Abstract
Background
Surgical procedures are related to the activation of the inflammatory reaction. This is called surgical stress. It is believed that diminished surgical trauma reduces surgical stress. The laparoscopic approach reduces trauma, but the systemic immune responses are still invariably activated. Cytokines and C-reactive protein (CRP) are the main markers in the study of inflammatory or stress response. α-Defensins play an important role in host defense, acting early in phagocytosis. α-Defensins, as early markers—earlier than cytokines—of the inflammatory response, have been used, together with high-sensitivity CRP (hs-CRP) and interleukin-6 (IL-6), to determine the inflammatory response in laparoscopic and open colectomy for cancer.
Materials and methods
A total of 40 patients with colorectal cancer were randomized to two groups: group A (n = 20), open colectomy; group B (n = 20), laparoscopic colectomy. One hour preoperatively an epidural catheter was placed in all patients and rupivacaine was administered perioperatively and again 48 h postoperatively. Blood samples were taken for calculating α-defensins, IL-6, and hs-CRP levels preoperatively, 5 min after division of the colon (group A), or 5 min after deflation of pneumoperitoneum (group B), 6 h and 24 h postoperatively.
Results
The mean operative time was 115 min for group A and 142 min for group B (p < 0.05). The mean blood loss was 240 ml and 105 ml, respectively (p < 0.001). The mean hospital stay was 8 days and 5 days, respectively (p < 0.05). α-Defensin levels were statistically significantly lower in group B than in group A, 5 min and 24 h postoperatively (p < 0.002 and p < 0.007, respectively). The IL-6 levels were statistically significantly lower in group B than in group A, 6 h and 24 h postoperatively (p < 0.0001 at both time intervals), whereas the levels of hs-CRP were significantly lower in group B than in group A 24 h postoperatively (p < 0.001).
Conclusions
The present study confirms the results of previous studies, that the inflammatory immune response and surgical stress are significantly less after laparoscopic colectomy versus open colectomy for colorectal cancer. More investigation is needed to study if surgical stress has any influence on survival of these patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Surgical stress induces hormonal and cytokine response proportionally to the extent of the surgical injury [1, 2]. This stress response can go beyond its protective objectives and become harmful to the body, resulting in immunologic impairment, which increases postoperative morbidity [2].
Laparoscopic surgery provides tremendous benefits to patients, including faster recovery, shorter hospital stay, and prompt return to normal activities [3]. Additionally, laparoscopic surgery provides better cosmesis and greater patient satisfaction, resulting in greater patient demand for new less invasive procedures [3, 4].
While laparoscopy is “minimally invasive,” systemic immune responses are still invariably activated. Responses to surgery in general are reflected in terms of cytokine function and cellular messenger systems. While cytokine levels do not directly reflect immune status, they provide a framework for understanding systemic immunity in terms of underlying immune activations [3]. Cytokines, as interleukin-6 (IL-6), are thought to play a pivotal role in the pathogenesis of surgical trauma. Increased cytokine levels are believed to correlate with the magnitude of surgical trauma [5, 6].
C-reactive protein (CRP) is a liver-derived acute phase protein that serves as a nonspecific marker of an acute phase reaction caused by trauma or inflammation. The postoperative increment in serum CRP values can be used to monitor the magnitude of surgical trauma [7]. The calculation of the high-sensitivity C-reactive protein (hs-CRP) gives more precise results than CRP. A hs-CRP test using laser nephelometry measures low levels of CRP. The test gives results in 25 min with a sensitivity as low as 0.04 mg/l. So hs-CRP is CRP measured with a sensitive method.
Defensins, contained in Paneth cells, are a well-characterized family of antimicrobial peptides that are divided into two main classes, the α-defensins and the β-defensins, on the basis of their disulfide bond pairing pattern [8, 9]. α-Defensins, were first described as components of animal neutrophil granules, and four α-defensins (human neutrophil peptides; HNP 1–4) were subsequently discovered in human neutrophil granules [10].
α-Defensins are broadly effective against gram-positive and gram-negative bacteria, fungi, spirochetes, protozoa, and enveloped viruses [11]. It seems likely that α-defensins play an important role in host defense, acting early in phagocytosis. Paneth cell defensins are early markers—earlier than cytokines—and are therefore useful in studies of innate immunity [12].
In view of this information, we assumed that by determining the α-defensin levels, because they rise earlier than cytokines markers[9–12], we could draw more precise conclusions about the innate immune response system during colorectal surgery and, consequently, about the surgical stress in open and laparoscopic colectomy for colorectal cancer. This could be of importance, as there had been suggestions by Lacy et al. [13] that the survival rate after laparoscopic colectomy was better compared to open colectomy, due to less surgical stress in laparoscopic colectomy.
The present trial was designed to study and compare the inflammatory response during laparoscopic and open colectomy for colorectal cancer by determining the plasma levels of α-defensins as an early marker. The inflammatory response was also studied by measuring the plasma levels of hs-CRP, and IL-6, as standard markers of the stress response.
Materials and methods
Randomization
A total of 40 patients with a diagnosis of colorectal cancer were randomized to two groups of 20 patients each, using a closed envelope containing information regarding assignment to group A or group B. The criteria for exclusion, before the randomization, were: American Society of Anesthesiologists (ASA) physical status of 3 or more, body mass index (BMI) >30, anemia (Hb <11 g/dl), tumor stage T4, metastatic disease, or previous therapy for cancer. The first 40 patients not having any of the exclusion criteria were included in the study. Perioperative exclusion criteria were the conversion of laparoscopic to open procedure, concomitant abdominal diseases, or perioperative tumor stage more than the preoperative one. Patients with Hb = 11–12 g/dl (4 in the open group and 3 in the laparoscopic group) were treated preoperatively with administration of epoetin-a (subcutaneously) and ferrum (intravenously). In group A (n = 20), the patients underwent open colectomy, whereas in group B (n = 20), the patients underwent laparoscopic colectomy. All procedures were performed by the same group of surgeons at “G. Hatzikosta” General Hospital of Ioannina, Greece. All patients were informed of the planned interventional technique, and all of them provided written informed consent according to the principles of the Ethical Committee of “G. Hatzikosta” General Hospital.
Tumor staging
Preoperatively, patients underwent colonoscopy, computed tomography (CT), and in selected cases (rectal cancer) magnetic resonance imaging (MRI) for staging. On the day of hospital admission the surgeon was informed of whether the patient was enrolled in group A or group B (laparoscopic or open). One of the researchers was assigned to collect blood from the patient at the appropriate time intervals and to mark the sample vials with a serial number, starting with 1 for the first patient and ending with 40 for the last patient. The researcher who would analyze the blood samples, so as to calculate the α-defensins levels, IL-6, and the hs-CRP, was blinded to the type of operation and was given only the blood sample and its serial number.
Anesthesia
One hour prior to the operation, an epidural catheter was placed at 10th thoracic level and a bolus 10 ml of ropivacaine (Naropin) 7.5% was administered. During the operation, a solution of 4 ml morphine 0.03% and 116 ml ropivacaine 2% was placed in a Baxter pump with a steady flow of 5 ml/h, and this was administered perioperatively and for up to 48 h postoperatively [14]. During the whole period of epidural anesthesia, the patient was controlled by the anesthesiologist who had placed the catheter.
Blood sampling
Blood samples for determining the levels of α-defensins, IL-6, and hs-CRP were taken from these patients via a peripheral vein. Samples were taken at four different time intervals: (1) preoperatively, (2) 5 min after deflation of the pneumoperitoneum for the laparoscopic group or, for the open group, 5 min after the distal division of the colon in left colectomies, or the division of the small bowel in right colectomies; (3) 6 h postoperatively; and (4) 24 h postoperatively. At interval (2) in the laparoscopic group, the deflation in left colectomies was performed immediately after the distal division of the colon, whereas in right colectomies the deflation was performed immediately after the intraperitoneal division of the small bowel, resulting in the same time intervals as in the open group. The specimens then were exteriorized through a protected mini-laparotomy 3–6 cm in the right subcostal area or in the left iliac fossa (for right or left colectomy, respectively). In right colectomies, a side-to-side stapled anastomosis was performed extracorporeally after the division of the colon. In left colectomies, after the proximal division of the colon, the anvil of the circular stapler was inserted in the proximal colonic stump and secured with a purse-string suture extracorporeally, while a double-stapled anastomosis was performed intracorporeally after the bowel was returned to the abdominal cavity and the pneumoperitoneum was re-established. In the open group the anastomoses, in right and left colectomies, were performed by the same manner as in laparoscopic group. The samples taken at each time interval were marked “a” for preoperative, “b” for the 5-min sample, “c” for the 6-h postoperative sample, and “d” for the 24-h postoperative sample.
Measurement of α-defensins expression
α-Defensins levels in plasma were calculated by enzyme-linked immunosorbent assays (ELISA). The blood that had been collected was centrifuged at 2,500 rpm for 5 min. The plasma was then collected and placed in Cryovial vials (Simport, Canada) and then frozen at −82°C. These would be used for determining the levels of α-defensins. The levels of α-defensin were determined by enzyme-linked immunosorbent assay (ELISA) with the kit HC4014 (catalog no.; Hycult Biotech, Uden, the Netherlands), and the values were expressed in ng/ml.
Measurment of IL-6 and hs-CRP
At the same time intervals blood had been collected for determining IL-6 and hs-CRP levels. The levels of IL-6 in plasma were measured by ELISA with kits obtained from R&D Systems Inc. (Minneapolis, MN). Tests were performed according to the manufacturer’s instructions. Samples were tested in duplicate, and a standard curve with human recombinant cytokine was built in each plate. The sensitivity of ELISA was 0.5 pg/ml. High-sensitivity CRP was calculated in an automated analyzer, and the values were expressed in mg/dl.
Power analysis was performed with StudySize 2.04 for Mann–Whitney U-test. Setting p = 0.9 and α < 0.05, a sample of 20 patients was mandatory for each group. The outcomes of patients were compared between groups A and B and between the preoperative period and the postoperative period in each group. The differences were tested with the Mann–Whitney U-test, Student’s t-test, and the chi-square test, with a p value <0.05 being considered significant. SPSS version 15.0 was used throughout the statistical analysis (SPSS Inc., Chicago, IL).
Results
In group A, 9 patients were men and 11 were women, whereas in group B, the male/female ratio was 8/12 (p = 0.49). The demographic data are presented in Table 1. No statistical difference was found for age or sex between the two groups. In each group, 4 right colectomies and 16 left colectomies were performed. The mean operative time was 115 min (range: 85–152 min) in the open group (group A) and 142 min (range: 110–185 min) in the laparoscopic group (group B) (p < 0.05). The mean blood loss was 240 ± 124 ml (range: 65–380 ml) in group A and 105 ± 74 ml (mean 30–165 ml) in group B (p < 0.001). No blood or plasma infusion was used during the perioperative period.
No major intraoperative or postoperative complications developed in either group. The mean hospital stay was 8 ± 1.4 days (range: 7–12 days) in group A and 5 ± 1.1 days (range: 4–7 days) in group B (p < 0.05). No patients were excluded according the perioperative exclusion criteria, thus all 40 patients were included in the study.
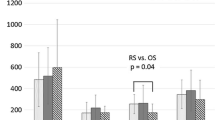
Expression of the α-defensins (Fig. 1) showed that in group A, at the 5-min and 24-h postoperative intervals, the levels were statistically significantly higher than in group B (p < 0.011 and p < 0.018, respectively, with Mann–Whitney U-test). The IL-6 results (Fig. 2) were significantly lower in group B than in group A, at the postoperative 6 h and 24 h intervals (p < 0.0001 at both time intervals). The analysis of the levels of hs-CRP (Fig. 3) showed significantly lower levels 24 h postoperatively in group B versus group A (p < 0.001).
Discussion
Laparoscopic colorectal surgery is as safe as the open approach. In addition to the ongoing discussion about the oncologic safety of the laparoscopic approach, it is not completely clear whether the laparoscopic approach offers significant immunological advantages over the conventional open approach[15–17]. Trauma induced by open surgery is substantially higher than with laparoscopic surgery, and it seems to be more aggravating to the immune system [18].
Recently Whelan et al. [19] found a better-preserved cell-mediated immune response in patients after laparoscopic colorectal surgery. Postoperative immune dysfunction is an important aspect of treatment for patients undergoing colorectal surgery for cancer, because, apart from the increase in infectious complications, immune dysfunction influences the growth of disseminated tumor cells and may lead to aggravated long-term oncologic results [20, 21].
A multicenter prospective randomized controlled trial with 872 patients, who underwent laparoscopy-assisted or open colectomy for cancer provides good evidence to suggest that the laparoscopic approach is an acceptable alternative to open surgery for colon cancer [22]. That study supports the results of Lacy et al. [13], who reported even better oncologic and clinical results of laparoscopic surgery in comparison with open surgery.
The systemic stress response is less affected after laparoscopic surgery than after conventional open surgery [18, 23]. This difference was found for cytokine and cell-mediated immune responses in both animal experiments and clinical trials [24–26]; in addition, serum CRP and IL-6 levels are appreciably lower after laparoscopy than after laparotomy [17, 18].
Cytokines, which until now have been the main markers in the study of the inflammatory response, are, in fact, not the first sensory-recognition receptors signaling the reaction of the stress response in the innate immune system; they are a result of stress, and they follow pattern-recognition receptors like the α-defensins, which are early markers, acting in phagocytosis [12, 27]. Phagocytosis in neutrophils and macrocytes follows from a cascade of reactions, leading to the production of cytokines. Thus, the α-defensins are earlier sensory-recognition receptors than the cytokines; they recognize the invading pathogens and initiate the innate immune response [9–12, 27]. Thus α-defensins, as early markers, are useful in the study of the inflammatory response, whereas levels of cytokines, which increase during the immune reactions, could be influenced by other parameters, changing the precise results of the innate immune response.
In the present study, the significant differences in serum levels of α-defensins between the groups was observed immediately after the end of the operative procedure, whereas the significant differences in IL-6 were first observed 6 h postoperatively. This difference between α-defensins and IL-6 may identify the earlier α-defensin plasma concentration as an earlier sensory-recognition receptor than IL-6 in the innate immune response.
A potential role for defensins in innate defense of the intestinal mucosa came with the discovery of an α-defensin in Paneth cell granules of the mouse small intestine [28]. Paneth cells, expressing a variety of rapidly acting microbicidal peptides, including human defensin-5 (HD-5), may thus play an important role in keeping the small intestine relatively free from bacteria so that nutrient absorption can take place effectively [10]. In chronic inflammatory bowel diseases, such as Crohn’s disease and ulcerative colitis, the colonic mucosal surface is breached and vulnerable to microbial penetration. Metaplastic Paneth cells expressing HD-5 are seen in the colonic epithelium affected by these conditions. It seems plausible that α-defensin secreting Paneth cells may be involved in protecting the host against microbial invasion across an already damaged epithelial surface [10]. In addition to enteric α-defensins, the neutrophil α-defensins HNP 1–3 are also expressed in intestinal epithelial cells [29], and they may play a role in mucosal defense after the loss of epithelial barrier integrity in inflammatory bowel disease [10].
Abnormal concentration of the α-defensins HNP 1–3 in blood has been demonstrated in connection with benign conditions, such as bacterial and nonbacterial infection [30], septicemia, and bacterial meningitis [31]. α-Defensins HNP 1–3 were found in elevated concentrations in blood after tumor growth [32]. Usually tumor-expressed peptides like α-defensins are not easily detected in serum or plasma. Perhaps the HNP 1–3 are detectable in serum only because they are expressed in exceptionally high amounts in the tumor microenvironment [32]. The high concentration of HNP 1–3 observed in tumors and the observation that HNP 1–3 are capable of lysing mammalian cells [33] may indicate that these peptides are of benefit to the host by killing tumor cells.
In the present study, the decreased serum levels of α-defensins immediately after the laparoscopic procedure, as compared with the preoperative levels, may reflect high levels of α-defensins in serum associated with the tumor microenvironment, which rapidly decrease after radical resection of the tumor. At the same time, in comparison with preoperative numbers, the decreased levels of α-defensins observed 5 min postoperatively in group B and the significantly increased levels noted in group A at the same time may reflect a significant difference in the surgical stress experienced by patients in the two groups. In group A the high rise in the α-defensin levels due to the stress response may neutralize the reduction in α-defensins associated with changes in the tumor microenvironment after radical resection of the tumor. This is a hypothesis that should be studied.
Defensins also regulate the systemic immune response through interaction with the chemokine receptor CCR6 [34] and are capable of recruiting leukocytes to the sites of infection [35]. Upregulated immune responses are known to stimulate tumor proliferation; immune cells are actively recruited by tumors to exploit their pro-angiogenic and pro-metastatic effects [36, 37].
The postoperative expression of α-defensins, IL-6, and hs-CRP present higher levels in open colectomy than in laparoscopic colectomy. This finding strengthens the idea that the laparoscopic approach, in surgical treatment of colorectal cancer, minimizes the surgical stress. This result may protect the immune responses leading to suppression of tumor proliferation [35–37].
There is an indication that laparoscopic colectomy in the treatment of cancer gives better oncologic and clinical results than open surgery [13, 22]. It is known that the laparoscopic approach minimizes the inflammatory response and then the surgical stress. There is an obvious question if this reduced surgical stress, which protects the immune response, is able to improve the oncologic results, leading to better survival of colorectal cancer patients treated by the laparoscopic approach than by open colectomy. Until now the answer is unclear, and well-scheduled, multicenter prospective randomized trials, with long-term follow-up may provide the answer. At present the only certainty is that laparoscopic colectomy for colorectal cancer minimizes surgical stress to a level significantly lower than that observed after open colectomy for cancer.
In conclusion, the present study confirms the results of previous studies, that both the inflammatory immune response and consequent surgical stress are significantly lower after laparoscopic colectomy than after open colectomy for colorectal cancer. The levels of α-defensins, an early marker of the innate immune response, give strong evidence for this conclusion. More investigation is needed to study if the surgical stress has any influence on survival of these patients.
References
Weissman C (1990) The metabolic response to stress: an overview and update. Anesthesiology 73:308–327
Chambier C, Chassard D, Bienvenu J et al (1996) Cytokine and hormonal changes after cholecystectomy. Ann Surg 224:178–182
Vittimberga FJ, Foley DP, Meyers WC et al (1998) Laparoscopic surgery and the systemic immune response. Ann Surg 227:326–334
Sawyers JL (1996) Current status of conventional (open) cholecystectomy versus laparoscopic cholecystectomy. Ann Surg 223:1–3
Allendorf JDF, Bessler M, Kayton ML et al (1995) Increased tumor establishment and growth after laparotomy vs laparoscopy in a murine model. Arch Surg 130:649–653
Hildebrandt U, Kessler K, Plusczyl T et al (2003) Comparison of surgical stress between laparoscopic and open colonic resections. Surg Endosc 17:242–246
Baigrie RJ, Lamont PM, Kwiatkowski D et al (1992) Systemic cytokine response after major surgery. Br J Surg 79:757–760
Nissen-Meyer J, Nes IF (1997) Ribosomally synthesized antimicrobial peptides: their function, structure, biogenesis, and mechanism of action. Arch Microbiol 167:67–77
Lehrer R, Bevins C, Ganz T (1999) Defensins and other antimicrobial peptides. In: Ogra P et al (eds) Mucosal immunology. Academic Press, New York, pp 89–99
Cunliffe RN (2003) α-Defensins in the gastrointestinal tract. Mol Immunol 40:463–467
Martin E, Ganz T, Lehrer RI (1995) Defensins and other endogenous peptide antibiotics of vertebrates. J Leukoc Biol 58:128–136
Ouellette AJ, Selsted ME (1996) Paneth cell defensins: endogenous peptide components of intestinal host defense. FASEB J 10:1280–1289
Lacy AM, Garcia-Valdecasas JC, Delgado S et al (2002) Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomized trial. Lancet 359:2224–2229
Turunen P, Carpelan-Holmström M, Kairaluoma P et al (2009) Epidural analgesia diminished pain bud did not otherwise improve enhanced recovery after laparoscopic sigmoidectomy: a prospective randomized study. Surg Endosc 23:31–37
Wichmann MW, Meyer G, Angele MK et al (2002) Recent advances in minimally invasive colorectal cancer surgery. Onkologie 25:318–323
Braga M, Vignali A, Gianotti L et al (2002) Laparoscopic versus open colorectal surgery: a randomized trial on short-term outcome. Ann Surg 236:759–767
Wichmann MW, Hüttl TP, Winter H et al (2005) Immunological effects of laparoscopic vs open colorectal surgery. Arch Surg 140:692–697
Buunen M, Gholghesaei M, Veldkamp R et al (2004) Stress response to laparoscopic surgery. Surg Endosc 18:1022–1028
Whelan RL, Franklin M, Holubar SD et al (2003) Postoperative cell mediated immune response is better preserved after laparoscopic vs open colorectal resection in humans. Surg Endosc 17:972–978
Salo M (1992) Effects of anaesthesia and surgery on the immune response. Acta Anaesthesiol Scand 36:201–220
Shigemitsu Y, Saito T, Kinoshita T et al (1992) Influence of surgical stress on bacterial activity of neutrophils and complications of infection in patients with esophageal cancer. J Surg Oncol 50:90–97
Clinical Outcomes of Surgical Therapy Study Group (2004) A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med 350:2050–2059
Gupta A, Watson DI (2001) Effect of laparoscopy on immune function. Br J Surg 88:1296–1306
Hajri A, Mutter D, Wack S et al (2000) Dual effect of laparoscopy on cell-mediated immunity. Eur Surg Res 32:261–266
Holub Z (2002) Impact of laparoscopic surgery on immune function. Clin Exp Obstet Gynecol 29:77–81
Wu FP, Cuesta MA, Sietses C (2001) Randomized clinical trial of the effect of open versus laparoscopically assisted colectomy on systemic immunity in patients with colorectal cancer. Br J Surg 88:801–807
Royet J, Dziarski R (2007) Peptidoglycan recognition proteins: pleiotropic sensors and effectors of antimicrobial defences. Nat Rev Microbiol 5:264–277
Quellette AJ, Greco RM, James M et al (1989) Development regulation of cryptdin, a corticostatin/defensin precursor mRNA in mouse small intestinal crypt epithelium. J Cell Biol 108:1687–1695
Cunliffe RN, Kamal M, Rose FR et al (2002) Expression of antimicrobial neutrophil defensins in epithelial cells of active inflammatory bowel disease mucosa. J Clin Pathol 55:298–304
Ihi T, Nakazato M, Mukae H et al (1997) Elevated concentrations of human neutrophil peptides in plasma blood, and body fluids from patients with infections. Clin Infect Dis 25:1134–1140
Panyutich AV, Panyutich EA, Krapivin VA et al (1993) Plasma defensin concentrations are elevated in patients with septicemia or bacterial meningitis. J Lab Clin Med 122:202–207
Alberhsen J, Bøgebo R, Gammeltoft S et al (2005) Upregulated expression of human neutrophil peptides 1, 2 and 3 (HNP 1–3) in colon cancer serum and tumours: a biomarker study. BMC Cancer 5:1–10
Lichtenstein A, Ganz T, Selsted ME et al (1986) In vitro tumor cell cytolysis mediated by peptide defensins of human and rabbit granulocytes. Blood 68:1407–1410
Yang D, Chertov O, Bykovskaia SN et al (1999) Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science 286:525–528
Welling MM, Hiemstra PS, van den Barselaar MT et al (1998) Antibacterial activity of human neutrophil defensins in experimental infections in mice is accompanied by increased leucocyte accumulation. J Clin Invest 102:1583–1590
Balkwill F, Mantovani A (2001) Inflammation and cancer: back to Virchow? Lancet 357:539–545
Van Kempen LC, Ruiter DJ, van Muijen GN et al (2003) The tumor microenvironment: a critical determinant of neoplastic evolution. Eur J Cell Biol 82:539–548
Acknowledgments
The authors are grateful to Professor Charalampos Moutsopoulos, Chair, Pathologic Physiology, Athens University, School of Medicine, for help in defensins knowledge. This work is identified as Registered Clinical Trial number NCT00942461 at www.clinicaltrials.gov.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsimogiannis, K.E., Telis, K., Tselepis, A. et al. α-Defensin Expression of Inflammatory Response in Open and Laparoscopic Colectomy for Colorectal Cancer. World J Surg 35, 1911–1917 (2011). https://doi.org/10.1007/s00268-011-1140-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-011-1140-5