Abstract
Background
In sentinel node surgery for breast cancer, procedural accuracy is assessed by calculating the false-negative rate. It is important to measure this since there are potential adverse outcomes from missing node metastases. We performed a meta-analysis of published data to assess which method has achieved the lowest false-negative rate.
Methods
We found 3,588 articles concerning sentinel nodes and breast cancer published from 1993 through mid-2011; 183 articles met our inclusion criteria. The studies described in these 183 articles included a total of 9,306 patients. We grouped the studies by injection material and injection location. The false-negative rates were analyzed according to these groupings and also by the year in which the articles were published.
Results
There was significant variation related to injection material. The use of blue dye alone was associated with the highest false-negative rate. Inclusion of a radioactive tracer along with blue dye resulted in a significantly lower false-negative rate. Although there were variations in the false-negative rate according to injection location, none were significant.
Conclusions
The use of blue dye should be accompanied by a radioactive tracer to achieve a significantly lower false-negative rate. Location of injection did not have a significant impact on the false-negative rate. Given the limitations of acquiring appropriate data, the false-negative rate should not be used as a metric for training or quality control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The false-negative rate (FNR) of the sentinel node (SN) biopsy procedure is an important measure of procedural accuracy in the surgical management of breast cancer. Potential adverse outcomes from missing node metastases include understaging the patient and an increased risk of cancer recurrence. The FNR is measured by comparing the pathological status of the SNs to that of the remainder of the axillary nodes present in a completion axillary node resection (ANR).
A large randomized trial has demonstrated that when cancer has not metastasized to the SN, survival and recurrence are equivalent in SN biopsy only and ANR [1]. Since the morbidity is lower with SN biopsy only, ANR is not typically justifiable in the SN-negative case. The era of gathering new data on FNR is essentially over.
There are many different methods for removing SNs. These have been devised to achieve practical and theoretical advantages such as decreased pain, increased success rate, and ease of performing the procedure. Many of these outcomes can be reliably evaluated with the number of patients typically seen in a single center. However, establishing an accurate FNR involves observations on a very large number of patients. For example, to establish a 5 % FNR (in a range of 0–7 %) for a single surgeon would take about 300 patients with positive axillary nodes [2]. Comparing the differences in FNR between different SN biopsy methods requires a very large number of patients. Few if any published reports on single-patient populations can define with sufficient statistical certainty what the FNR is for any given SN biopsy method.
We present here a meta-analysis of the FNR observed with different SN biopsy methods. The FNR analysis was based upon grouping published data according to the type of material injected and the location of injection. Only the axillary nodes were considered for this analysis; nonaxillary nodes were not included.
Materials and methods
Search strategy
An information service provided a manually categorized list of the 3,588 published articles related to SNs and breast cancer that were listed in PubMed from 1993 through mid-2011 (Sentinel Nodes and Breast Cancer, www.treeofmedicine.com). Of the 3,588 articles, 302 were categorized as having reported a FNR. These 302 articles were reviewed for inclusion in this meta-analysis. Additionally, a PubMed search was performed for meta-analyses that have already addressed SN biopsy in breast cancer and FNR [3–8]. Citations from these meta-analyses were also reviewed.
Selection criteria
To be included in the meta-analysis, articles must have contained groups or subgroups of patients who had pathologically negative SN biopsies followed by ANR. Injection material(s) and injection location(s) must have been clearly specified. A FNR reported for a group of patients must have been from a group that had the same injection material(s) and injection location(s) for each patient in the group. An article could have more than one group of patients and be included in our study. The number of patients with positive axillae and the number of patients with false-negative SNs had to be described separately for each group in the article.
Articles that reported results based largely on the same group of patients as a previous article were excluded. Articles that were not available in English were also excluded.
Data collection
A false-negative event is when a patient has a pathologically negative SN and has at least one pathologically positive non-SN. For each article, the number of false-negative cases and the number of cases with positive axillary nodes were recorded for each group of patients that met our inclusion criteria.
Patients were grouped according to the injection location and the injection material. Injection material was classified as dye alone, radioactive tracer alone, and combined dye and tracer. Dyes included isosulfan blue, methylene blue, patent blue, indocyanine green, indigo carmine, and Evan’s blue. Tracers included 99mTc-colloidal albumin, technetium sulfur colloid, 99mTc tin, 99mTc phytate, 99mTc dextran, antimony sulfide, and 99mTc rhenium colloid.
Location of injection was classified as around the tumor (including peritumoral and subcutaneous over the tumor), areolar or periareolar, intratumoral, and intradermal. Combinations of injection locations were classified as around the tumor and areolar; around the tumor and intradermal; and intradermal and areolar. The classification of multiple injection locations was based on groups of patients for whom data were available and who met our inclusion criteria.
Statistical analysis
For a set of patients who had a successful SN biopsy, the FNR was defined as the number of false-negative cases divided by the number of cases with any axillary nodal metastases (FNR = FN/(TP + FN). The reported FNRs were in the form of sample proportions (p = x/n), where n is the number of cases with axillary dissections and x is the number of false-negative cases from each citation. This sample proportion p can be viewed as being an estimate of an unknown binomial parameter that represents the true FNR (π) for a given study. It is known that the variance of the sample proportion p as an estimate of the binomial parameter π is equal to (π) × (1 − π)/n and that the usual method of estimating this sampling variance is to substitute the observed proportion p for π. However, in the two extreme cases where the observed FNR proportion value p equals zero or 1.0, such a substitution can result in a zero value for the sampling variance. Such a simple substitution scenario does not provide a reasonable estimate for the sampling variance of p that reflects the influence of the sample size n. The arc sine transformation of the sample proportion p (x/n) does provide a method that reflects the sample size in the transformed data scale. The arc sine transformation is the result of taking the arc sine of the square root of (x/n) [9]. On the transformed arc sine scale, the transformed FNR has a sampling distribution with a variance of approximately 1/(4 × n). Thus, the sample size n from each citation can be included to reflect the precision of the reported data. Formal meta-analysis of the transformed proportions (p = x/n) can be conducted and then the use of the appropriate back transformation allows summary effect size estimates to be converted back to the original FNR scale.
The arc sine-transformed FNR and sample size for each citation were entered into a data file with the corresponding covariate information, such as year of publication (broken down into approximate quartiles), the injection agents, and the locations used for injections. Furthermore, citations were divided into 17 possible groups by the combination of injection agent and location of injection used. Each covariate was examined relative to variation among the transformed FNR data with the use of a mixed model where citations within any given covariate subgroup were assumed to represent a random effect and the covariate subgroups were assumed to be a fixed effect. Summary forest plots for each of the three covariate subgroups were created based upon the random-effects models. Forest plots of the transformed FNR data were created for those covariate groups, which showed reasonable mixed-model statistical heterogeneity based upon the Q test [10]. Significant Q statistic values were followed with pairwise comparisons to isolate the source of the FNR heterogeneity. The summary effect sizes and 95 % confidence limits within covariate subgroups were back-transformed to create summary covariate-specific subgroup FNR point estimates and their 95 % confidence intervals. A sensitivity analysis was conducted by deleting those citations with FNR equal to zero to determine if any of the primary results were altered.
All primary data transformations and back transformations as well as recoding were conducted using SYSTAT ver. 11 (Systat, Chicago, IL). Mixed-model meta-analysis calculations and graphical displays were obtained using Comprehensive Meta-Analysis ver. 2.2 (Biostat, Englewood, NJ).
Results
Of the 302 articles that reported a FNR value, there were 183 articles that met the inclusion criteria. Overall, these articles produced 202 unique patient groups for analysis. Seventeen articles had two clearly defined groups with either different injection materials or different locations and gave FNRs for each group. One article presented three separate groups. The total number of patients included in these studies was 9,220. The total number of patients with false-negative axillae was 794. The crude overall FNR was 8.61 % (CI = 8.05–9.2 %). Using a fixed-effects model assuming homogeneity between studies, the overall FNR was calculated to be 7.5 % (CI = 7.0–8.1 %). Dropping the homogeneity assumption, it was estimated to be 7.0 % (CI = 6.1–7.9 %) using a random-effects model.
By year
The year of publication for the 202 groups shows growth from a few before 1997, with rapid growth from 1997 to a peak in 2000 and 2001, and a steady decline over the decade (Fig. 1). The dates of publication for the 202 groups were divided into approximate quartiles by year of publication as follows: 1993–1999, 2000–2001, 2002–2004, and 2005–2011. The FNRs for these quartiles were 5.4 % (CI = 3.8–7.3 %), 7.4 % (CI = 6.0–8.9 %), 6.1 % (CI = 4.5–7.8 %), and 8.9 % (CI = 7.1–11.5 %), respectively. There was no significant variation between quartiles (p = 0.09).
By injection material
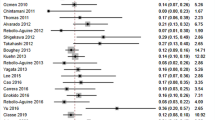
FNRs were calculated for three categories of injection materials: dye-only, tracer-only, and dye-and-tracer. One study was excluded from this analysis because each patient received two different types of dye [11]. FNRs were 8.6 % (CI = 6.7–10.8 %) for dye-only, 7.4 % (CI = 5.6–9.3) for tracer-only, and 5.9 % (CI = 4.8–7.1 %) for dye-and-tracer (Fig. 2 shows overall group data and Fig. 3a–c shows individual study data). All three groups were first compared to determine if their FNRs were equal. The Q statistic for heterogeneity indicated that they were not all equal (p = 0.050). Subsequent pairwise comparisons indicated that there was a difference between the dye-only and the dye-and-tracer categories (p = 0.018). However, there was no difference between tracer-only and dye-only (p = 0.370), or tracer-only and dye-and-tracer (p = 0.178).
By location of injection
There were seven categories based on location of injection. Four categories consisted of a single location site while three categories represented a combination of locations. The intratumoral location had the lowest FNR at 2.5 % (CI = 0.2–12.6 %), while FNRs at the other locations ranged from 4.9 to 8.3 % (Fig. 4). However, there was no statistically significant variation between these seven location categories (p = 0.95).
By location and by injection material
A total of 17 groups of patients could be identified based on the combination of materials injected and the location of injection (Table 1). The categories were first compared as a whole, and the Q statistic indicated a statistically significant variation existed among the FNRs of the 17 categories (p = 0.034).
There was significant FNR variation between the injection material categories for those injected around the tumor and those injected intradermally. In the patients who had injections restricted to around the tumor, there was a significant variation in the FNR depending on the injection material (p = 0.0069). The difference in FNR between the dye-only group (9.4 %: CI = 7.3–11.6 %) and the dye-and-tracer group (5.4 %: CI = 4.2–6.9 %) was statistically significant (p = 0.002). There was no significant difference in the FNR between the tracer-only and the other two injection material categories. For the patients in whom the location of injection was intradermal, the FNR was significantly different between dye-only (14.3 %: CI = 6–25.3 %) and tracer-only (0 %: CI = 0–10.3 %) (p = 0.03). However, there were only two groups of patients with injections restricted to this site.
There was no significant FNR variation detected between locations no matter the type of injection material(s) used.
Sensitivity analysis
A sensitivity analysis was performed by dropping all groups that had a FNR of 0 % from the analysis. After this, 166 groups remained. The resulting FNR was 8.7 % (CI = 8.2–9.3 %) using a fixed-effects model and 8.5 % (CI = 7.8–9.3 %) using a random-effects model. There continues to be no significant variation in FNR between the quartiles based on year of publication (p = 0.125). or between injection locations (p = 0.450). The significant variation between injection materials persists (p = 0.005).
Conclusions
This meta-analysis includes data from over 9,000 cases from 183 articles that met the inclusion criteria. As shown in Fig. 1, the number of articles reporting FNR peaked in the year 2000 and declined at a rate similar to the ramp-up rate. There was a nonsignificant trend for the FNR to increase with the last time-period quartile, with the highest at 9.2 %. It is unclear whether the most current quartile truly represents the stabilized rate (~9 %) or whether the trend for even higher FNRs will continue.
We focused on injection materials and injection locations as the procedural variables most likely to impact the FNR. These are the variables that can be most easily modified to achieve the best results. There are too few cases available in the literature to make strong conclusions about how other variables impact the FNR, such as those related to institution, surgeon, and the patient. Injection materials were grouped into three categories: dye-only, tracer-only, and dye-and-tracer. There were insufficient cases to perform a more detailed analysis of materials within these categories. Figure 2 shows that dye-only had the highest FNR of the three categories. No difference was observed between tracer-only and dye-and-tracer. These data support the inclusion of tracer when performing SN biopsy in order to achieve the lowest possible FNR.
Injection locations were grouped into seven categories: four single-site injection locations and three multiple-site injection locations. There were no statistically significant variations among these seven location categories. Intratumor injections had the lowest numerical FNR, but there were too few cases to establish that rate as statistically significant. Intradermal-only injections also had a low FNR, but fewer cases resulted in an even wider confidence interval range. Despite the trends, no location stands out as a compelling choice to achieve the lowest FNR. In the absence of such information, the surgeon may consider other factors such as convenience or success rate when choosing the injection location.
More detailed analysis based on material injected and injection location allowed 17 groups of patients to be identified. The results from this more detailed analysis provided no new conclusions. When compared within individual injection materials, location did not make a difference in the FNR. Interestingly, injection material made a difference only when it was injected around the tumor and intradermally.
The results of this meta-analysis are limited to the cases available in the published literature. As a consequence, the potential for publication bias may exist due to the use of only English language citations in peer-reviewed journals. In addition, since many small studies may be prone to reporting of small FNRs and a true FNR of zero would be extremely unlikely in practice, the sensitivity analysis conducted excluded citations with FNR = 0. The subsequent analysis of this reduced set of citations did not impact on the basic conclusions based upon the full set of citations. Other limitations relate to results for the injection sites. Here, intratumor injection (with intradermal injection close behind) may have the lowest FNR, but there are insufficient cases to determine this with certainty. The available data do not support any one injection location over another. There may also be a persistent trend of higher false-negative cases over time. This seemingly increasing trend, however, is not likely ever to be confirmed since at this point confirmatory ANR is not tenable.
This meta-analysis was restricted to axillary nodes and the status of nonaxillary nodes was not considered. There is considerable variation between the different SN biopsy methods to identify nonaxillary SNs. For example, intratumoral or deep tracer injections identify nonaxillary nodes in 15 % or more of patients. About 1 % of clinically node-negative patients have extra-axillary metastases when there are no axillary metastases [12, 13]. Ignoring nonaxillary nodes means that about 1 % of SN metastases will be missed. An interesting question is whether this 1 % of missed SN metastases should be added to the FNR of methods that are restricted to the axilla.
These results also are relevant to training and quality control. The number of cases needed to assess performance accuracy of an individual surgeon cannot be based on that surgeon’s FNR. This has always been true, given the large number of cases necessary to establish an individual surgeon’s FNR. Now, without the ability to perform confirmatory ANR, it will be impossible to determine a surgeon’s FNR. For the same reasons, long-term surgeon performance also cannot be pegged to a FNR. Other metrics for gauging surgeon performance of SN biopsy will need to be established.
In conclusion, the results of this meta-analysis indicate that the material injected had an impact on FNR and that blue dye alone was associated with the highest FNR. Location of injection did not have a significant impact on FNR, indicating that any of the reported locations will achieve a similar FNR. There was a trend for higher FNRs over time and FNR for the population of surgeons currently performing this procedure is unknown. Lastly, FNR should not be used as a training or quality control metric.
References
Krag DN et al (2010) Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol 11(10):927–933
Tanis PJ et al (2002) The illusion of the learning phase for lymphatic mapping. Ann Surg Oncol 9(2):142–147
Cox CE et al (2000) Lymphatic mapping and sentinel lymph node biopsy in patients with breast cancer. Annu Rev Med 51:525–542
Fraile M et al (2000) Sentinel node biopsy as a practical alternative to axillary lymph node dissection in breast cancer patients: an approach to its validity. Ann Oncol 11(6):701–705
Kim T, Giuliano AE, Lyman GH (2006) Lymphatic mapping and sentinel lymph node biopsy in early-stage breast carcinoma: a metaanalysis. Cancer 106(1):4–16
McMasters KM et al (1998) Sentinel-lymph-node biopsy for breast cancer: not yet the standard of care. N Engl J Med 339(14):990–995
Miltenburg DM et al (1999) Meta-analysis of sentinel lymph node biopsy in breast cancer. J Surg Res 84(2):138–142
Orr RK, Hoehn JL, Col NF (1999) The learning curve for sentinel node biopsy in breast cancer: practical considerations. Arch Surg 134(7):764–767
Dixon W, Massey F (1969) Introduction to statistical analysis, 3rd edn. McGraw-Hill, New York
Borenstein M et al (2009) Introduction to meta-analysis. Wiley, Chichester
Anan K et al (2006) Double mapping with subareolar blue dye and peritumoral green dye injections decreases the false-negative rate of dye-only sentinel node biopsy for early breast cancer: 2-site injection is more accurate than 1-site injection. Surgery 139(5):624–629
Heuts EM et al (2009) Results of tailored treatment for breast cancer patients with internal mammary lymph node metastases. Breast 18(4):254–258
Heuts EM et al (2009) Internal mammary lymph drainage and sentinel node biopsy in breast cancer—a study on 1008 patients. Eur J Surg Oncol 35(3):252–257
Giuliano AE et al (1994) Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg 220(3):391–398 discussion 398–401
Giuliano AE et al (1997) Sentinel lymphadenectomy in breast cancer. J Clin Oncol 15(6):2345–2350
Turner RR et al (1997) Histopathologic validation of the sentinel lymph node hypothesis for breast carcinoma. Ann Surg 226(3):271–276 discussion 276–278
Guenther JM, Krishnamoorthy M, Tan LR (1997) Sentinel lymphadenectomy for breast cancer in a community managed care setting. Cancer J Sci Am 3(6):336–340
Flett MM et al (1998) Sentinel node localization in patients with breast cancer. Br J Surg 85(7):991–993
Ratanawichitrasin A et al (1998) Experience with lymphatic mapping in breast cancer using isosulfan blue dye. J Womens Health 7(7):873–877
Kapteijn BA et al (1998) Identification and biopsy of the sentinel lymph node in breast cancer. Eur J Surg Oncol 24(5):427–430
Imoto S, Hasebe T (1999) Initial experience with sentinel node biopsy in breast cancer at the National Cancer Center Hospital East. Jpn J Clin Oncol 29(1):11–15
Cserni G (1999) Estimating the overlap between sentinel lymph nodes and axillary node samples in breast cancer. Pathol Oncol Res 5(2):129–133
Morgan A et al (1999) Initial experience in a community hospital with sentinel lymph node mapping and biopsy for evaluation of axillary lymph node status in palpable invasive breast cancer. J Surg Oncol 72(1):24–30 discussion 30–31
Bobin JY et al (1999) Tagging sentinel lymph nodes: a study of 100 patients with breast cancer. Eur J Cancer 35(4):569–573
Kern KA (1999) Sentinel lymph node mapping in breast cancer using subareolar injection of blue dye. J Am Coll Surg 189(6):539–545
Cserni G, Boross G, Baltas B (2000) Value of axillary sentinel nodal status in breast cancer. World J Surg 24(3):341–344. doi:10.1007/s002689910054 discussion 344
Motomura K et al (1999) Sentinel node biopsy guided by indocyanine green dye in breast cancer patients. Jpn J Clin Oncol 29(12):604–607
Canavese G et al (2000) Sentinel lymph node mapping in early-stage breast cancer: technical issues and results with vital blue dye mapping and radioguided surgery. J Surg Oncol 74(1):61–68
Altinyollar H et al (2000) Lymphatic mapping and sentinel lymphadenectomy in early stage breast carcinoma. J Exp Clin Cancer Res 19(2):141–144
Ilum L et al (2000) Sentinel node localization in breast cancer patients using intradermal dye injection. Acta Oncol 39(3):423–428
Rodier JF et al (2000) Lymphatic mapping and sentinel node biopsy of operable breast cancer. World J Surg 24(10):1220–1225. doi:10.1007/s002680010240. discussion 1225–1226
Noguchi M et al (1998) Dye-guided sentinel lymphadenectomy in clinically node-negative and node-positive breast cancer patients. Breast Cancer 5(4):381–387
Miyauchi M et al (1999) Computed tomography for preoperative evaluation of axillary nodal status in breast cancer. Breast Cancer 6(3):243–248
Motomura K et al (1999) Sentinel node biopsy in breast cancer patients with clinically negative lymph-nodes. Breast Cancer 6(3):259–262
Motomura K et al (2001) Combination technique is superior to dye alone in identification of the sentinel node in breast cancer patients. J Surg Oncol 76(2):95–99
Nos C et al (2001) Sentinel lymph node detection for breast cancer: which patients are best suited for the patent blue dye only method of identification? Ann Surg Oncol 8(5):438–443
Mokbel K, Mostafa A (2001) The role of subareolar blue dye in identifying the sentinel node in patients with invasive breast cancer. Curr Med Res Opin 17(2):93–95
Shimazu K et al (2002) Comparison between periareolar and peritumoral injection of radiotracer for sentinel lymph node biopsy in patients with breast cancer. Surgery 131(3):277–286
Cserni G et al (2002) Comparison of vital dye-guided lymphatic mapping and dye plus gamma probe-guided sentinel node biopsy in breast cancer. World J Surg 26(5):592–597. doi:10.1007/s00268-001-0274-2
Yu JC et al (2002) Sentinel node biopsy in early breast cancer in Taiwan. World J Surg 26(11):1365–1369. doi:10.1007/s00268-002-6390-9
Ozmen V et al (2002) Increased false negative rates in sentinel lymph node biopsies in patients with multi-focal breast cancer. Breast Cancer Res Treat 76(3):237–244
Nos C et al (2003) Macroscopic quality control improves the reliability of blue dye-only sentinel lymph node biopsy in breast cancer. Ann Surg Oncol 10(5):525–530
Aihara T, Takatsuka Y (2003) Dye-guided sentinel node biopsy revisited; validation and observational study from a single institute. Breast Cancer 10(3):254–259
Meyer-Rochow GY, Martin RC, Harman CR (2003) Sentinel node biopsy in breast cancer: validation study and comparison of blue dye alone with triple modality localization. ANZ J Surg 73(10):815–818
D’Eredita G et al (2003) Subareolar injection may be more accurate than other techniques for sentinel lymph node biopsy in breast cancer. Ann Surg Oncol 10(8):942–947
Shenoy V, Ravichandran D, Ralphs DN (2002) Is massage following dye injection necessary in sentinel node biopsy in breast cancer? Breast 11(3):273–274
Nour A (2004) Efficacy of methylene blue dye in localization of sentinel lymph node in breast cancer patients. Breast J 10(5):388–391
Radovanovic Z et al (2004) Blue dye versus combined blue dye-radioactive tracer technique in detection of sentinel lymph node in breast cancer. Eur J Surg Oncol 30(9):913–917
Langer S et al (2004) Lymphatic mapping improves staging and reduces morbidity in women undergoing total mastectomy for breast carcinoma. Am Surg 70(10):881–885
Deo S et al (2004) Sentinel lymph node biopsy assessment using intraoperative imprint cytology in breast cancer patients: results of a validation study. Asian J Surg 27(4):294–298
Peintinger F, Reitsamer R, Ralph G (2005) Implementation of sentinel lymph node biopsy with blue dye outside a specialized center: Can we improve quality assurance? Breast J 11(2):103–107
Ratanachaikanont T, Karalak A (2005) Sentinel node localization in breast cancer using intradermal dye injection: results, influencing factors and learning curve. J Med Assoc Thai 88(3):386–390
Gui GP et al (2005) Continued axillary sampling is unnecessary and provides no further information to sentinel node biopsy in staging breast cancer. Eur J Surg Oncol 31(7):707–714
Asoglu O et al (2005) The role of sentinel lymph node biopsy with blue dye alone in breast cancer patients with excisional biopsy. Acta Chir Belg 105(3):291–296
Kanwal S, Hussain R (2005) Sentinel lymph node biopsy in early carcinoma breast with blue dye. J Coll Physicians Surg Pak 15(8):515–516
Gurleyik G et al (2005) Accuracy of sentinel lymph node biopsy for the assessment of axillary status in patients with early (T1) breast carcinoma. J Coll Physicians Surg Pak 15(11):697–700
Hung WK et al (2005) Randomized clinical trial comparing blue dye with combined dye and isotope for sentinel lymph node biopsy in breast cancer. Br J Surg 92(12):1494–1497
D’Eredita G et al (2006) A further validation of subareolar injection technique for breast sentinel lymph node biopsy. Ann Surg Oncol 13(5):701–707
Lamichhane N et al (2007) Initial experience in a cancer hospital in Nepal with sentinel lymph node mapping and biopsy for evaluation of axillary lymph node status in palpable invasive breast cancer. Nepal Med Coll J 9(1):22–26
Soares CT, Frederigue-Junior U, Luca LA (2007) Anatomopathological analysis of sentinel and nonsentinel lymph nodes in breast cancer: hematoxylin-eosin versus immunohistochemistry. Int J Surg Pathol 15(4):358–368
Kavallaris A, Camara O, Runnebaum IB (2008) Subareolar blue dye only injection sentinel lymph node biopsy could reduce the numbers of standard axillary lymph node dissection in environments without access to nuclear medicine. J Cancer Res Clin Oncol 134(6):667–672
Koukouraki S et al (2009) Is there any benefit from sentinel lymph node biopsy using the combined radioisotope/dye technique in breast cancer patients with clinically negative axilla? Nucl Med Commun 30(1):48–53
Hirche C et al (2010) ICG fluorescence-guided sentinel node biopsy for axillary nodal staging in breast cancer. Breast Cancer Res Treat 121(2):373–378
Yamashita K, Shimizu K (2009) Evaluation of sentinel lymph node metastasis alone guided by three-dimensional computed tomographic lymphography in video-assisted breast surgery. Surg Endosc 23(3):633–640
Chintamani et al (2011) Sentinel lymph node biopsy using dye alone method is reliable and accurate even after neo-adjuvant chemotherapy in locally advanced breast cancer—a prospective study. World J Surg Oncol 9:19. doi:10.1186/1477-7819-9-19
Albertini JJ et al (1996) Lymphatic mapping and sentinel node biopsy in the patient with breast cancer. JAMA 276(22):1818–1822
Borgstein PJ, Meijer S, Pijpers R (1997) Intradermal blue dye to identify sentinel lymph-node in breast cancer. Lancet 349(9066):1668–1669
Barnwell JM et al (1998) Sentinel node biopsy in breast cancer. Ann Surg Oncol 5(2):126–130
O’Hea BJ et al (1998) Sentinel lymph node biopsy in breast cancer: initial experience at Memorial Sloan-Kettering Cancer Center. J Am Coll Surg 186(4):423–427
Nwariaku FE et al (1998) Sentinel lymph node biopsy, an alternative to elective axillary dissection for breast cancer. Am J Surg 176(6):529–531
Cox CE et al (1998) Sentinel lymphadenectomy: A safe answer to less axillary surgery? Recent Results Cancer Res 152:170–179
Hill AD et al (1999) Lessons learned from 500 cases of lymphatic mapping for breast cancer. Ann Surg 229(4):528–535
Carcoforo P et al (1999) Lymphatic mapping for the dissection of the sentinel node in the treatment of breast carcinoma. J Exp Clin Cancer Res 18(1):75–77
Rehman S et al (1999) Sentinel lymph node mapping for staging breast cancer: preliminary results of a prospective study. Md Med J 48(3):105–110
Burak WE Jr et al (1999) Routine preoperative lymphoscintigraphy is not necessary prior to sentinel node biopsy for breast cancer. Am J Surg 177(6):445–449
Jaderborg JM et al (1999) The feasibility and accuracy of the sentinel lymph node biopsy for breast carcinoma. Am Surg 65(8):699–703 discussion 704–705
Bass SS et al (1999) The role of sentinel lymph node biopsy in breast cancer. J Am Coll Surg 189(2):183–194
Eary JF et al (1999) Sentinel lymph node mapping for breast cancer: analysis in a diverse patient group. Radiology 213(2):526–529
Kollias J et al (1999) Reliability of sentinel node status in predicting axillary lymph node involvement in breast cancer. Med J Aust 171(9):461–465
Molland JG, Dias MM, Gillett DJ (2000) Sentinel node biopsy in breast cancer: results of 103 cases. Aust N Z J Surg 70(2):98–102
Reintgen D et al (1997) The role of selective lymphadenectomy in breast cancer. Cancer Control 4(3):211–219
Doting MH et al (2000) Lymphatic mapping with intralesional tracer administration in breast carcinoma patients. Cancer 88(11):2546–2552
Borgstein PJ et al (2000) Functional lymphatic anatomy for sentinel node biopsy in breast cancer: echoes from the past and the periareolar blue method. Ann Surg 232(1):81–89
Mariani G et al (2000) Mapping sentinel lymph node in breast cancer by combined lymphoscintigraphy, blue-dye, and intraoperative gamma-probe. Cancer Biother Radiopharm 15(3):245–252
Lauridsen MC et al (2000) Sentinel lymph node biopsy in breast cancer—the Aarhus experience. Acta Oncol 39(3):421–422
Villa G et al (2000) Localization of the sentinel lymph node in breast cancer by combined lymphoscintigraphy, blue dye and intraoperative gamma probe. Tumori 86(4):297–299
Tsugawa K et al (2000) Dye- and gamma probe-guided sentinel lymph node biopsy in breast cancer patients: using patent blue dye and technetium-99m-labeled human serum albumin. Breast Cancer 7(1):87–94
Ikeda T et al (2000) Sentinel lymph node biopsy using tin colloid RI and blue dye method. Breast Cancer 7(4):284–286
Tafra L et al (2001) Multicenter trial of sentinel node biopsy for breast cancer using both technetium sulfur colloid and isosulfan blue dye. Ann Surg 233(1):51–59
Mann BG et al (2000) High incidence of micrometastases in breast cancer sentinel nodes. Aust N Z J Surg 70(11):786–790
Smith LF, Cross MJ, Klimberg VS (2000) Subareolar injection is a better technique for sentinel lymph node biopsy. Am J Surg 180(6):434–437 discussion 437–438
Boolbol SK et al (2001) Intradermal isotope injection: a highly accurate method of lymphatic mapping in breast carcinoma. Ann Surg Oncol 8(1):20–24
Hodgson N et al (2001) A new radiocolloid for sentinel node detection in breast cancer. Ann Surg Oncol 8(2):133–137
Frisell J et al (2001) Sentinel node in breast cancer—a Swedish pilot study of 75 patients. Eur J Surg 167(3):179–183
Dupont E et al (2001) Learning curves and breast cancer lymphatic mapping: institutional volume index. J Surg Res 97(1):92–96
McMasters KM et al (2001) Dermal injection of radioactive colloid is superior to peritumoral injection for breast cancer sentinel lymph node biopsy: results of a multiinstitutional study. Ann Surg 233(5):676–687
Canavese G et al (2001) Technical issues and pathologic implications of sentinel lymph node biopsy in early-stage breast cancer patients. J Surg Oncol 77(2):81–87 discussion 88
Smillie T et al (2001) Evaluation of feasibility and accuracy of sentinel node biopsy in early breast cancer. Am J Surg 181(5):427–430
Sabel MS et al (2001) Accuracy of sentinel node biopsy in predicting nodal status in patients with breast carcinoma. J Surg Oncol 77(4):243–246
Sato K et al (2001) Management of the axilla in breast cancer: a comparative study between sentinel lymph node biopsy and four-node sampling procedure. Jpn J Clin Oncol 31(7):318–321
Martin RC et al (2001) Intradermal isotope injection is superior to intramammary in sentinel node biopsy for breast cancer. Surgery 130(3):432–438
Xavier NL et al (2001) Sentinel lymph node identification and sampling in women with early breast cancer using 99mTc labelled dextran 500 and patent blue V dye. Nucl Med Commun 22(10):1109–1117
Kim R et al (2001) Significance of lymphoscintigraphic mapping with Tc-99m human serum albumin and tin colloid in sentinel lymph node biopsy in breast cancer. Int J Oncol 19(5):991–996
Tavares MG et al (2001) The use of 99mTc-phytate for sentinel node mapping in melanoma, breast cancer and vulvar cancer: a study of 100 cases. Eur J Nucl Med 28(11):1597–1604
Donahue EJ (2001) Sentinel node imaging and biopsy in breast cancer patients. Am J Surg 182(4):426–428
Manecksha R et al (2001) Value of sentinel node biopsy in the management of breast cancer. Ir J Med Sci 170(4):233–235
Euhus DM et al (2002) Validating the performance of the mammary sentinel lymph node team. J Surg Oncol 79(4):209–215
Shivers S et al (2002) Final results of the department of defense multicenter breast lymphatic mapping trial. Ann Surg Oncol 9(3):248–255
Lloyd LR, Wesen CA, McCallum S (2002) An analysis of filtration and volume of radionucleotide in sentinel lymph node biopsy in breast cancer patients. Am Surg 68(4):373–375 discussion 375–376
Sardi A et al (2002) The benefit of using two techniques for sentinel lymph node mapping in breast cancer. Am Surg 68(1):24–28
Motomura K et al (2002) Patient age affects identification rate of sentinel nodes in breast cancer. Biomed Pharmacother 56(Suppl 1):209s–212s
Motomura K et al (2003) Intradermal radioisotope injection is superior to subdermal injection for the identification of the sentinel node in breast cancer patients. J Surg Oncol 82(2):91–96 discussion 96–97
Koizumi M et al (2003) Sentinel node detection using 99mTc-rhenium sulphide colloid in breast cancer patients: evaluation of 1 day and 2 day protocols, and a dose-finding study. Nucl Med Commun 24(6):663–670
Classe JM et al (2003) Learning curve for the detection of axillary sentinel lymph node in breast cancer. Eur J Surg Oncol 29(5):426–433
Yong WS et al (2003) Single institution’s initial experience with sentinel lymph node biopsy in breast cancer patients. ANZ J Surg 73(6):416–421
Hoar FJ, Stonelake PS (2003) A prospective study of the value of axillary node sampling in addition to sentinel lymph node biopsy in patients with breast cancer. Eur J Surg Oncol 29(6):526–531
Mahajna A et al (2003) Sentinel lymph node biopsy in early breast cancer: the first 100 cases performed in a teaching institute. Isr Med Assoc J 5(8):556–559
Kennedy RJ et al (2003) Removal of two sentinel nodes accurately stages the axilla in breast cancer. Br J Surg 90(11):1349–1353
Hughes M et al (2004) Obesity and lymphatic mapping with sentinel lymph node biopsy in breast cancer. Am J Surg 187(1):52–57
McIntosh SA et al (2001) Sentinel lymph node biopsy in impalpable breast cancer. Breast 10(1):82–83
Snoj M et al (2003) Added value of blue dye in sentinel node biopsy for breast cancer. J Exp Clin Cancer Res 22(4):551–555
Lauridsen MC et al (2004) Sentinel lymph node biopsy in breast cancer—experience with the combined use of dye and radioactive tracer at Aarhus University Hospital. Acta Oncol 43(1):20–26
Peley G et al (2004) Subareolar injection of radioactive colloid for sentinel lymph node identification in breast cancer patients. Am Surg 70(7):625–629
van Wessem KJ, Meijer WS (2004) Sentinel lymph node biopsy in breast cancer: results of intradermal periareolar tracer injection and follow-up of sentinel lymph node-negative patients. Breast 13(4):290–296
Goyal A, Newcombe RG, Mansel RE (2005) Clinical relevance of multiple sentinel nodes in patients with breast cancer. Br J Surg 92(4):438–442
Knox SM, Ley CA (2002) Comparison of intraparenchymal and intradermal injection for identification of the sentinel node in patients with breast cancer. Proc (Bayl Univ Med Cent) 15(4):366–368
Krag DN et al (2007) Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol 8(10):881–888
Canavese G et al (2009) Sentinel node biopsy compared with complete axillary dissection for staging early breast cancer with clinically negative lymph nodes: results of randomized trial. Ann Oncol 20(6):1001–1007
Moghimi M et al (2009) Accuracy of sentinel node biopsy in breast cancer patients with a high prevalence of axillary metastases. Scand J Surg 98(1):30–33
Nakashima K et al (2010) Preoperative dynamic lymphoscintigraphy predicts sentinel lymph node metastasis in patients with early breast cancer. Breast Cancer 17(1):17–21
Cheung TT, Suen DT, Kwong A (2009) Is sentinel lymph node biopsy after neoadjuvant chemotherapy feasible in Chinese patients with invasive breast cancers? ANZ J Surg 79(10):719–723
Nason KS et al (2000) Increased false negative sentinel node biopsy rates after preoperative chemotherapy for invasive breast carcinoma. Cancer 89(11):2187–2194
Martin RC 2nd et al (2000) Practical guidelines for optimal gamma probe detection of sentinel lymph nodes in breast cancer: results of a multi-institutional study. For the University of Louisville Breast Cancer Study Group. Surgery 128(2):139–144
de Kanter AY et al (2000) Controlled introduction of the sentinel node biopsy in breast cancer in a multi-centre setting: the role of a coordinator for quality control. Eur J Surg Oncol 26(7):652–656
Yalcin H et al (2010) Comparison of efficacy of Tc-99m HIG and Tc-99m nanocolloid on sentinel lymph node mapping in patients with breast cancer. Nucl Med Commun 31(10):903–909
Dalus K et al (2011) Lymphoscintigraphy in breast cancer patients after neoadjuvant chemotherapy. Diagnostic value and the work-up of sentinel node negative patients. Nuklearmedizin 50(1):33–38
Krag DN et al (1993) Surgical resection and radiolocalization of the sentinel lymph node in breast cancer using a gamma probe. Surg Oncol 2(6):335–339 discussion 340
Pijpers R et al (1997) Impact of lymphoscintigraphy on sentinel node identification with technetium-99m-colloidal albumin in breast cancer. J Nucl Med 38(3):366–368
Veronesi U et al (1997) Sentinel-node biopsy to avoid axillary dissection in breast cancer with clinically negative lymph-nodes. Lancet 349(9069):1864–1867
Roumen RM, Valkenburg JG, Geuskens LM (1997) Lymphoscintigraphy and feasibility of sentinel node biopsy in 83 patients with primary breast cancer. Eur J Surg Oncol 23(6):495–502
Borgstein PJ et al (1998) Sentinel lymph node biopsy in breast cancer: guidelines and pitfalls of lymphoscintigraphy and gamma probe detection. J Am Coll Surg 186(3):275–283
Offodile R et al (1998) Minimally invasive breast carcinoma staging using lymphatic mapping with radiolabeled dextran. Cancer 82(9):1704–1708
Miner TJ et al (1998) Ultrasonographically guided injection improves localization of the radiolabeled sentinel lymph node in breast cancer. Ann Surg Oncol 5(4):315–321
Crossin JA et al (1998) Gamma-probe-guided resection of the sentinel lymph node in breast cancer. Am Surg 64(7):666–668 discussion 669
Gulec SA et al (1998) Sentinel lymph node localization in early breast cancer. J Nucl Med 39(8):1388–1393
Krag D et al (1998) The sentinel node in breast cancer—a multicenter validation study. N Engl J Med 339(14):941–946
Snider H et al (1998) Sentinel node biopsy in the staging of breast cancer. Am J Surg 176(4):305–310
Rubio IT et al (1998) Sentinel lymph node biopsy for staging breast cancer. Am J Surg 176(6):532–537
Feldman SM et al (1999) Limitation in gamma probe localization of the sentinel node in breast cancer patients with large excisional biopsy. J Am Coll Surg 188(3):248–254
Winchester DJ et al (1999) Sentinel lymphadenectomy for breast cancer: experience with 180 consecutive patients: efficacy of filtered technetium 99m sulphur colloid with overnight migration time. J Am Coll Surg 188(6):597–603
Miner TJ et al (1999) Sentinel lymph node biopsy for breast cancer: the role of previous biopsy on patient eligibility. Am Surg 65(6):493–498 discussion 498–499
Bembenek A et al (1999) Sentinel lymph node dissection in breast cancer. Swiss Surg 5(5):217–221
Moffat FL Jr et al (1999) Unfiltered sulfur colloid and sentinel node biopsy for breast cancer: technical and kinetic considerations. Ann Surg Oncol 6(8):746–755
Zurrida S et al (2000) Radioguided sentinel node biopsy to avoid axillary dissection in breast cancer. Ann Surg Oncol 7(1):28–31
Weaver DL et al (2000) Pathologic analysis of sentinel and nonsentinel lymph nodes in breast carcinoma: a multicenter study. Cancer 88(5):1099–1107
Kowolik JH et al (2000) Detection of micrometastases in sentinel lymph nodes of the breast applying monoclonal antibodies AE1/AE3 to pancytokeratins. Oncol Rep 7(4):745–749
Casalegno PS et al (2000) Sentinel lymph node and breast cancer staging: final results of the Turin Multicenter Study. Tumori 86(4):300–303
Gucciardo G et al (2000) The success rate of identification of the sentinel lymph node in breast cancer: our training series. Tumori 86(4):312–313
Galli A et al (2000) Study of sentinel lymph node in breast cancer: our experience in 46 cases. Tumori 86(4):317–319
Mechella M et al (2000) A study of sentinel node biopsy in T1 breast cancer treatment: experience of 48 cases. Tumori 86(4):320–321
Paolo de Rubeis G et al (2000) The sentinel lymph node in T1N0 breast cancer. Tumori 86(4):327–328
Hsieh PP et al (2000) Histopathologic analysis of sentinel lymph nodes in breast carcinoma. Zhonghua Yi Xue Za Zhi (Taipei) 63(10):744–750
Sato K et al (1998) Efficacy and significance of sentinel lymph node identification with technetium-99m-labeled tin colloids for breast cancer. Breast Cancer 5(4):389–393
Rink T et al (2001) Lymphoscintigraphic sentinel node imaging and gamma probe detection in breast cancer with Tc-99 m nanocolloidal albumin: results of an optimized protocol. Clin Nucl Med 26(4):293–298
Watanabe T et al (2001) Sentinel node biopsy with technetium-99m colloidal rhenium sulphide in patients with breast cancer. Br J Surg 88(5):704–707
Krag DN et al (2001) Radiolabeled sentinel node biopsy: collaborative trial with the National Cancer Institute. World J Surg 25(6):823–828. doi:10.1007/s00268-001-0012-9
Rink T et al (2001) Results of a standardized protocol for sentinel node imaging in breast cancer with Tc-99m labeled nanocolloidal albumin. Nuklearmedizin 40(3):80–85
Ishida M et al (2002) Detection of micrometastasis in the sentinel lymph nodes in breast cancer. Surgery 131(1 Suppl):S211–S216
Quan ML et al (2002) Biology of lymphatic metastases in breast cancer: lessons learned from sentinel node biopsy. Ann Surg Oncol 9(5):467–471
Fonti R et al (2002) Sentinel lymph node identification in breast cancer patients. Radiol Med 103(4):370–377
Xu M et al (2002) Sentinel lymph nodes lymphoscintigraphy and biopsy in breast cancer. Chin Med J (Engl) 115(8):1137–1140
Mariotti S et al (2002) Minimal sentinel node procedure for staging early breast cancer. Tumori 88(3):S45–S47
Fernandez A et al (2002) Sentinel node localization in patients with non-palpable breast cancer. Nucl Med Commun 23(12):1165–1169
Maza S et al (2003) Peritumoural versus subareolar administration of technetium-99m nanocolloid for sentinel lymph node detection in breast cancer: preliminary results of a prospective intra-individual comparative study. Eur J Nucl Med Mol Imaging 30(5):651–656
Bourgeois P et al (2003) How ‘hot’ is the pathologically positive sentinel lymph node in breast cancer patients? Nucl Med Commun 24(5):513–518
Pargaonkar AS et al (2003) Evaluation of immunohistochemistry and multiple-level sectioning in sentinel lymph nodes from patients with breast cancer. Arch Pathol Lab Med 127(6):701–705
Veronesi U et al (2003) A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med 349(6):546–553
Zavagno G et al (2000) Sentinel node biopsy in breast cancer. Breast 9(3):139–143
Ellis RL et al (2004) Periareolar injection for localization of sentinel nodes in breast cancer patients. Breast J 10(2):94–100
Lamichhane N et al (2004) Sentinel lymph node biopsy in breast cancer patients after overnight migration of radiolabelled sulphur colloid. Postgrad Med J 80(947):546–550
Classe JM et al (2005) Prospective comparison of 3 gamma-probes for sentinel lymph node detection in 200 breast cancer patients. J Nucl Med 46(3):395–399
Kebudi A et al (2005) The safety and accuracy of sentinel-node biopsy in early-stage invasive breast cancer: Turkish experience. J Invest Surg 18(3):129–134
Bajen MT et al (2006) Subdermal re-injection: a method to increase surgical detection of the sentinel node in breast cancer without increasing the false-negative rate. Eur J Nucl Med Mol Imaging 33(3):338–343
Namwongprom S et al (2005) Breast lymphoscintigraphy for sentinel node identification in breast cancers with clinically-negative axillary nodes. Singapore Med J 46(12):688–692
Lo YF et al (2006) Radioguided sentinel lymph node biopsy in early breast cancer: experience at Chang Gung Memorial Hospital. Chang Gung Med J 29(5):458–467
Song XY et al (2007) Different criteria for radioactive sentinel lymph nodes has different impact on sentinel node biopsy in breast cancer patients. J Surg Oncol 95(8):635–639
Yen RF et al (2007) Radio-guided sentinel lymph node biopsy using periareolar injection technique for patients with early breast cancer. J Formos Med Assoc 106(1):44–50
Zavagno G et al (2008) Clinical impact of false-negative sentinel lymph nodes in breast cancer. Eur J Surg Oncol 34(6):620–625
Seok JW et al (2010) Sentinel lymph node identification with radiopharmaceuticals in patients with breast cancer: a comparison of 99mTc-tin colloid and 99mTc-phytate efficiency. Breast Cancer Res Treat 122(2):453–457
Veronesi U et al (2010) Sentinel lymph node biopsy in breast cancer: ten-year results of a randomized controlled study. Ann Surg 251(4):595–600
Seok JW et al (2009) Comparison of subareolar injection lymphoscintigraphy with the 1-day and the 2-day protocols for the detection of sentinel lymph nodes in patients with breast cancer. Ann Nucl Med 23(5):465–469
Mudun A et al (2008) Comparison of different injection sites of radionuclide for sentinel lymph node detection in breast cancer: single institution experience. Clin Nucl Med 33(4):262–267
Gimbergues P et al (2008) Sentinel lymph node biopsy after neoadjuvant chemotherapy is accurate in breast cancer patients with a clinically negative axillary nodal status at presentation. Ann Surg Oncol 15(5):1316–1321
Acknowledgments
This study was funded by grant 4R01CA074137-12, Sentinel Node Versus Axillary Dissection in Breast Cancer.
Conflict of interest
Dr. David Krag has a financial interest in treeofmedicine.com. Sarah Pesek, Taka Ashikaga, and Lars Erik Krag have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pesek, S., Ashikaga, T., Krag, L.E. et al. The False-Negative Rate of Sentinel Node Biopsy in Patients with Breast Cancer: A Meta-Analysis. World J Surg 36, 2239–2251 (2012). https://doi.org/10.1007/s00268-012-1623-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-012-1623-z