA.0b013e31818a157d
Hollenbeck BK, Hong J, Zaojun Y, Birkmeyer JD (2007) Misclassification of hospital volume with surveillance, epidemiology, and end results medicare data. Surg Innov 14(3):192–198. doi:10.1177/1553350607307274
van den Brink M, Stiggelbout AM, van den Hout WB, Kievit J, Klein Kranenbarg E, Marijnen CA, Nagtegaal ID, Rutten HJ, Wiggers T, van de Velde CJ (2004) Clinical nature and prognosis of locally recurrent rectal cancer after total mesorectal excision with or without preoperative radiotherapy. J Clin Oncol 22(19):3958–3964. doi:10.1200/JCO.2004.01.023
Dahlberg M, Stenborg A, Pahlman L, Glimelius B (2002) Cost-effectiveness of preoperative radiotherapy in rectal cancer: results from the Swedish Rectal Cancer Trial. Int J Radiat Oncol Biol Phys 54(3):654–660. doi:S0360301602028808
Simunovic M, Gafni A, Levine M (2004) Economics of preoperative radiotherapy with total mesorectal excision: what can we learn from the Dutch experience? J Clin Oncol 22(2):217–219. doi:10.1200/JCO.2004.11.918
Daniels IR, Fisher SE, Heald RJ, Moran BJ (2007) Accurate staging, selective preoperative therapy and optimal surgery improves outcome in rectal cancer: a review of the recent evidence. Colorectal Dis 9(4):290–301. doi:10.1111/j.1463-1318.2006.01116.x
Sharma A, Sharp DM, Walker LG, Monson JR (2008) Colorectal MDTs: the team’s perspective. Colorectal Dis 10(1):63–68. doi:10.1111/j.1463-1318.2007.01209.x
Fleissig A, Jenkins V, Catt S, Fallowfield L (2006) Multidisciplinary teams in cancer care: are they effective in the UK? Lancet Oncol 7(11):935–943. doi:10.1016/S1470-2045(06)70940-8
Kusters M, Marijnen CA, van de Velde CJ, Rutten HJ, Lahaye MJ, Kim JH, Beets-Tan RG, Beets GL (2010) Patterns of local recurrence in rectal cancer; a study of the Dutch TME trial. Eur J Surg Oncol 36(5):470–476. doi: 10.1016/j.ejso.2009.11.011
Polglase AL, Grodski SF, Tremayne AB, Chee JB, Bhathal PS (2004) Local recurrence following surgical treatment for carcinoma of the lower rectum. ANZ J Surg 74(9):745–750. doi:10.1111/j.1445-1433.2004.03136.x
Heald RJ, Moran BJ, Ryall RD, Sexton R, MacFarlane JK (1998) Rectal cancer: the Basingstoke experience of total mesorectal excision, 1978–1997. Arch Surg 133(8):894–899
Stocchi L, Nelson H, Sargent DJ, O’Connell MJ, Tepper JE, Krook JE, Beart R Jr (2001) Impact of surgical and pathologic variables in rectal cancer: a United States community and cooperative group report. J Clin Oncol 19(18):3895–3902
Chiappa A, Biffi R, Zbar AP, Luca F, Crotti C, Bertani E, Biella F, Zampino G, Orecchia R, Fazio N, Venturino M, Crosta C, Pruneri GC, Grassi C, Andreoni B (2005) Results of treatment of distal rectal carcinoma since the introduction of total mesorectal excision: a single unit experience, 1994–2003. Int J Colorectal Dis 20(3):221–230. doi:10.1007/s00384-004-0670-9
Porter GA, Soskolne CL, Yakimets WW, Newman SC (1998) Surgeon-related factors and outcome in rectal cancer. Ann Surg 227(2):157–167
Engel J, Kerr J, Eckel R, Gunther B, Heiss M, Heitland W, Siewert JR, Jauch KW, Holzel D (2005) Influence of hospital volume on local recurrence and survival in a population sample of rectal cancer patients. Eur J Surg Oncol 31(5):512–520. doi:10.1016/j.ejso.2005.02.027
Acknowledgements
We would like to thank Ms. Eimear Mannion and Dr. Sandra Deady for their help in the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
KH Chang and MJ Smith are joint first authors.
Rights and permissions
About this article
Cite this article
Chang, K.H., Smith, M.J., McAnena, O.J. et al. Increased use of multidisciplinary treatment modalities adds little to the outcome of rectal cancer treated by optimal total mesorectal excision. Int J Colorectal Dis 27, 1275–1283 (2012). https://doi.org/10.1007/s00384-012-1440-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-012-1440-8