Abstract
Background
Lymph node metastasis is considered one of the most important prognostic factors in gastric cancer. However, the optimal system for accurate staging of lymph node metastasis for patients with gastric cancer remains controversial. This study was designed to investigate the prognostic significance of the metastatic lymph node ratio (MLR), which is calculated by dividing the number of metastatic lymph nodes by the total number of nodes harvested from patients with gastric cancer.
Methods
We retrospectively analyzed the clinical data of 186 consecutive patients diagnosed with gastric cancer who underwent curative gastrectomy at our hospital. The lymph node status was classified according to three systems: the International Union Against Cancer/American Joint Committee on Cancer (UICC/AJCC) system; the Japanese Gastric Cancer Association (JGCA) system; and an MLR-based system (MLR0: 0, MLR1: 0.01–0.19, MLR2: ≥0.2). The influence of the MLR on patient survival was determined using univariate Kaplan-Meier survival analysis, the generalized Wilcoxon test, and analysis with the multivariate Cox proportional hazards model.
Results
The 5-year survival rate of the patients with MLR0, MLR1, and MLR2 was 88.6%, 59.4%, and 13.4%, respectively. In addition to the MLR, the UICC/AJCC N category, JGCA n category, tumor stage (pT category), and tumor diameter significantly influenced the 5-year survival rate, as determined by univariate analysis. Multivariate analyses revealed that of the three factors used to stage lymph node involvement, MLR was the most significant prognostic factor.
Conclusions
The MLR is an important and easy-to-assess prognostic factor that should be considered for staging lymph node metastasis in patients with gastric cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lymph node metastasis is one of the most important prognostic factors of gastric cancer [1–3]. Current staging of lymph node metastasis in gastric cancer is variable, depending on number of metastatic nodes for the UICC system or, in the case of the JGCA system, the anatomical location of the affected nodes relative to the primary tumor. A standardized, globally accepted, and sufficiently reliable staging system has yet to be adopted. The Japanese Gastric Cancer Association (JGCA) system [4], wherein the n stage is defined considering the location of the nodal metastasis relative to the primary tumor, is complicated; therefore, this system is not commonly adopted in western countries. On the other hand, the TNM classification of malignant tumors (TNM) staging system developed by the International Union Against Cancer/American Joint Committee on Cancer (UICC/AJCC) [5] is less complicated, because as per this system, N stage classification is based on the number of lymph nodes involved rather than their location. However, a disadvantage of this system is that the number of metastatic lymph nodes is influenced by the number of resected nodes, which may lead to stage migration. Many studies have shown that staging based on the metastatic lymph node ratio (MLR), which is calculated by dividing the number of metastatic lymph nodes by the total number of nodes harvested, is a highly reliable system that can minimize stage migration errors [6–12]. This study was designed to (1) evaluate the prognostic significance and clinical impact of the MLR by assessing the clinical data of patients diagnosed with gastric cancer who underwent curative resection and (2) discuss our findings in light of the relevant available literature.
Materials and methods
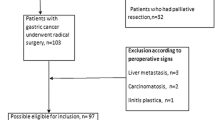
We retrospectively reviewed a cohort of 186 consecutive patients with gastric cancer who had undergone curative resection at our institution between January 2001 and December 2007. The tumors were staged according to the JGCA system [4]. Patients with metastatic disease who had undergone palliative resection were excluded from this study. All patients had undergone distal partial gastrectomy, proximal partial gastrectomy, or total gastrectomy along with regional lymphadenectomy; all surgical procedures were performed with curative intent and in accordance with the JGCA guidelines [13]. D2 lymphadenectomy dissecting all group 1 and group 2 lymph nodes according to the JGCA recommendations was performed in 158 patients. Another 28 cases underwent D1 + No. 7, 8a, 9 dissection because of early gastric cancer [13]. The mean number of examined lymph nodes was 33.7 (range, 4–94). Depending on the mean number of lymph nodes removed, the patients were classified into two groups (≤33 or >33). A member of the surgical team meticulously dissected all of the lymph nodes from the en block resected specimens and classified and numbered them as per the JGCA system [4], after which the nodes were pathologically examined. The following clinicopathological data were recorded for each patient: number of examined lymph nodes [≤33 or >33 (mean value)], number of metastatic lymph nodes (N0, N1, N2, and N3) [5], anatomical location of metastatic lymph nodes (n0, n1, and n2) [4], MLR, tumor stage (T1, invasion of the mucosa and submucosa; T2, invasion of the muscularis and subserosa; T3, invasion of the serosa; and T4, invasion to adjacent structures), tumor diameter (≤45 mm or >45 mm (mean value)), and histological type (differentiated or undifferentiated). Considering that patients with no nodal involvement and those with nodal involvement should not be grouped together, and that the mean MLR in patients with lymph node metastasis was 0.19, the patients were classified into the following three groups on the basis of the MLR: MLR0: 0, MLR1: 0.01–0.19, MLR2: ≥0.2.
The observation period ended on December 31, 2008. The median follow-up duration from the date of surgery was 35 (range, 1–91) months. Thirty-four patients (18.3%) were given postoperative adjuvant chemotherapy using UFT for nine cases, S-1 for eight cases, paclitaxel for seven cases, 5′-DFUR for five cases, and others for five cases. Thirty-nine patients (21%) died during follow-up period. Of them, 30 were related to recurrence of gastric cancer, 2 were due to another malignancy, and 7 were due to another disease or accident.
The cumulative survival was determined by the Kaplan-Meier method, and univariate comparisons between the groups were performed using the generalized Wilcoxon test. Covariates that remained significant throughout the univariate analysis were selected for multivariate analysis, which was performed using the Cox proportional hazard model and a step-wise procedure. Differences at p values <0.05 were considered significant.
Results
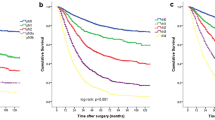
Our study involved 126 men (67.7%) and 60 women (32.3%) aged 30–88 (mean, 67.8) years. In total, 98 patients (52.7%) had early gastric carcinoma. Lymph node metastasis was observed in 71 cases (38.2%). The patient characteristics are presented in Table 1. The 5-year overall survival rate for the entire cohort was 73%. The clinicopathological records of the 186 patients and the 5-year survival rates are shown in Table 2. The 5-year survival rate was influenced by the UICC/AJCC N category (p < 0.01), JGCA n category (p < 0.001), MLR (p < 0.001), pT category (p < 0.001), and tumor diameter (p < 0.01). The number of lymph nodes dissected and their histological classification were not significant prognostic factors. The 5-year survival rates of the patients staged by the UICC/AJCC system were 88.6%, 52.1%, 36.3%, and 0.0% for N0, N1, N2, and N3, respectively. The 5-year survival rates of the patients staged by JGCA system were 88.6%, 54.4%, and 38.1% for n0, n1, and n2, respectively. The 5-year survival rates of the patients in the MLR0, MLR1, and MLR2 groups were 88.6%, 59.4%, and 13.4%, respectively, with statistically significant differences between the groups: MR0 versus MR1, p < 0.05; MLR0 versus MLR2, p < 0.001; and MLR1 versus MLR2, p < 0.05 (Fig. 1). Because three categories of lymph node metastasis (UICC/AJCC, JGCA, MLR) were already reported as multicollinear variables and simultaneous use of these variables might miss important prognostic factors, three multivariate analyses were conducted to know which N classification is important, using the following variables: (1) UICC N category, pT, tumor diameter, (2) JGCA n category, pT, tumor diameter, (3) MLR, pT, tumor diameter. Among variables of UICC N category, pT, and tumor diameter, UICC N category and pT were independent prognostic factors. Among variables of JGCA n category, pT, and tumor diameter, JGCA n category and pT were independent prognostic factors. On the other hand, among variables of MLR, pT, and tumor diameter, MLR was a sole independent prognostic factor. The hazard ratios of lymph node category calculated in each multivariate analysis were 1.7098 for UICC N category, 1.8547 for JGCA n category, and 2.2963 for MLR (Table 3). According to the above-mentioned results, MLR was the most significant prognostic factor for the patient with gastric cancer compared with the UICC/AJCC and JGCA systems.
Discussion
The staging system for the classification of lymph nodes around malignant tumors must be feasible, precise, and reproducible, thus enabling prognosis prediction, treatment planning, and comparison of the results from different institutions. The JGCA [4] and UICC/AJCC [5] systems are currently used for lymph nodes metastases in gastric cancer. The JGCA classification system [4] is based on the location of the metastatic lymph nodes relative to the primary tumor, whereas the UICC/AJCC is based on the number of metastatic lymph nodes. The JGCA system may be useful for the systematic lymphadenectomy in gastric cancer; however, surgeons and pathologists from western countries consider it a very complicated classification system for clinical use. On the other hand, several researchers have evaluated the UICC/AJCC system developed in 1977 and have suggested that it is associated with higher reproducibility and better prognostic stratification than the JGCA system [14–16]. One significant limitation of the UICC/AJCC system is that the number of metastatic nodes is influenced by the number of resected nodes. Extended lymph node dissection, which is commonly adopted in Japan, is not a routine procedure in gastric cancer surgery in many western countries. A published review on gastric cancer treatment in the United States reported that D2 lymph node dissection is performed on only 4–7% of all patients with gastric cancer who undergo gastrectomy [17]. In most cases of gastric cancer, the number of metastatic lymph nodes increases in proportion to the number of dissected nodes. Therefore, gastric cancer classified as N1 after limited lymph node dissection may be classified as N2 or N3 after extensive lymphadenectomy. At present, classification per the UICC/AJCC N stage system may be influenced by the surgeon’s attitude toward the lymph node dissection, especially in western countries, where D1 lymph node dissection is widely performed [6, 7]. Stage migration occurs with both the JGCA and UICC/AJCC classification systems at a rate of more than 15% [7].
The MLR, which is calculated by dividing the number of metastatic lymph nodes by the total number of nodes harvested, has been proposed as a new prognostic factor that is independent of the number of dissected lymph nodes with the goal of reducing stage migration. In 1990, Okusa et al. [18] were the first to demonstrate the impact of the “frequency of the metastases.” Thereafter, several studies on the significance of the MLR in gastric cancer have been reported in international literature; it has been considered a powerful prognostic parameter after radical resection for gastric cancer [6–12]. Siewert et al. [19] performed prospective multicenter trial to evaluate the 10-year outcomes of 1,653 patients with gastric cancer; they identified the MLR (≤0.2 vs. >0.2), together with the residual tumor status (R classification) as a major independent prognostic factor. Yu et al. [20] studied the cases of 886 patients who underwent R0 gastrectomy with D2 lymphadenectomy and found a significant prognostic difference between each subgroup classified on the basis of the MLR (N0: 0; N1: 0.01–0.25; N2: >0.25). The authors also emphasized that MLR-based classification is a simple, convenient, and reproducible method that can be used to predict surgical results. Several studies involving multivariate analysis have demonstrated the superiority of the MLR-based system compared with the UICC/AGCC and JGCA systems for staging lymph node metastasis in gastric cancer [6, 7, 11]. In this study, multivariate analyses to examine three modalities used for staging lymph node involvement (UICC/AJCC, JGCA, and MLR) revealed that MLR was the most significant prognostic factor for the patient of gastric cancer compared with the UICC/AJCC and JGCA systems. Because the number of gastric lymph nodes varies among individuals [7, 21], the total number of lymph nodes harvested varies even if standard D2 lymphadenectomy is performed for all patients. In contrast, the number of metastatic lymph nodes, which is influenced by the total number of dissected lymph nodes, depends on the attitude of the surgeon and on the accuracy of the pathological evaluation undertaken. The prognostic value of MLR may obviate the possible confounding factors related to variations between individuals in terms of the number of lymph nodes and the number of dissected lymph nodes [6]. In fact, patients with gastric cancer staged with the same grade of lymph node involvement as per the MLR-based system are found to have the same clinical outcomes regardless of the number of lymph nodes dissected [7]. Thus, compared with the UICC/AJCC and JGCA systems, the MLR classification system offers the advantage of a more accurate prognostic evaluation after D2 lymphadenectomy in a patient with advanced-stage gastric cancer.
There has been no consensus about the appropriate MLR cutoff value for gastric carcinoma and different cutoffs have been used in previous studies [4, 7, 8, 11, 20, 22]. In our study, lymph node involvement was classified as follows on the basis of MLR cutoffs: MLR0: 0, MLR1: 0.01–0.19, MLR2: ≥ 0.2. The MLR1 and MLR2 subgroups were separated at the cutoff point of 0.19, which was the mean value of MLR in patients with lymph node involvement. Because of small sample size (n = 186) of this study, we stratified the patients into three MLR groups with two cutoff points rather than four MLR groups. Cutoff point of 0 was adopted in most studies for MLR of gastric cancer [8, 11, 20] based on the idea that patients with no nodal involvement and those with nodal involvement should not be grouped together. The mean value of MLR in the patients with positive node (0.19) was selected for the second cutoff point, because it seemed to be the boundary between the groups in which lymph node metastasis was great or little. In fact, univariate analysis revealed a significant prognostic difference between each subgroup classified on the basis of the MLR using two cutoff points of 0 and 0.19 (Fig. 1). We also tried to analyze the 5-year survival rate of patients with gastric cancer classified by MLR staging with other cutoff points of 0 and 0.25 [20]; the result did not exceed the above-mentioned study using cutoff points of 0 and 0.19 (data were not shown). Although the mean MLR value may vary when a subject is examined at different institutions and may be influenced by the number of the cases examined, MLR-based stratification using two cutoff points of 0 and the mean value in the patients with lymph node involvement seems to be a simple, effective, and reproducible method for evaluating the prognosis of patients with gastric cancer who undergo curative gastrectomy.
Conclusions
Compared with the UICC/AJCC and JGCA systems, MLR-based staging is more effective for evaluating lymph node metastasis in patients with gastric cancer.
References
Adachi Y, Kamakura T, Mori M et al (1994) Prognostic significance of the number of positive lymph nodes in gastric carcinoma. Br J Surg 81:414–416
Yokota T, Ishiyama S, Saito T et al (2004) Lymph node metastasis as a significant prognostic factor in gastric cancer: a multi logistic regression analysis. Scand J Gastroenterol 39:380–384
Wu ZY, Li JH, Abe S et al (2007) Effect of lymph node micrometastases on prognosis of gastric carcinoma. World J Gastroenterol 13:4122–4125
Japanese Gastric Cancer Association (1998) Japanese classification of gastric carcinoma, 2nd English edition. Gastric Cancer 1:10–24
Sobin LH, Fleming ID (1997) TNM classification of malignant tumors, Fifth edition Union Internationale Contre le Cancer and American Joint Committee on Cancer. Cancer 80:1803–1804
Nitti D, Marchet A, Olivieri M et al (2003) Ratio between metastatic and examined lymph nodes is an independent prognostic factor after D2 resection for gastric cancer: analysis of a large European monoinstitutional experience. Ann Surg Oncol 10:1077–1085
Bando E, Yoneyama Y, Taniguchi K et al (2002) Outcome of ratio of lymph node metastasis in gastric carcinoma. Ann Surg Oncol 9:775–784
Inoue K, Nakane Y, Iiyama H et al (2002) The superiority of ratio-based lymph node staging in gastric carcinoma. Ann Surg Oncol 9:27–34
Ozgüc H, Sönmer Y, Yerci O (2008) Metastatic/resected lymph nodes ratio-based classification in gastric cancer. Turk J Gastroenterol 19:2–7
Saito H, Fukumoto Y, Osaki T et al (2008) Prognostic significance of the ratio between metastatic and dissected lymph nodes (n ratio) in patients with advanced gastric cancer. J Surg Oncol 97:132–135
Persiani R, Rausei S, Biondi A et al (2008) Ratio of metastatic lymph nodes: impact on staging and survival of gastric cancer. Euro J Surg Oncol 34:519–524
Huang CM, Lin BJ, Lu HS et al (2008) Prognostic impact of metastatic lymph node ratio in advanced gastric cancer from cardia and fundus. World J Gastroenterol 14:4383–4388
Yamaguchi T (2003) JGCA gastric cancer treatment guideline: a new trend in gastric cancer treatment. Jpn Medical Association J 46:238–245
Ichikura T, Tomimatsu S, Uefuji K et al (1999) Evaluation of the New American Joint Committee on Cancer/International Union against cancer classification of lymph node metastasis from gastric carcinoma in comparison with the Japanese classification. Cancer 86:553–558
Fujii K, Isozaki H, Okajima K et al (1999) Clinical evaluation of lymph node metastasis in gastric cancer defined by the fifth edition of the TNM classification in comparison with the Japanese system. Br J Surg 86:685–689
Hemaneck P (2000) The superiority of the new International Union Against Cancer and American Joint Committee on Cancer TNM staging of gastric carcinoma. Cancer 88:1763–1765
Hundahl SA, Phillips JL, Menck HR (2000) National Cancer Data Base report on poor survival of U.S. gastric carcinoma patients treated with gastrectomy: Fifth Edition American Joint Committee on Cancer staging, proximal disease, and the “different disease” hypothesis. Cancer 88:921–932
Okusa T, Nakane Y, Boku T et al (1990) Quantitative analysis of nodal involvement with respect to survival rate after curative gastrectomy for carcinoma. Surg Gynecol Obstet 170:488–494
Siewert JR, Bottcher FK, Stein HJ, Roder JD (1998) Relevant prognostic factors in gastric cancer: ten-year results of the German Gastric Cancer Study. Ann Surg 228:449–461
Yu W, Choi GS, Whang J et al (1997) Comparison of five systems for staging lymph node metastasis in gastric cancer. Br J Surg 84:1305–1309
Wagner PK, Ramaswamy A, Schmitz-Moormann P et al (1991) Lymph node counts in the upper abdomen: anatomical basis for lymphadenectomy in gastric cancer. Br J Surg 78:825–827
Kodera Y, Yamamura Y, Shimizu Y et al (1998) Lymph node status assessment for gastric carcinoma: is the number of metastatic lymph nodes really practical as a parameter for N categories in the TNM classification? J Surg Oncol 69:15–20
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fukuda, N., Sugiyama, Y., Midorikawa, A. et al. Prognostic Significance of the Metastatic Lymph Node Ratio in Gastric Cancer Patients. World J Surg 33, 2378–2382 (2009). https://doi.org/10.1007/s00268-009-0205-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-009-0205-1