Abstract
Background
The lymph node ratio (LNR) and log odds of positive lymph nodes (LODDS) have been proposed to minimize the stage migration phenomenon. The value of the LODDS and LNR staging systems to predict and discriminate prognosis was assessed and compared to the International Union Against Cancer (UICC) TNM classification (pN).
Methods
Three hundred and twenty-six patients with gastric carcinoma were retrospectively studied. Disease-specific survival rates were calculated for every pN, LNR, and LODDS category.
Results
Four LNR categories (0, 1–25, 26–75, and >76 %) and four LODDS categories (−5 to −3, > −3 to −1, > −1 to 3, and >3 to 5) were established. In the multivariate analysis, only the stage pT3-4 versus pT1-2 (HR 1.88, 95 % CI 1.11–3.20, p = 0.02) and LODDS as continuous variable (HR 1.40, 95 % CI 1.21–1.61, p < 0.001) remained as independent prognostic factors. In patients with <16 lymph nodes retrieved, only the LODDS system could discriminate different disease-specific survival curves for every category. LODDS categories were able to discriminate subgroups with different prognoses in pN stages and LNR categories.
Conclusions
The LODDS staging system was superior to the pN classification and LNR system to discriminate risk prognosis especially in patients with an insufficient number of retrieved lymph nodes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lymph node (LN) status and the depth of invasion are the most important prognostic factors in gastric carcinomas when peritoneal or distant dissemination is absent.1 , 2 In the setting of an R0 surgical resection, the prognosis of gastric cancer is poor when LN metastasis exists in spite of the performance of extended lymphadenectomies.2 , 3 Currently, the benefits of extended D2 lymphadenectomy are not well established in Western countries,4 , 5 although current clinical guidelines recommend the spleen- and pancreas-preserving D2 lymphadenectomy for gastric resections.6 , 7
The seventh edition of the International Union Against Cancer (UICC) TNM staging classification for gastric carcinomas is currently widely accepted for LN staging.8 It recommends a minimum of 16 LNs to be analyzed even though gastric tumors can be staged as negative LN (pN0) with fewer retrieved LNs. Studies on Western population registries have reported that 15 or more LNs were only retrieved in 18–31 % of the cases.9 – 11 Recently, an upward trend in the mean numbers of retrieved LNs over the last decade has been reported in US institutions, but the percentage of sufficient retrieved LNs still remains below 50 %.12
Some studies have shown that the LN ratio (LNR), which takes into account the total number of retrieved LNs, is more accurate in differentiating the prognosis than in considering the number of positive LNs alone (pN),13 – 15 even when fewer than 15 LNs are analyzed.16 , 17 The log odds of positive LNs (LODDS) is a new staging system that considers the probability that an LN is positive and the probability that an LN is negative when one LN is retrieved. This system can discriminate between the prognoses for pN0 tumors, and it was shown to be more reliable than the pN or LN ratio classifications in gastric cancer patients when insufficient numbers of LNs were retrieved.18
The aim of our study was to establish a prognostic stratification of gastric cancer calculating the LNR and LODDS. Subsequently, the 5-year survival rates of these staging systems were compared to pN from the UICC TNM classification to assess the usefulness of LNR and LODDS staging systems to predict and discriminate prognoses.
Methods
Patients
Data from patients with non-metastatic gastric carcinoma who underwent surgical R0 resection between 2004 and 2010 were retrospectively collected from Elche University General Hospital, Castellon University General Hospital, and La Fe University and Technological Hospital. These three hospitals are located in the Comunitat Valenciana at the Mediterranean coast in the east of Spain (Europe).
Patients with positive peritoneal cytology and non-resectable tumors or those who were treated with induction chemotherapy prior resection were excluded. Written informed consent was obtained from all patients before treatment.
Perigastric LN stations (1 to 6) were removed in the D1 lymphadenectomies. LN stations 7, 8a, 9, and 11p were additionally removed in the modified D2 lymphadenectomies. D1 or modified D2 lymphadenectomies were chosen according to the surgeon’s criteria. The tumors were staged according to the 7th edition UICC TNM classification.8
The following variables were studied: age, gender, location, histological type, type of gastrectomy, depth of invasion (pT), total and positive LNs retrieved (pN), extent of the LN dissection as stated by the surgeon, adjuvant treatment, recurrence, and overall and disease-specific mortality.
Patients were followed up after surgery every 4 months for the first and second years and every 6 months thereafter. The routine examination during follow-up included a clinical anamnesis and physical examination, blood analysis, and abdominal ultrasound or CT scan every 6 months.
LNR and LODDS Intervals
LNR values represent the ratio of the number of positive lymph nodes to the total number of retrieved LNs and they range from 0 to 1. Initially, the metastatic LNR was stratified into five subgroups (0–100 every 25 %) to evaluate the increased risk. Subsequently, these LNR intervals were regrouped in fewer categories considering patients’ disease-specific survival (log-rank statistic).
LODDS values were defined as the log e ([pLN + 0.5]/[nLN + 0.5]), where pLN is the number of positive lymph nodes and nLN the number of negative lymph nodes. A value of 0.5 was added to both numerator and denominator to avoid singularity. The LODDS value was calculated for each case. Then, the different risk groups from best to worst were chosen by means of the final cutoffs established according to the patients’ disease-specific survival (log-rank statistic).
Statistical Analysis
The Fisher exact test, Student’s t test, and Spearman’s rho correlation coefficient were used in the univariate analysis.
A Kaplan-Meier survival analysis was performed with the log-rank test and univariate hazard ratio (HR) to estimate differences in the overall survival and disease-specific survival. Multivariate survival analysis was used by means of Cox’s regression and the hazard ratio with a 95 % confidence interval. To strengthen the prognostic value of LNR and LODDS in our series, a multivariate survival analysis including the variables LODDS, LNR, and pN as continuous variables was performed.
Then, disease-specific survival rates, based on pN, LNR, and LODDS classifications according to the number of retrieved LNs, were compared by the log-rank pairwise test. Stata for Windows, version 12 (StataCorp, College Station, Texas), was used to perform the statistical analysis.
Results
Clinical and Pathological Data
Three hundred and twenty-six patients were included in the study. The clinical and pathological data of the patients are summarized in Table 1. No differences were found in the median age and sex distributions among the three centers. One hundred eleven patients were older than 75 years (34 %). Two hundred and thirty-one (71 %) patients underwent modified D2 lymphadenectomies. A higher number of modified D2 lymphadenectomies were carried out in patients ≤75 years (79 %) opposite to patients >75 years (54 %) being the extent of the LN dissection correlated negatively with the patient’s age (Spearman coefficient = −0.304, p < 0.001).
The percentage of pT3 or pT4 tumors (60.3–64.7 %) and the percentages of pN0 (38.6–47 %) and pN positive (53–61.4 %) tumors at the three institutions were not significantly different. A similar percentage of pN2 tumors was diagnosed in each hospital (13.7–16 %), but differences were observed in pN1 (8.5–18 %) and pN3 (19–39.2 %) carcinomas. Sixteen or more LNs were harvested in 63 % of the patients although there were significant differences between one hospital (83.7 %) and the other two (42–49.3 %). A significant correlation was found between the number of involved lymph nodes and the number of removed lymph nodes (Spearman coefficient = 0.387, p < 0.001).
Fourteen patients died during the postoperative period (4.3 %). Adjuvant treatment was administered to 21 patients (15.6 %) with pN0 and 138 patients (72.3 %) with pN-positive carcinomas without differences among the three hospitals. Median follow-up was 28 months (range 2–105, interquartile range 12–50).
LNR and LODDS Categories
LNR and LODDS categories were stratified according to the results of the log-rank test. Finally, four LNR categories were established (LNR 0 = 0 %, LNR 1 = 1–25 %, LNR 2 = 26–75 %, LNR 3 ≥ 76 %), and the differences were significant between each category in the univariate log-rank test (Table 2). Related to LODDS, four risk categories were also established (LODDS 1 = −5 to −3, LODDS 2 = more than −3 to −1, LODDS 3 = more than −1 to 3, LODDS 4 = more than 3 to 5). The disease-specific survival rates decreased with increasing LODDS in the univariate log-rank test (Table 2). To show how the LODDS system is related with pN classification, a diagram of the relationship between LODDS and the pN seventh edition groups were plotted (Fig. 1).
With each pN stage, LNR and LODDS categories were evaluated with the log-rank pairwise test (Table 3), and it was found that the LODDS 1 carried the lowest disease-specific mortality, included pN0 tumors with more than 10 LNs analyzed, and pN1 tumors with an LNR up to 3 %. Any pN2 or pN3 tumors could be included in this category. The next LODDS 2 category carried a higher disease-specific mortality than LODDS 1, included pN0 tumors with less than 10 LNs analyzed, and pN1, pN2, and pN3 tumors with an LNR from 3 to 26 %. The LODDS 3 category, which carried a higher disease-specific mortality than LODDS 2, included pN1, pN2, and pN3 tumors with an LNR of ≥25 %. Any pN0 tumor was present in this category. Finally, the LODDS 4 category, which carried ominous risk prognoses, included only pN3a or pN3b tumors with LNR of 100 %; all these patients had a disease-specific survival less than 20 months (Fig. 2).
Survival Univariate and Multivariate Analysis
Univariate and multivariate analysis of the disease-specific cumulated survival is shown in Table 2. After a multivariate survival analysis, only the stage pT3-4 versus pT1-2 (HR 1.88, 95 % CI 1.11–3.20, p = 0.020) and LODDS as a continuous variable (HR 1.40, 95 % CI 1.21–1.61, p < 0.001) remained as independent prognostic factors.
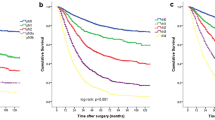
The disease-specific long-term survival curves corresponding to the pN, LNR, and LODDS staging systems are shown in Fig. 2. All three staging systems could properly discriminate between categories, as the differences were significant with the log-rank pairwise comparison. However, when the three staging systems were assessed depending on whether <16 LNs or ≥16 LNs were retrieved, only the LODDS system could clearly discriminate disease-specific survival curves for every category (Fig. 3). Thus, in patients with <16 LNs retrieved, the disease-specific survival curves between pN2 and pN3, LNR 1 and LNR 2, and LNR 2 and LNR 3 were not significantly different in the log-rank pairwise test. On the other hand, the differences were significant between all categories of LODDS. In patients with ≥16 retrieved LNs, the pN0 and pN1 and the LNR 2 and LNR 3 disease-specific survival curves were not significantly different in the log-rank pairwise comparison. In this group, the disease-specific survival curves of LODDS 3 and LODDS 4 did not reach significant differences (p = 0.074), but all the six patients included in LODDS 4 category died within a period of 20 s (Fig. 3). In addition, when LODDS, LNR, and pN were analyzed as continuous variables, only LODDS resulted as a strong independent prognostic variable (Table 4).
When the pN0 and pN1 tumors were assessed together, the survival curves of those having 10 or more analyzed LNs were significantly better than the survival curves of those with less than 10 LNs analyzed (p < 0.005).
Discussion
In this study, LODDS classification and pT staging remained as the only independent variables in the multivariate survival analysis, which support the usefulness of LODDS to discriminate patients with different prognoses attending to disease-specific survival. In addition, using a log-rank pairwise comparison, the LODDS categories were able to discriminate subgroups in pN stages and LNR categories with different prognoses. Since Sun et al. emphasized the value of LODDS for prognostic assessment of LN metastasis in gastric cancer,18 some recent reports have compared pN classification to LNR and LODDS staging systems19 – 23 with disappointing conclusions, most likely due to the use of different statistical tools for assessment. While some of these studies have certified a superiority of LODDS staging discriminating patients with the same pN or LNR categories but having different survival rates,18 – 20 others did not find a superiority of LODDS over pN21 or LNR.22 , 23 Altogether, these studies strongly support that not only the number of metastatic LNs but also the number of retrieved LNs are important to discriminate patients with different survival rates.
The superiority of LODDS classification over pN classification has been mainly attributed to its potential of minimizing the stage migration phenomenon when an insufficient number of LNs are analyzed.18 , 19 The optimal number of retrieved LNs to be considered sufficient for avoiding stage migration is still controversial,8 , 11 , 24 – 26 and it is an important point in Western countries where the retrieval of more than 15 LNs is achieved in less than half of the patients.9 – 12 In our study, the LODDS staging system was able to discriminate patients with different survival rates whether ≥16 or <16 LNs were retrieved. On the contrary, pN classification and LNR categories could not properly discriminate patients with different survival rates in patients with <16 LNs analyzed. These results are substantially in agreement with those reported by Wang et al. where LODDS showed a clear advantage over pN classification regardless of the total number of retrieved LNs; a superiority of LODDS over LNR was shown although no significant difference was found between these two staging systems.20 Additionally, in the study of Sun et al., the LODDS staging system was shown to be superior to pN and LNR classifications, but this was restricted to patients in whom an insufficient number of LNs were retrieved.18 Two other studies that compared LN staging systems did not demonstrate superiority of LODDS over LNR,22 , 23 but in one of them, only patients with more than 15 LNs analyzed were included.22
Some studies have previously reported that the LNR staging system can also be useful to discriminate prognosis among pN1 and pN2 patients even when fewer than 15 LNs are analyzed.16 , 17 However, the LNR system can only differentiate prognoses between patients with positive LNs; moreover, the LNR seems not accurate enough in discriminating survival rates from patients in which all LNs are metastatic.20 Clinical consequence of the pN0 misclassification when few LNs are retrieved is critical because migration from negative to positive LNs currently determines the need to administer adjuvant treatment in gastric carcinomas.6 , 7 For pN0 patients with few LNs harvested, the LODDS staging system seems to be especially useful to detect different subgroups of risk prognosis.18 – 20 This fact was also confirmed in our study in which two different categories of LODDS with different disease-specific survival rates were identified in the group of pN0 patients depending on whether <10 or ≥10 LNs were analyzed. A minimum number of 10 LNs retrieved has been shown as crucial in other studies using different approaches to minimize the stage migration.10 , 11 , 27
In our study, the retrieval of more than 15 LNs was only achieved in 64 % of patients. This percentage is sparse compared to those of Asian series and reports from some esophagogastric units, but it is better than those of other Western studies based on cancer registries.9 – 12 In this context, the LODDS staging system can be very useful to discriminate prognosis in patients classified with UICC TNM classification especially when an insufficient number of LNs are retrieved. Although the tumor burden of LNs is better assessed with the LNR and LODDS staging systems that take into account the number of LNs analyzed, UICC TNM classification continues to be the gold standard to classify the nodal status of gastric cancer. Harvesting as many LNs as possible must be a challenge for both surgeons and pathologists to decrease the effects of stage migration.11 , 26 , 28 This was also confirmed in our study where a significant correlation was found between the number of metastatic LN detected and the retrieved LNs. Finally, the most important limit of LODDS is that there are no universal cutoff points that can be used to assess the LN-related prognosis because they depend on the characteristics of the particular series.
Conclusions
Our study supports the usefulness of LODDS to discriminate risk prognosis especially in patients where an insufficient number of LNs are retrieved. The LODDS system was able to identify and discriminate different subgroups of risk prognosis in pN stages and LNR categories.
References
Maruyama K, Gunven P, Okabayashi K, Sasako M, Kinoshita T. Lymph node metastases of gastric cancer. General pattern in 1931 patients. Ann Surg 1989; 210 (5): 596–602
Siewert JR, Bottcher K, Stein HJ, Roder JD. Relevant prognostic factors in gastric cancer. Ten-year results of the German Gastric Cancer Study. Ann Surg 1998; Vol. 228 (4), 449–461
Kim JP. Surgical Results in Gastric Cancer Semin Surg Oncol 1999; 17:132–138
Cuschieri A, Weeden S, Fielding J J Bancewicz, J Craven, V Joypaul, et al. Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MCR randomized surgical trial. Br J Cancer 1999; 79:1522–1530
Bonenkamp JJ, Hermans J, Sasako M, van de Velde CJH, et al. Extended lymph-node dissection for gastric cancer. N Engl J Med 1999; 340: 908–914
van Cutsem, Dicato M, Geva R, Arber N, Bang Y, Bernson A, et al. The diagnosis and management of gastric cancer: expert discussion and recommendations from the 12th ESMO/World Congress on Gastrointestinal Cancer, Barcelona, 2010. Ann Oncol 2011; 22 (Suppl 5): 1–9
Gastric Cancer. NCCN Clinical Practice Guidelines in Oncology. V1.2013. www.nccn.org
Sobin LH, Gospodarowicz MK, Wittekind C (2009) TNM classification of malignant tumours, 7th edn. Wiley-Blackwell, New York: pp 73–77
Mullaney PJ, Wadley MS, Hyde C, Wyatt J, Lawrence G, Hallissey MT, et al. Appraisal of compliance with the UICC/AJCC staging system in the staging of gastric cancer. Br J Surg 2002; 89: 1405–1408.
Bouvier AM, Haas O, Piard F, Roignot Ph, Bonithon-Kopp C, Faivre J. How many nodes must be examined to accurately stage gastric carcinomas? Results from a population based study. Cancer 2002; 94: 2862–2866.
Smith DD, Schwartz RR, Schwarz RE. Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: Data from a large US-population database. J Clin Oncol 2005; 23:7114–7124.
Dubecz A, Solymosi N, Schweigert M, Stadlhuber RJ, Peters JH, Ofner D, et al. Time trends and disparities in lymphadenectomy for gastrointestinal cancer in the United States: a population-based analysis of 326,243 patients. J Gastrointest Surg 2013; 17(4):611–619
Inoue K, Nakane Y, Iiyama H, Sato M, Kanbara T, Nakai K, et al. The superiority of ratio-based lymph node staging in gastric carcinoma. Ann Surg Oncol 2002; 9(1):27–34.
Nitti D, Marchet A, Olivieri M, Ambrosi A, Mencarelli R, Belluco C et al. Ratio between metastatic and examined lymph nodes is an independent prognostic factor after D2 resection for gastric cancer: analysis of a large European monoinstitutional experience. Ann Surg Oncol 2003; 10: 1077–1085.
Rodriguez Santiago JM, Munoz E, Marti M, Quintana S, Veloso E, Marco C. Metastatic lymph node ratio as a prognostic factor in gastric cancer. Eur J Surg Oncol 2005; 31:59–66.
Marchet A, Mocellin S, Ambrosi A, Morgagni P, Garcea D, Marrelli D. The ratio between metastatic and examined lymph nodes (N ratio) is an independent prognostic factor in gastric cancer regardless of the type of lymphadenectomy: results from an Italian multicentric study in 1853 patients. Ann Surg 2007; 245(4): 543–52
Wang J, Dang P, Raut CP, Pandalai PK, Maduekwe UN, Rattner DW, et al. Comparison of a lymph node ratio–based staging system with the 7th AJCC system for gastric cancer. Analysis of 18,043 patients from the SEER database. Ann Surg 2012; 255:478–485
Sun Z, Xu Y, Li DM, Wang ZN, Zhu GL, Huang BJ, et al. Log Odds of positive lymph nodes. A novel prognostic indicator superior to the number-based and the ratio-based N category for gastric cancer patients with R0 resection. Cancer 2010; 116:2571–2580
Qiu MZ, Qiu HJ, Wang ZQ, Ren C, Wang DS, Zhang DS, et al. The Tumor-Log Odds of Positive Lymph Nodes-Metastasis Staging System, a Promising New Staging System for Gastric Cancer after D2 Resection in China. PLoS One 2012; 7(2):e31736. doi: 10.1371/journal.pone.0031736
Wang X, Appleby DH, Zhang X, Gan L, Wang JJ, Wan F. Comparison of three lymph node staging schemes for predicting outcome in patients with gastric cancer. Br J Surg 2013; 100(4):505–514
Xu J, Bian Y, Jin X, Cao H. Prognostic assessment of different metastatic lymph node staging methods for gastric cancer after D2 resection. World J Gastroenterol 2013; 19(12): 1975–1983
Liu H, Deng J, Zhang R, Hao X, Jiao X, Liang H. The RML of lymph node metastasis was superior to the LODDS for evaluating the prognosis of gastric cancer. Int J Surg 2013; 11: 419–424
Smith DD, Nelson RA, Schwarz RE. A comparison of five competing lymph node staging schemes in a cohort of resectable gastric cancer patients. Ann Surg Oncol 2014; 21 (3): 875–882.
Roder JD, Bottcher K, Busch R, Wittekind C, Hermanek P, Siewert JR. Classification of regional lymph node metastasis from gastric carcinoma. German Gastric Cancer Study Group. Cancer 1998; 82: 621–631.
Lee HK, Yang HK, Kim WH, Lee KU, Choe KJ, Kim JP. Influence of the number of lymph nodes examined on staging of gastric cancer. Br J Surg 2001; 88(10):1408–1412.
Seevaratnam R, Bocicariu A, Cardoso R, Yohanathan L, Dixon M, Law C, et al. How many lymph nodes should be assessed in patients with gastric cancer? A systematic review. Gastric Cancer 2012; 15 (Suppl 1):S70–S88
Martinez-Ramos D, A. Calero, J. Escrig-Sos, Mingol F, Daroca-Jose JM, Sauri M, Arroyo A, et al. Prognosis for gastric carcinomas with an insufficient number of examined negative lymph nodes Eur J Surg Oncol 2014; 40(3): 358–365.
Ichikura T, Ogawa T, Chochi K, Kawabata T, Sugasawa H, Mochizuki H. Minimum number of lymph nodes that should be examined for the International Union Against Cancer/American Joint Committee on Cancer TNM classification of gastric carcinoma. World J Surg. 2003; 27(3):330–333
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Calero, A., Escrig-Sos, J., Mingol, F. et al. Usefulness of the Log Odds of Positive Lymph Nodes to Predict and Discriminate Prognosis in Gastric Carcinomas. J Gastrointest Surg 19, 813–820 (2015). https://doi.org/10.1007/s11605-014-2728-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-014-2728-5